Feasibility Study of Single-Port Laparoscopic Techniques for Pancreatic Exploration, Ultrasound, and Biopsy in Dogs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
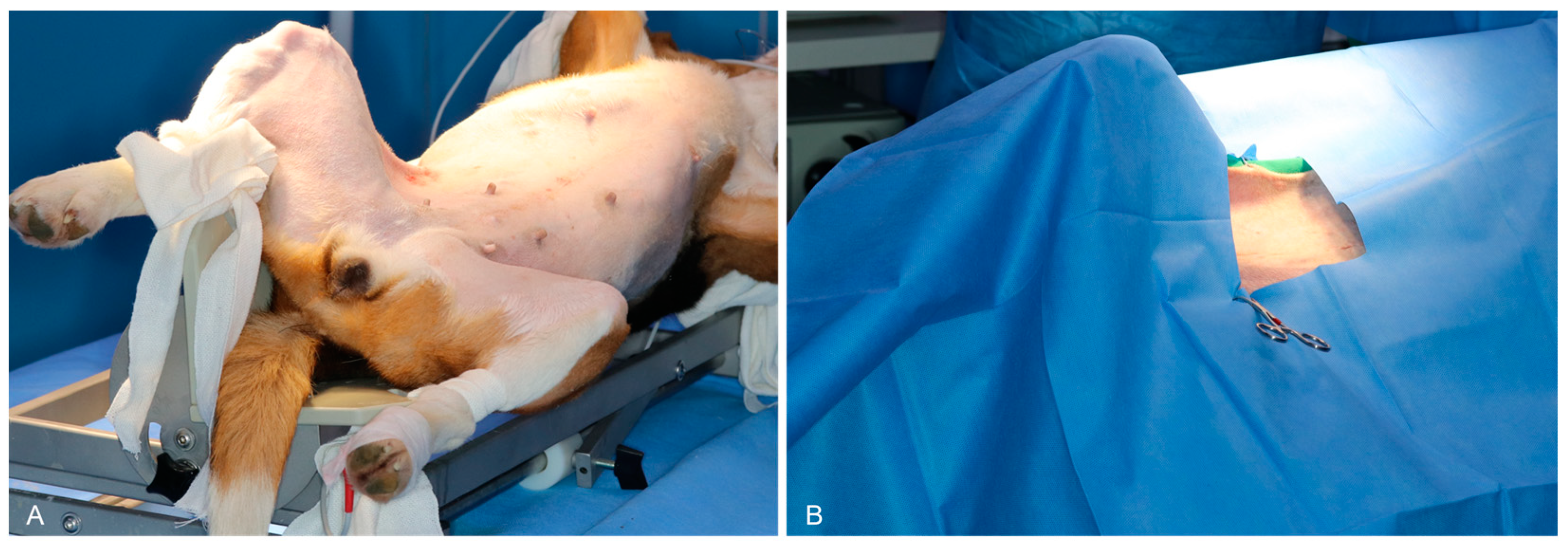
2.2. Anesthesia
2.3. Surgical Procedures
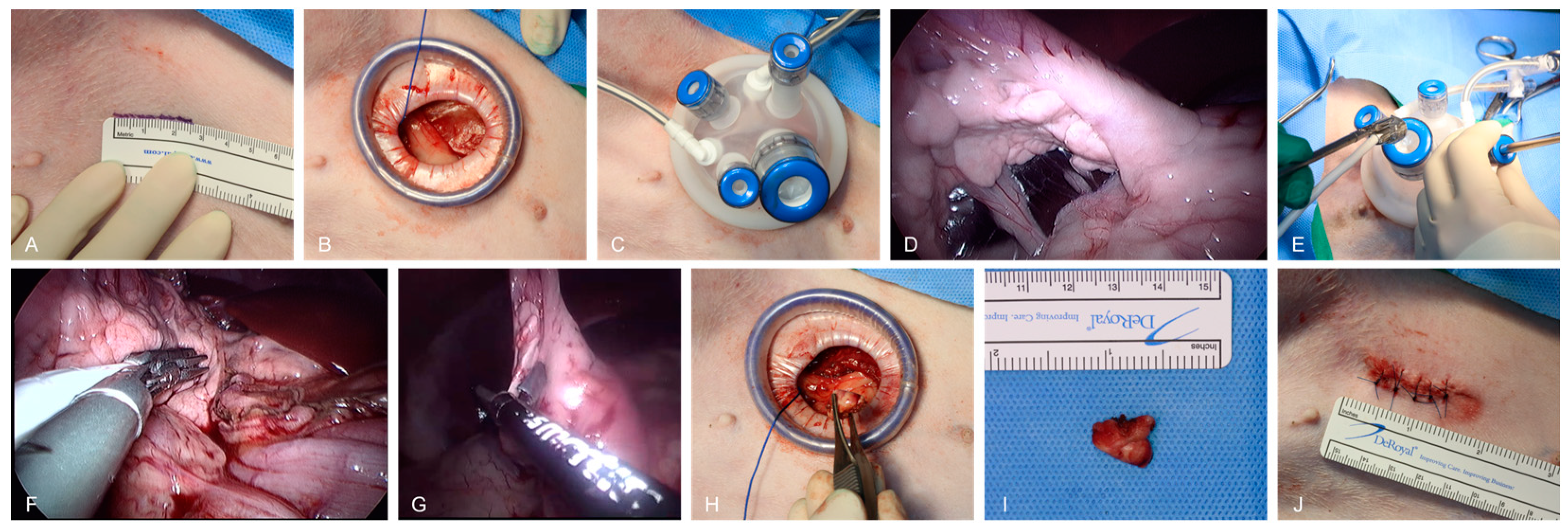
2.3.1. Pancreatic Exploration
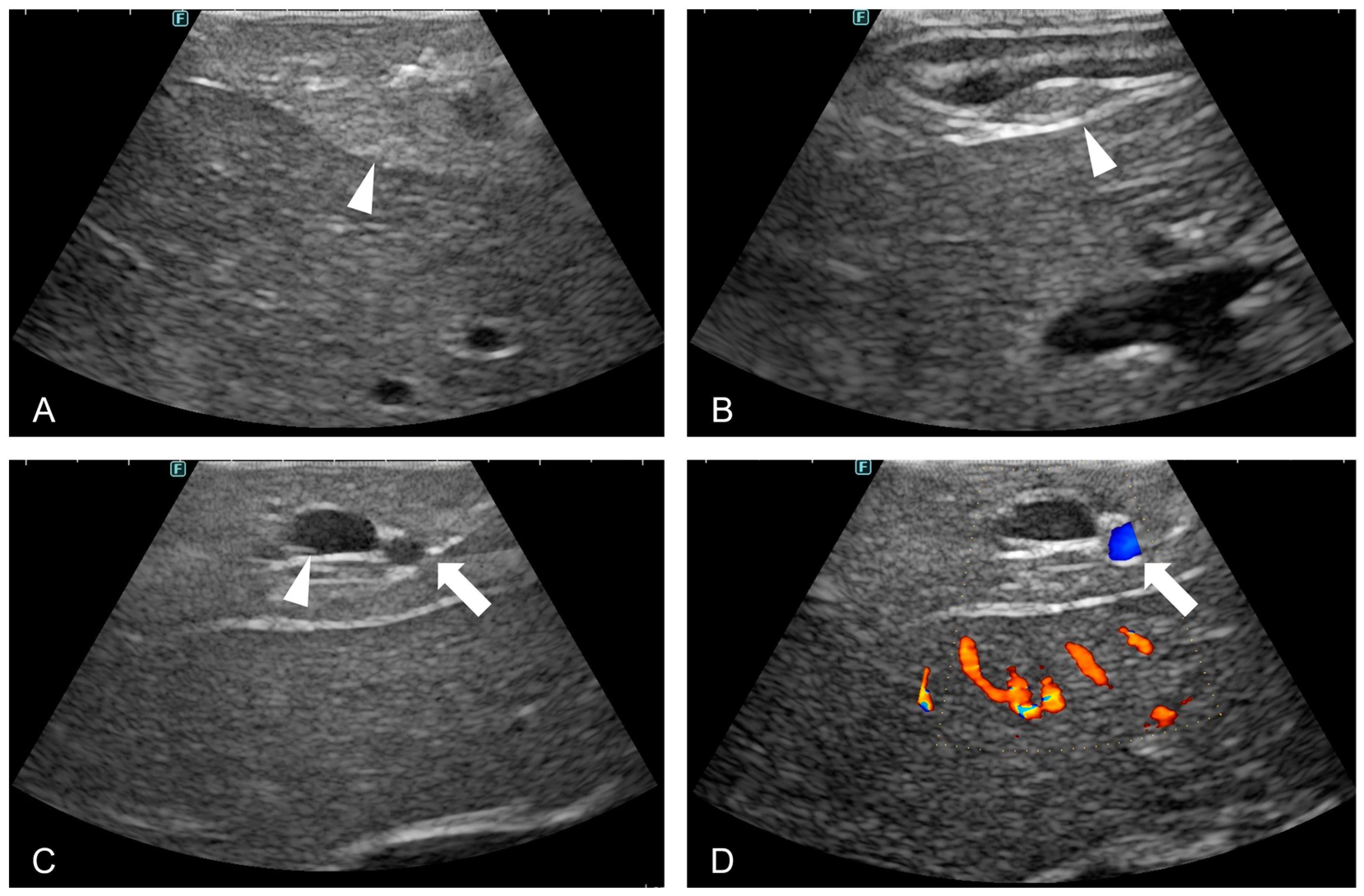
2.3.2. Laparoscopic Ultrasound
2.3.3. Laparoscopic Pancreatic Biopsy
2.4. Closure
2.5. Postoperative Care
2.6. Data Collection
3. Results
3.1. Pancreatic Exploration
3.2. Laparoscopic Ultrasound
3.3. Pancreatic Biopsy
3.4. Incision Length
3.5. Postoperative Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Remedios, A.M.; Ferguson, J. Minimally invasive surgery: Laparoscopy and thoracoscopy in small animals. Compend. Contin. Educ. Pract. Vet. 1996, 11, 1191–1199. [Google Scholar]
- Dusterdieck, K.F.; Pleasant, R.S.; Lanz, O.I.; Hooper, R.N.; Howard, R.D. Evaluation of the harmonic scalpel for laparoscopic bilateral ovariectomy in standing horses. Vet. Surg. 2003, 32, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, C.A.; Mahaffey, M.B.; Bement, S.; Canalis, C. Prospective evaluation of laparoscopic-assisted gastropexy in dogs susceptible to gastric dilatation. J. Am. Vet. Med. Assoc. 2002, 221, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.P. Laparoscopy in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2001, 31, 707–727. [Google Scholar] [CrossRef] [PubMed]
- Petre, S.L.; Kovak McClaran, J.; Bergman, P.J.; Monette, S. Safety and efficacy of laparoscopic hepatic biopsy in dogs: 80 cases (2004–2009). J. Am. Vet. Med. Assoc. 2012, 240, 181–185. [Google Scholar] [CrossRef]
- Simons, M.; Isaaz, K.; Le Moine, F.; Klompenhouwer, A.J.; van Lienden, K.P. Surgical pancreatic biopsies for cases with locally advanced pancreatic cancer with inconclusive histology after interventional biopsy. Surg. Oncol. 2023, 43, 101882. [Google Scholar]
