Structure and Function Analyses of the Thioredoxin 2 and Thioredoxin Reductase Gene in Pacific White Shrimp (Litopenaeus vannamei)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Shrimp
2.2. Ammonia-N Stress and LPS Challenge
2.3. RNA Extraction and Complementary DNA (cDNA) Synthesis
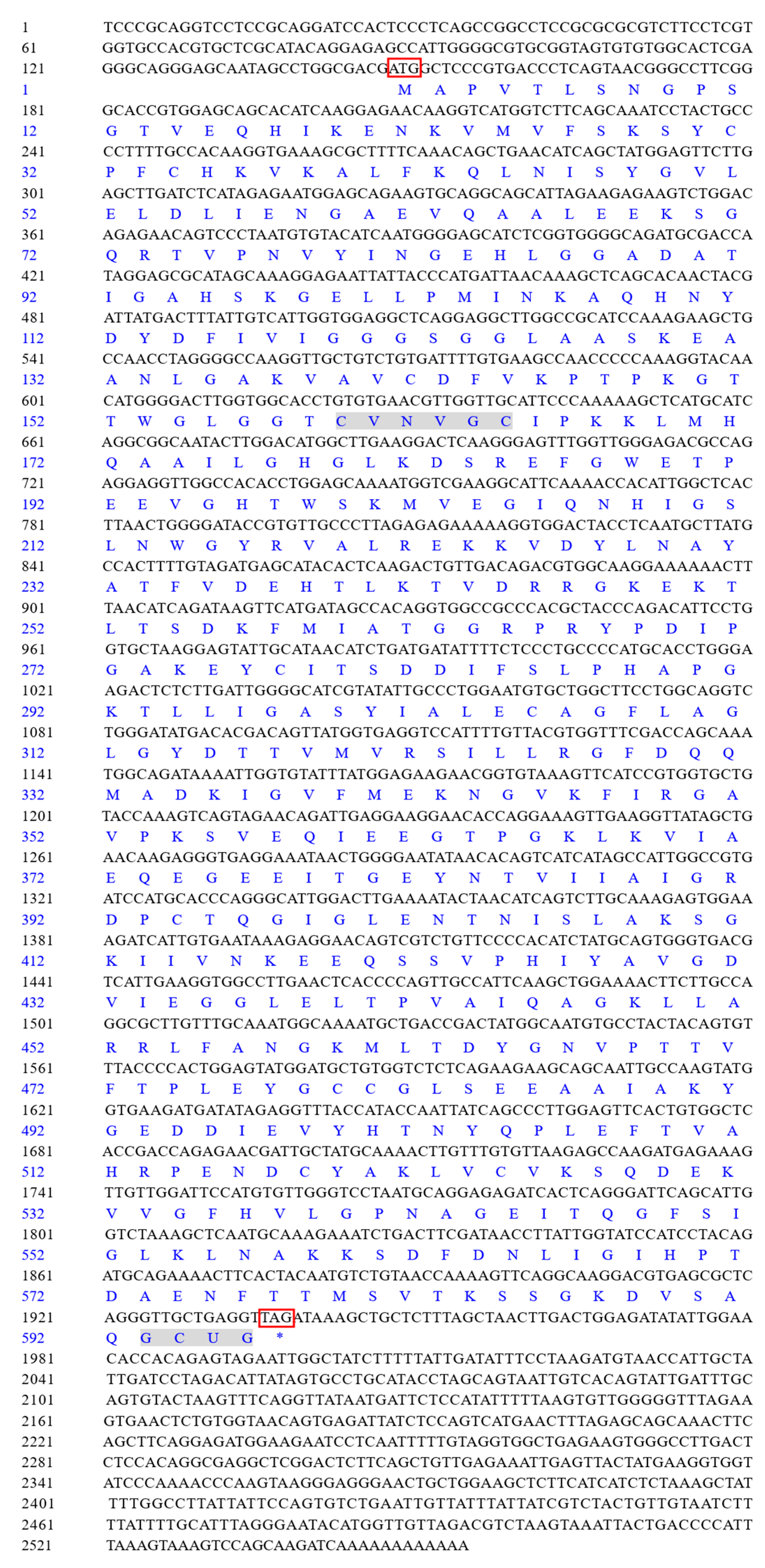
2.4. Cloning of Trx2 and TrxR and Sequence Analysis
2.5. Rapid Amplification of cDNA Ends (RACE)
2.6. Bioinformatics Analysis
2.7. Tissue Expression
2.8. Gene Expression Analysis Under Different Stress Conditions
2.9. LvTrx2 Gene Silence Experiment
2.9.1. Double-Stranded RNA (dsRNA) Synthesis
2.9.2. Gene Silencing and qRT-PCR
2.9.3. Effects of 4-NP Stress on LvTrx2-Interfered Shrimp
2.9.4. Expression Levels of LvTrx2 and Antioxidant-Related Genes
2.9.5. Malondialdehyde (MDA) Content
2.10. Statistical Analyses
3. Results
3.1. Cloning and Sequence Analysis of LvTrx2 and LvTrxR Genes
3.2. Multiple Alignment and Phylogenetic Analysis
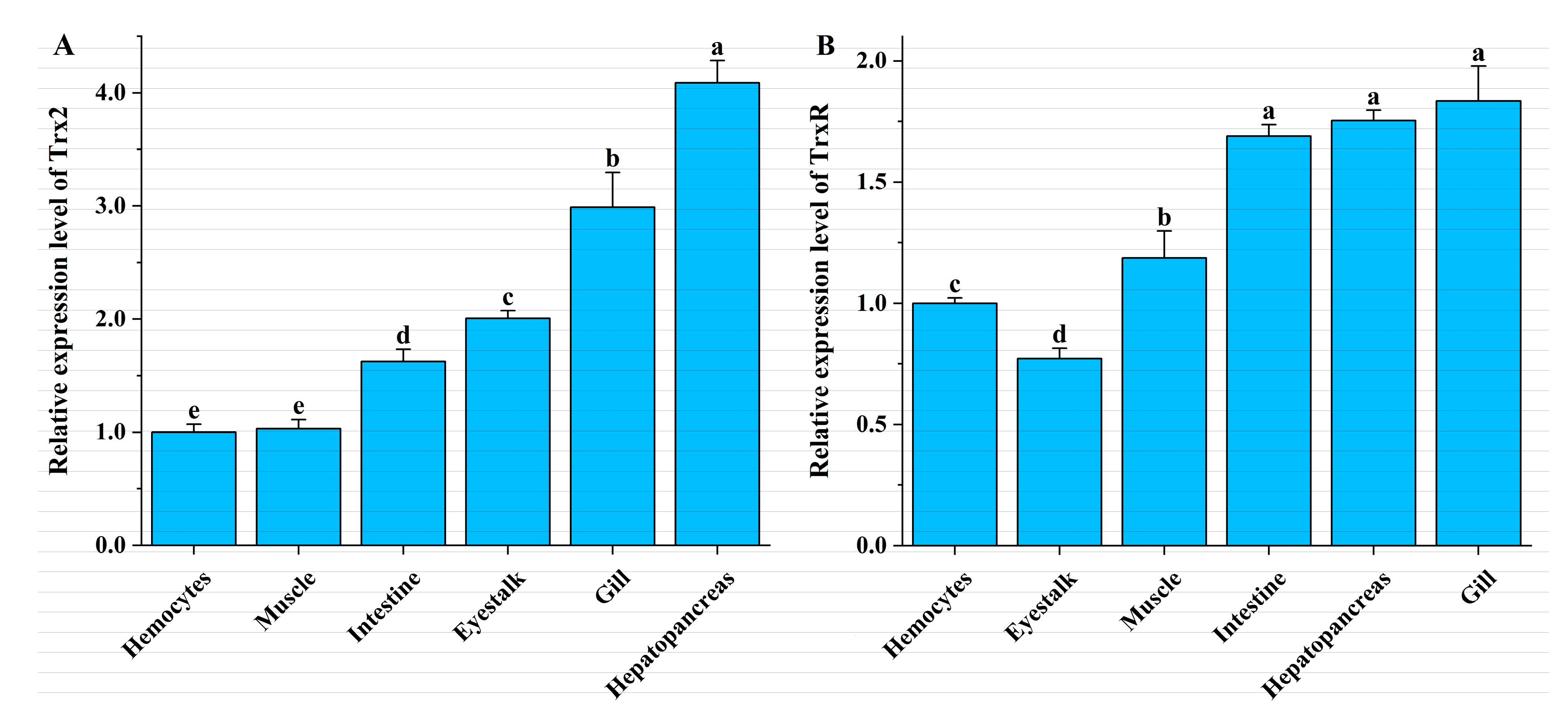
3.3. Tissue Expression in L. vannamei
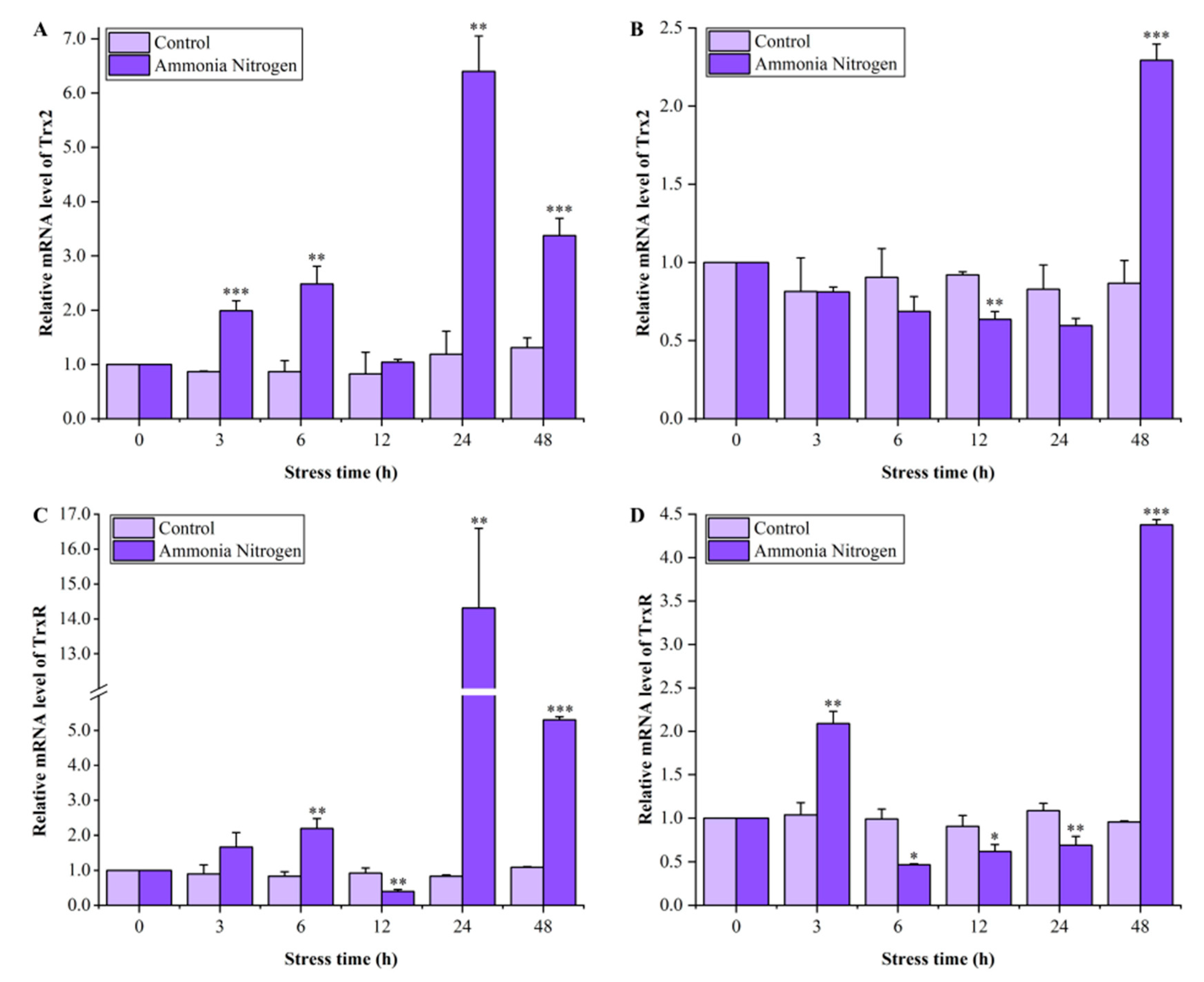
3.4. Expression Profiles of LvTrx2 and LvTrxR in Hepatopancreas and Gill During Ammonia-N Stress
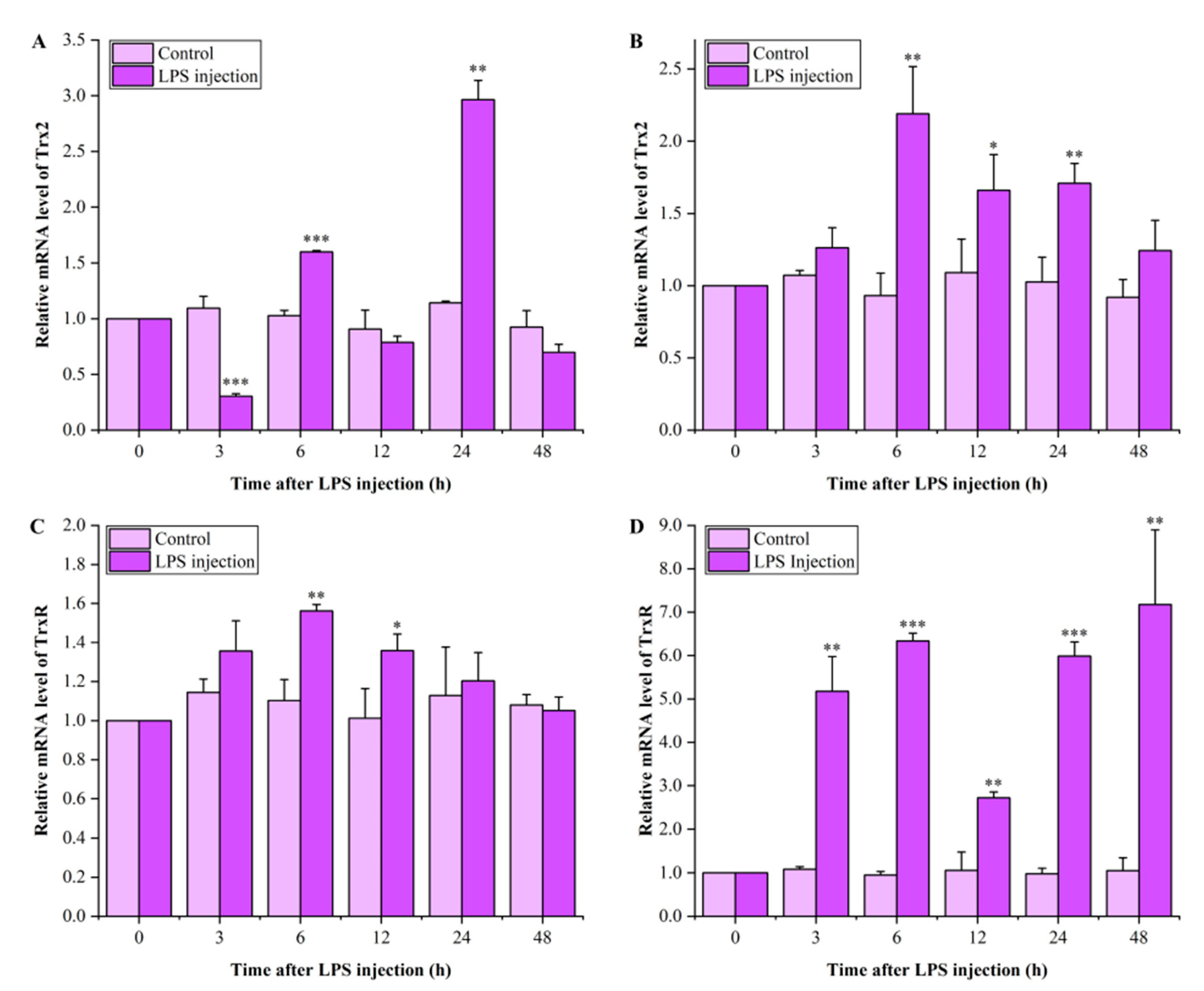
3.5. Expression Profiles of LvTrx2 and LvTrxR in Hepatopancreas and Gill After LPS Injection
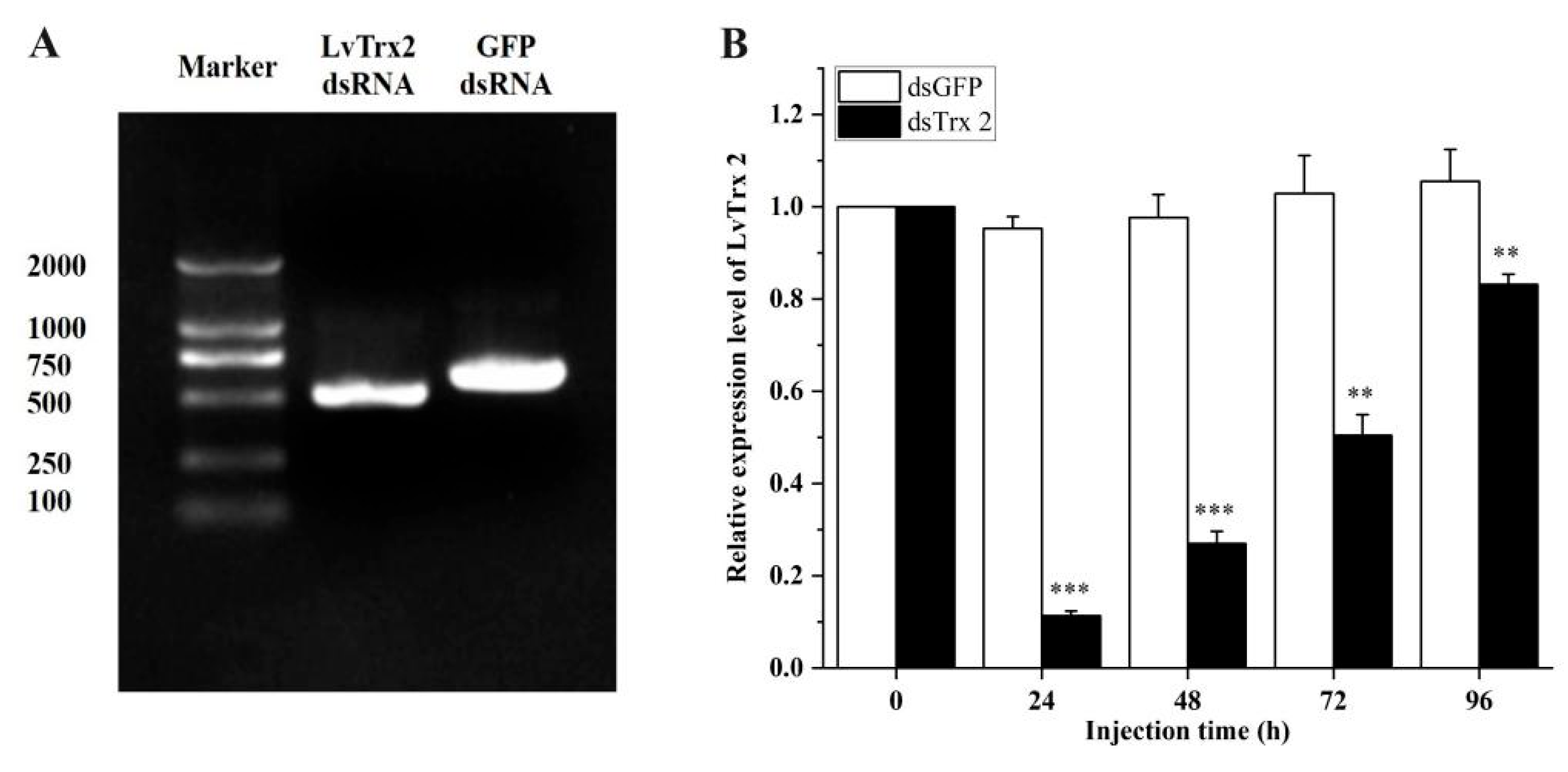
3.6. LvTrx2 Silencing Assay
3.7. Expression Patterns in LvTrx2-Silenced Shrimp Following 4-NP Challenge
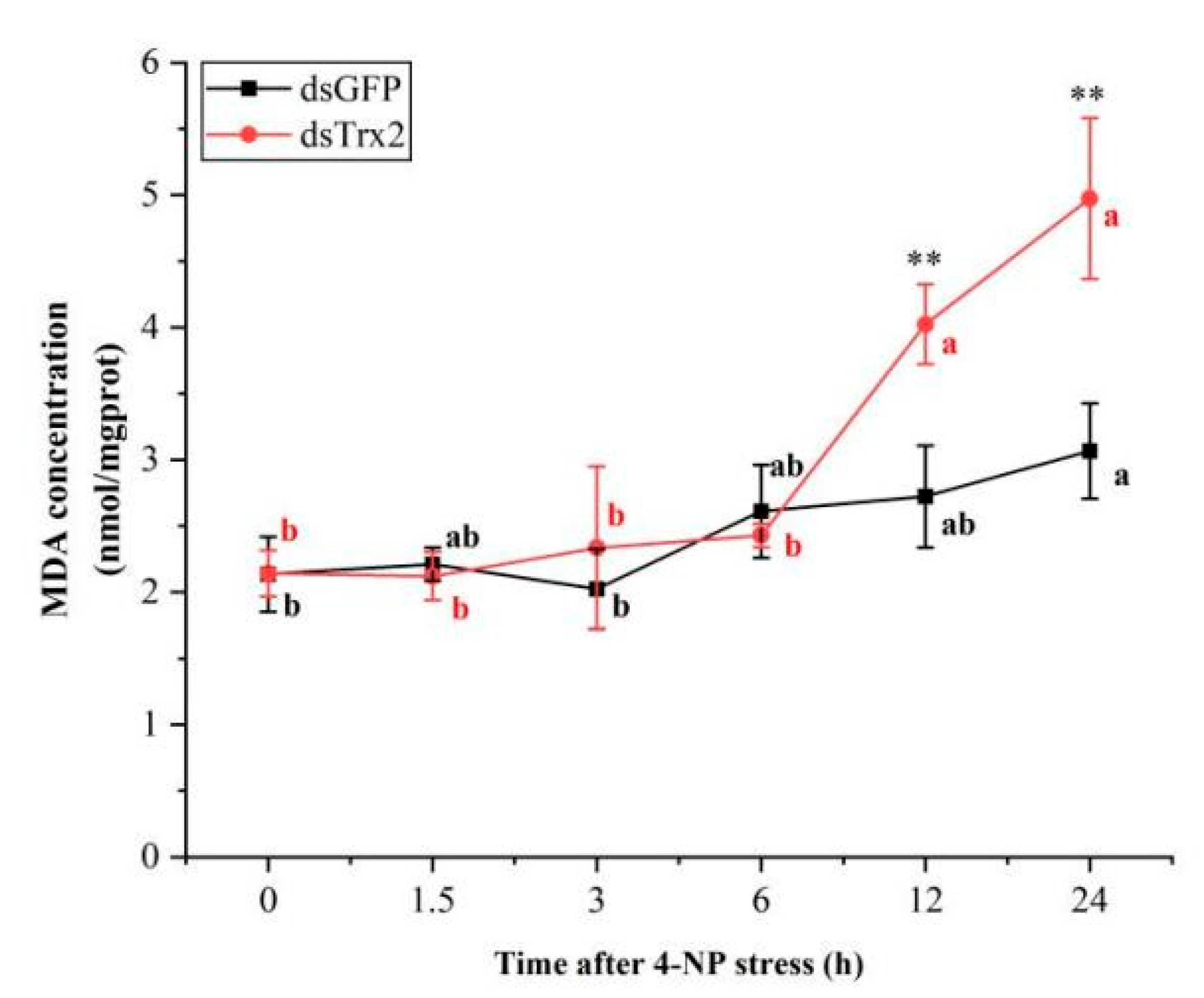
3.8. MDA Content in LvTrx2-Interfered Shrimp Under 4-NP Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2022; Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- CAFS. China Fishery Statistical Yearbook; Ministry of Agriculture and Rural Affairs: Beijing, China, 2022. [Google Scholar]
- Wang, F.F.; Huang, L.; Liao, M.Q.; Dong, W.N.; Liu, C.; Zhuang, X.Q.; Liu, Y.; Yin, X.L.; Liang, Q.J.; Wang, W.N. Pva-miR-252 participates in ammonia nitrogen-induced oxidative stress by modulating autophagy in Penaeus vannamei. Ecotoxicol. Environ. Saf. 2021, 225, 112774. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Restrepo, E.; Jaramillo-Colorado, B.E.; Duarte-Jaramillo, L. Effects of chlorpyrifos on the crustacean Litopenaeus vannamei. PLoS ONE 2020, 15, e0231310. [Google Scholar] [CrossRef] [PubMed]
- Frías-Espericueta, M.G.; Abad-Rosales, S.; Nevárez-Velázquez, A.C.; Osuna-López, I.; Páez-Osuna, F.; Lozano-Olvera, R.; Voltolina, D. Histological effects of a combination of heavy metals on Pacific white shrimp Litopenaeus vannamei juveniles. Aquat. Toxicol. 2008, 89, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Qu, Y.X.; Yan, M.T.; Li, J.Y.; Zou, J.X.; Fan, L.F. Physiological Responses of Pacific White Shrimp Litopenaeus vannamei to Temperature Fluctuation in Low-Salinity Water. Front. Physiol. 2019, 10, 1025. [Google Scholar] [CrossRef] [PubMed]
- Kır, M.; Sunar, M.C.; Topuz, M.; Sarıipek, M. Thermal acclimation capacity and standard metabolism of the Pacific white shrimp Litopenaeus vannamei (Boone, 1931) at different temperature and salinity combinations. J. Therm. Biol. 2023, 112, 103429. [Google Scholar] [CrossRef]
- Han, S.Y.; Wang, B.J.; Liu, M.; Wang, M.Q.; Jiang, K.Y.; Liu, X.W.; Wang, L. Adaptation of the white shrimp Litopenaeus vannamei to gradual changes to a low-pH environment. Ecotoxicol. Environ. Saf. 2018, 149, 203–210. [Google Scholar] [CrossRef]
- Powis, G.; Kirkpatrick, D.L. Thioredoxin signaling as a target for cancer therapy. Curr. Opin. Pharmacol. 2007, 7, 392–397. [Google Scholar] [CrossRef]