- Park, J.; Lee, J.; Lee, H.B.; Jeong, S.M. Laparoscopic kidney biopsy in dogs: Comparison of cup forceps and core needle biopsy. Vet. Surg. 2017, 46, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.B.; Trott, C. Laparoscopic diagnosis of pancreatic disease in dogs and cats. J. Vet. Intern. Med. 2008, 22, 1263–1266. [Google Scholar] [CrossRef] [PubMed]
- Culp, W.T.; Mayhew, P.D.; Brown, D.C. The effect of laparoscopic versus open ovariectomy on postsurgical activity in small dogs. Vet. Surg. VS 2009, 38, 811–817. [Google Scholar] [CrossRef]
- Kim, H.W.; Oh, Y.; Choi, J.H.; Kim, D.Y.; Youn, H.Y. Use of laparoscopy for diagnosing experimentally induced acute pancreatitis in dogs. J. Vet. Sci. 2014, 15, 551–556. [Google Scholar] [CrossRef]
- de Rooij, T.; Lu, M.Z.; Steen, M.W.; Gerhards, M.F. Minimally invasive versus open pancreatoduodenectomy: Systematic review and meta-analysis of comparative cohort and registry studies. Ann. Surg. 2016, 264, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.B. (Ed.) Laparoscopic Surgery: Principles and Procedures, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar] [CrossRef]
- Helton, W.S.; Rose, J.B. Intraoperative laparoscopic ultrasound during pancreatic surgery. In Abdominal Ultrasound for Surgeons; Hagopian, E., Machi, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Kirshtein, B.; Haas, E.M. Single port laparoscopic surgery: Concept and controversies of a new technique. Surg. Laparosc. Endosc. Percutaneous Tech. 2012, 22, 507–512. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dupré, G.; Fiorbianco, V.; Skalicky, M.; Gültiken, N.; Ay, S.S.; Findik, M. Laparoscopic ovariectomy in dogs: Comparison between single portal and two-portal access. Vet. Surg. 2009, 38, 818–824. [Google Scholar] [CrossRef]