- Jia, J.J.; Xu, G.T.; Zhu, D.S.; Liu, H.J.; Zeng, X.S.; Li, L. Advances in the Functions of Thioredoxin System in Central Nervous System Diseases. Antioxid. Redox Signal. 2023, 38, 425–441. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Vlamis-Gardikas, A.; Holmgren, A. Thioredoxin and glutaredoxin isoforms. Meth. Enzymol. 2002, 347, 286–296. [Google Scholar]
- Hirota, K.; Nakamura, H.; Masutani, H.; Yodoi, J. Thioredoxin superfamily and thioredoxin-inducing agents. Ann. N. Y. Acad. Sci. 2002, 957, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. The Mitochondrial Thioredoxin Is Required for Liver Development in Zebrafish. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2013. [Google Scholar]
- Kim, D.H.; Kim, J.W.; Jeong, J.M.; Park, H.J.; Park, C.I. Molecular cloning and expression analysis of a thioredoxin from rock bream, Oplegnathus fasciatus, and biological activity of the recombinant protein. Fish Shellfish Immunol. 2011, 31, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Santhekadur, P.K. Annual Reviews of Biochemistry. Curr. Sci. 2020, 118, 2000–2001. [Google Scholar]
- Hasan, A.A.; Kalinina, E.; Tatarskiy, V.; Shtil, A. The Thioredoxin System of Mammalian Cells and Its Modulators. Biomedicines 2022, 10, 1757. [Google Scholar] [CrossRef] [PubMed]
- Pacitti, D.; Wang, T.; Martin, S.A.; Sweetman, J.; Secombes, C.J. Insights into the fish thioredoxin system: Expression profile of thioredoxin and thioredoxin reductase in rainbow trout (Oncorhynchus mykiss) during infection and in vitro stimulation. Dev. Comp. Immunol. 2014, 42, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.W.; Zheng, X.B.; Gao, X.M.; Liu, Y.; Chen, Y.E.; Zhu, J.Q. Cadmium-Induced Oxidative Damage and the Expression and Function of Mitochondrial Thioredoxin in Phascolosoma esculenta. Int. J. Mol. Sci. 2024, 25, 13283. [Google Scholar] [CrossRef]
- Nadarajapillai, K.; Sellaththurai, S.; Liyanage, D.S.; Yang, H.; Lee, J. Molecular and functional explication of thioredoxin mitochondrial-like protein (Trx-2) from big-belly seahorse (Hippocampus abdominalis) and expression upon immune provocation. Fish Shellfish Immunol. 2020, 99, 495–504. [Google Scholar] [CrossRef]
- Xu, Z.H.; Liu, J.; Wang, E.M.; Zhao, C.Y.; Hu, X.L.; Chu, K.H.; Wang, L. Detoxification and recovery after cadmium exposure in the freshwater crab Sinopotamon henanense. Environ. Sci. Pollut. Res. Int. 2021, 28, 58050–58067. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, J.C. Acute toxicity of ammonia on Litopenaeus vannamei Boone juveniles at different salinity levels. J. Exp. Mar. Biol. Ecol. 2001, 259, 109–119. [Google Scholar] [CrossRef]
- Duan, Y.F.; Wang, Y.; Zhang, J.S.; Liu, Q.S.; Ding, X. Morphologic, digestive enzymes and immunological responses of intestine from Litopenaeus vannamei after lipopolysaccharide injection. J. Invertebr. Pathol. 2018, 153, 186–194. [Google Scholar] [CrossRef]
- Livak, K.; Schmittgen, T. Analysis of Relative Gene Expression Data using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Xian, J.A.; Zhang, X.X.; Guo, H.; Wang, D.M.; Wang, A.L. Cellular responses of the tiger shrimp Penaeus monodon haemocytes after lipopolysaccharide injection. Fish Shellfish Immunol. 2016, 54, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Alim, I.; Caulfield, J.T.; Chen, Y.; Swarup, V.; Geschwind, D.H.; Ivanova, E.; Seravalli, J.; Ai, Y.; Sansing, L.H.; Ste.Marie, E.J.; et al. Selenium Drives a Transcriptional Adaptive Program to Block Ferroptosis and Treat Stroke. Cell 2019, 177, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Xinastle-Castillo, L.O.; Landa, A. Physiological and modulatory role of thioredoxins in the cellular function. Open Med. 2022, 17, 2021–2035. [Google Scholar] [CrossRef]