- David, S.; de Rooster, H.; Van Goethem, B. Single-port laparoscopic-assisted abdominal cryptorchidectomy in 14 dogs. Vet. Surg. 2024, 53, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Khalaj, A.; Bakhtiari, J.; Niasari-Naslaji, A. Comparison between single and three portal laparoscopic splenectomy in dogs. BMC Vet. Res. 2012, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Jeong, J.; Lee, S.; Son, H.; Kweon, O.K.; Kim, W.H. Feasibility of single-port retroperitoneoscopic adrenalectomy in dogs. Vet. Surg. VS 2018, 47 (Suppl. 1), O75–O83. [Google Scholar] [CrossRef] [PubMed]
- Poggi, E.; Lillo-Araya, F.J.; Garcia Rubio, D.; Pérez Duarte, F.J.; Gutiérrez del Sol, J.; Izzo, F.; Cinti, F. Laparoscopic resection of pancreatic masses in 12 dogs. Vet. Surg. 2023, 53, 860–871. [Google Scholar] [CrossRef]
- Case, J.B.; Adin, C.; Crews, C.; Gilor, C. Laparoscopic partial pancreatectomy of the left limb using a harmonic scalpel in nine cats. Vet. Surg. 2023, 53, 350–356. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, H.; Tan, Z.; Li, G.; Xu, Z.; Zhou, J.; Fu, J.; Wu, M.; Xi, J.; Wang, Y. Long-term outcomes of single-incision plus one-port laparoscopic surgery versus conventional laparoscopic surgery for rectosigmoid cancer: A randomized controlled trial. BMC Cancer 2023, 23, 1204. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.Y.; Lee, C.S.; Lee, Y.S. Single incision laparoscopic appendectomy using a new multi-joint articulating instrument. J. Gastrointest. Surg. 2021, 25, 2437–2438. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, Y.S.; Kim, J.H. Early experience with the ARTISENTIAL® articulated instruments in laparoscopic low anterior resection. Tech. Coloproctol. 2022, 26, 385–387. [Google Scholar] [CrossRef]
- Hecht, S.; Henry, G. Sonographic evaluation of the normal and abnormal pancreas. Clin. Tech. Small Anim. Pract. 2007, 22, 115–121. [Google Scholar] [CrossRef]