- Guo, N.N.; Sun, X.J.; Xie, Y.K.; Yang, G.W.; Kang, C.J. Cloning and functional characterization of thioredoxin gene from kuruma shrimp Marsupenaeus japonicus. Fish Shellfish Immunol. 2019, 86, 429–435. [Google Scholar] [CrossRef]
- Hu, J.H. Study on Thioredoxin System and Severalgenes Related to Antioxidant in Mud Crab, Scylla paramam. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2014. [Google Scholar]
- Chen, M.N. Identification and Function Analysis of Thioredoxin and Thioredoxin Reductase from Larimichthys crocea. Master’s Thesis, Zhejiang Ocean University, Zhoushan, China, 2018. [Google Scholar]
- Hu, J.H.; Zhang, F.Y.; Jiang, K.J.; Fang, Y.B.; Wang, J.; Zhao, M.; Qiao, Z.G.; Ma, L.B. Molecular characterization of thioredoxin-1 and thioredoxin reductase activity in mud crab Scylla paramamosain. Genet. Mol. Res. 2014, 13, 10241–10255. [Google Scholar] [CrossRef]
- Pannala, V.R.; Dash, R.K. Mechanistic characterization of the thioredoxin system in the removal of hydrogen peroxide. Biophys. J. 2015, 108, 610a–611a. [Google Scholar] [CrossRef]
- Ruan, Z.L.; Liu, G.Y.; Guo, Y.F.; Zhou, Y.H.; Wang, Q.Q.; Chang, Y.L.; Wang, B.L.; Zheng, J.M.; Zhang, L.M. First report of a thioredoxin homologue in jellyfish: Molecular cloning, expression and antioxidant activity of CcTrx1 from Cyanea capillata. PLoS ONE 2017, 9, e97509. [Google Scholar] [CrossRef]
- Garcia-Orozco, K.D.; Sanchez-Paz, A.; Aispuro-Hernandez, E.; Gomez-Jimenez, S.; Lopez-Zavala, A.; Araujo-Bernal, S.; Muhlia-Almazan, A. Gene expression and protein levels of thioredoxin in the gills from the whiteleg shrimp (Litopenaeus vannamei) infected with two different viruses: The WSSV or IHHNV. Fish Shellfish Immunol. 2012, 32, 1141–1147. [Google Scholar] [CrossRef]
- Bjørklund, G.; Zou, L.; Peana, M.; Chasapis, C.T.; Hangan, T.; Lu, J.; Maes, M. The Role of the Thioredoxin System in Brain Diseases. Antioxidants 2022, 11, 2161. [Google Scholar] [CrossRef]
- Lin, W.; Wu, J.Y.; Luo, H.M.; Liu, X.L.; Cao, B.B.; Hu, F.; Liu, F.; Yang, J.F.; Yang, P.H. Sub-chronic ammonia exposure induces hepatopancreatic damage, oxidative stress, and immune dysfunction in red swamp crayfish (Procambarus clarkii). Ecotoxicol. Environ. Saf. 2023, 254, 114724. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Luo, L.; Zuo, F.Y.; Huang, X.L.; Zhong, L.; Liu, S.; Geng, Y.; Ou, Y.P.; Chen, D.F.; Cai, W.L.; et al. Ammonia nitrogen stress damages the intestinal mucosal barrier of yellow catfish (Pelteobagrus fulvidraco) and induces intestinal inflammation. Front. Physiol. 2023, 14, 1279051. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yao, T.; Shi, S.K.; Ye, L.T. Effects of acute ammonia nitrogen exposure on metabolic and immunological responses in the Hong Kong oyster Crassostrea hongkongensis. Ecotoxicol. Environ. Saf. 2022, 237, 113518. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.J.; Zhai, Y.F.; He, P.M.; Jia, R. Comprehensive Transcriptomic and Metabolomic Analysis of the Litopenaeus vannamei Hepatopancreas After WSSV Challenge. Front. Immunol. 2022, 13, 826794. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wu, Q.P.; Liao, G.W.; Fan, L.F. New insights into the regulation mechanism of Litopenaeus vannamei hepatopancreas after lipopolysaccharide challenge using transcriptome analyses. Fish Shellfish Immunol. 2022, 128, 466–473. [Google Scholar] [CrossRef]
- Guo, H.; Xian, J.A.; Li, B.; Ye, C.X.; Wang, A.L.; Miao, Y.T.; Liao, S.A. Gene expression of apoptosis-related genes, stress protein and antioxidant enzymes in hemocytes of white shrimp Litopenaeus vannamei under nitrite stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013, 157, 366–371. [Google Scholar] [CrossRef]