- Williams, J.M.; Panciera, D.L.; Larson, M.M. Ultrasonography of the pancreas. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 1–12. [Google Scholar] [CrossRef]
- Jin, H.Y.; Lee, C.S.; Kim, Y.S. Evaluation of articulated laparoscopic instruments in clinical practice: A systematic review. Surg. Endosc. 2022, 36, 450–458. [Google Scholar]
| Dog | Body Weight, kg | Exploration Time, Right Lobe, s | Exploration Time, Right Body, s | Incision Length, cm |
|---|---|---|---|---|
| 1 | 7.84 | 204 | 328 | 2.7 |
| 2 | 9.27 | 257 | 356 | 2.8 |
| 3 | 8.35 | 233 | 417 | 2.8 |
| 4 | 9.51 | 268 | 347 | 3.0 |
| 5 | 10.64 | 245 | 384 | 2.8 |
| 6 | 7.82 | 228 | 457 | 2.6 |
| Dog | Major Duodenal Papilla | Cranial Pancreatoduodenal Vein | Cranial Pancreatoduodenal Artery | LUS Time, s |
|---|---|---|---|---|
| 1 | ✕ | O | O | 878 |
| 2 | O | O | O | 729 |
| 3 | O | O | O | 851 |
| 4 | O | O | O | 825 |
| 5 | O | O | O | 741 |
| 6 | O | O | O | 855 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, C.; Yi, K.; Lee, S.; Yu, Y.; Heo, S. Feasibility Study of Single-Port Laparoscopic Techniques for Pancreatic Exploration, Ultrasound, and Biopsy in Dogs. Animals 2025, 15, 652. https://doi.org/10.3390/ani15050652
Jeong C, Yi K, Lee S, Yu Y, Heo S. Feasibility Study of Single-Port Laparoscopic Techniques for Pancreatic Exploration, Ultrasound, and Biopsy in Dogs. Animals. 2025; 15(5):652. https://doi.org/10.3390/ani15050652
Chicago/Turabian StyleJeong, Changwoo, Kangwoo Yi, Sangjun Lee, Yong Yu, and Suyoung Heo. 2025. "Feasibility Study of Single-Port Laparoscopic Techniques for Pancreatic Exploration, Ultrasound, and Biopsy in Dogs" Animals 15, no. 5: 652. https://doi.org/10.3390/ani15050652
APA StyleJeong, C., Yi, K., Lee, S., Yu, Y., & Heo, S. (2025). Feasibility Study of Single-Port Laparoscopic Techniques for Pancreatic Exploration, Ultrasound, and Biopsy in Dogs. Animals, 15(5), 652. https://doi.org/10.3390/ani15050652