- Su, X.B.; Li, T.; Zhu, X.W.; Zheng, P.H.; Pan, H.K.; Guo, H. Exploring the impact of nonylphenol exposure on Litopenaeus vannamei at the histological and molecular levels. Ecotoxicol. Environ. Saf. 2024, 279, 116475. [Google Scholar] [CrossRef]
- Holmgren, A.; Lu, J. Thioredoxin and thioredoxin reductase: Current research with special reference to human disease. Biochem. Biophys. Res. Commun. 2010, 396, 120–124. [Google Scholar] [CrossRef]




| Primers | Gene ID | Sequences (5′–3′) |
|---|---|---|
| Trx2-F1 | XM_027353791.2 | ATTCTTCAACTCCTGTCGTG |
| Trx2-R1 | TCTCCAATCAGCCTGCC | |
| TrxR-F1 | XM_027357384.1 | GCTCCCGTGACCCTCAGTAA |
| TrxR-R1 | CCAATGTGGTTTTGAATGCCTT | |
| Trx2-3′F1 | ATTCTTCAACTCCTGTCGTG | |
| Trx2-5′R1 | TGCCGACAAATGACTCAATCCG | |
| TrxR-3′F1 | AATGTGCCTACTACAGTGTTTACCCC | |
| TrxR-5′R1 | GCACATTGCCATAGTCGGTCAGC | |
| 3′RACE Outer Primer | TACCGTCGTTCCACTAGTGATTT | |
| UPM long primer | CTAATACGACTCACTATAGGGCAAGCAGTGGTATCAACGCAGAGT | |
| Short primer | CTAATACGACTCACTATAGGGC | |
| Trx2-RT-F1 | TGATAAGCAGCCTGATGGTG | |
| Trx2-RT-R1 | GCCTTGTTTCTGTTCCTCCA | |
| TrxR-RT-F1 | GTCGTCTGTTCCCCACATCTAT | |
| TrxR-RT-R1 | GCACATTGCCATAGTCGGTC | |
| Trx2i-F (with T7) | GGATCCTAATACGACTCACTATAGGTCAGCGACGAATAGGGC | |
| Trx2i-F | TCAGCGACGAATAGGGC | |
| Trx2i-R (with T7) | GGATCCTAATACGACTCACTATAGGCAACATTTACGGAAGAGGGA | |
| Trx2i-R | CAACATTTACGGAAGAGGGA | |
| GFPi-F (with T7) | TAATACGACTCACTATAGGGAGAGTGCCCATCCTGGTCGAGCT | |
| GFPi-F | GTGCCCATCCTGGTCGAGCT | |
| GFPi-R (with T7) | TAATACGACTCACTATAGGGAGATGCACGCTGCCGTCCTCGAT | |
| GFPi-R | TGCACGCTGCCGTCCTCGAT | |
| Trx1-F | XM_027377405.2 | TTAACGAGGCTGGAAACA |
| Trx1-R | AACGACATCGCTCATAGA | |
| Grx2-F | MG757219 | TGATAAGCAGCCTGATGGTG |
| Grx2-R | GCCTTGTTTCTGTTCCTCCA | |
| Grx 3-F | XM_070127026.1 | TTCAGCCGCACAACCATA |
| Grx 3-R | AGTCCTTGTCGCACTTCCTC | |
| GPx-F | AY973252 | AGGGACTTCCACCAGATG |
| GPx-R | CAACAACTCCCCTTCGGTA | |
| GST-F | AY573381 | AAGATAACGCAGAGCAAGG |
| GST-R | TCGTAGGTGACGGTAAAGA | |
| MGST3-F | XM_027382186.2 | TGTGCCGTTGGTGGTGC |
| MGST3-R | CAAGGAGCCCAGTAGCAAAAC | |
| β-Actin F | AF300705 | AACAGCGACTCCCGACAGA |
| β-Actin R | CCTTGGACCTAGTAATTTGCATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Zheng, P.-H.; Luan, K.-E.; Zhang, X.-X.; Li, J.-T.; Zhang, Z.-L.; Hou, W.-Y.; Zhang, L.-M.; Lu, Y.-P.; Xian, J.-A. Structure and Function Analyses of the Thioredoxin 2 and Thioredoxin Reductase Gene in Pacific White Shrimp (Litopenaeus vannamei). Animals 2025, 15, 629. https://doi.org/10.3390/ani15050629
Xu T, Zheng P-H, Luan K-E, Zhang X-X, Li J-T, Zhang Z-L, Hou W-Y, Zhang L-M, Lu Y-P, Xian J-A. Structure and Function Analyses of the Thioredoxin 2 and Thioredoxin Reductase Gene in Pacific White Shrimp (Litopenaeus vannamei). Animals. 2025; 15(5):629. https://doi.org/10.3390/ani15050629
Chicago/Turabian StyleXu, Tong, Pei-Hua Zheng, Ke-Er Luan, Xiu-Xia Zhang, Jun-Tao Li, Ze-Long Zhang, Wei-Yan Hou, Li-Min Zhang, Yao-Peng Lu, and Jian-An Xian. 2025. "Structure and Function Analyses of the Thioredoxin 2 and Thioredoxin Reductase Gene in Pacific White Shrimp (Litopenaeus vannamei)" Animals 15, no. 5: 629. https://doi.org/10.3390/ani15050629
APA StyleXu, T., Zheng, P.-H., Luan, K.-E., Zhang, X.-X., Li, J.-T., Zhang, Z.-L., Hou, W.-Y., Zhang, L.-M., Lu, Y.-P., & Xian, J.-A. (2025). Structure and Function Analyses of the Thioredoxin 2 and Thioredoxin Reductase Gene in Pacific White Shrimp (Litopenaeus vannamei). Animals, 15(5), 629. https://doi.org/10.3390/ani15050629







