Impact of Incorporating Defatted Black Soldier Fly Meal into Diet on Growth Performance, Serum Biochemical Parameters, Nutrient Digestibility, Morphology of the Intestinal Tract, and Immune Index of Brooding Laying Hens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of BSF Larva Powder
2.2. Animals and Experimental Design
2.3. Bird Husbandry
2.4. Assessment of Growth Performance
2.5. Determination of Serum Biochemical Parameters
2.6. Determination of Nutrient Digestibility
2.7. Intestinal Mucosal Immune Index
2.8. Statistical Analysis
3. Results
3.1. Effects of BSFM on the Growth Performance of Chicks
3.2. Effects of BSF on Serum Biochemical Parameters of Chicks
3.3. Effects of BSF on the Antioxidant Capacity of Birds
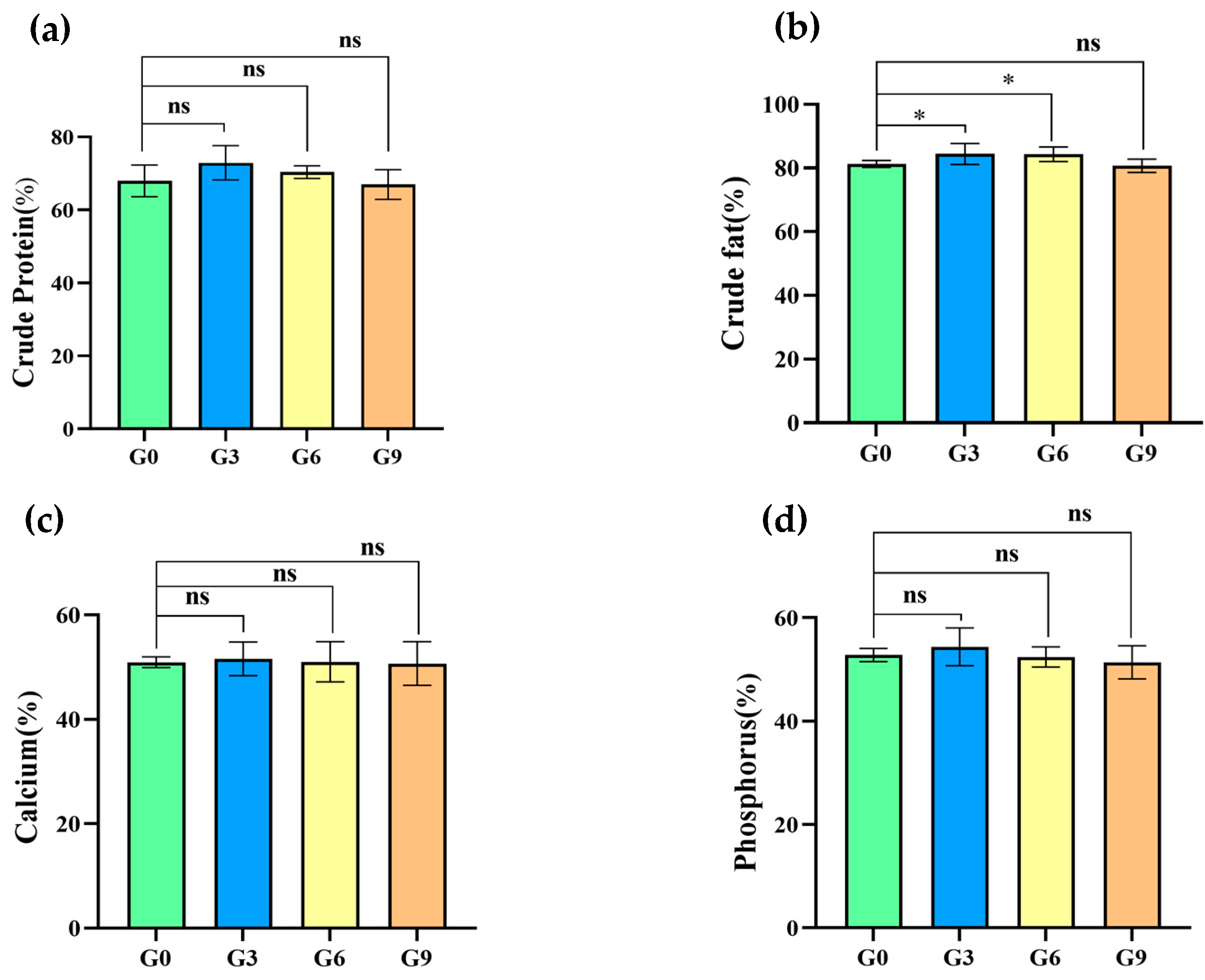
3.4. Effect of BSFM on Nutrient Digestibility
3.5. Effects of BSF on the Intestinal Mucosal Immunity of Chicks
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beller, S.; Grundmann, S.M.; Pies, K.; Most, E.; Schuchardt, S.; Seel, W.; Simon, M.C.; Eder, K.; Ringseis, R. Effect of replacing soybean meal with Hermetia illucens meal on cecal microbiota, liver transcriptome, and plasma metabolome of broilers. Poult. Sci. 2024, 5, 103635. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, G.; Goksen, G.; Khaneghah, A.M. Plant-based protein modification strategies towards challenges. J. Agric. Food Res. 2024, 15, 101017. [Google Scholar] [CrossRef]
- Zheng, X.; He, M. The impact of source diversification on soybean’s import prices of China. Resour. Sci. 2024, 46, 1358–1369. [Google Scholar]
- Aksoy, M.Y.; Eljack, R.; Aksoy, J.; Beck, B.H. Frass from Black Soldier Fly Larvae, Hermetia illucens, as a Possible Functional Dietary Ingredient in Channel Catfish Feed. Fishes 2023, 8, 542. [Google Scholar] [CrossRef]
- Khalifah, A.; Abdalla, S.; Rageb, M.; Maruccio, L.; Ciani, F.; El-Sabrout, K. Could Insect Products Provide a Safe and Sustainable Feed Alternative for the Poultry Industry? A Comprehensive Review. Animals 2023, 13, 1534. [Google Scholar] [CrossRef]
- Perez, J.T.; Casanova, F.; Queiroz, L.S.; Petersen, H.O.; Moreno, P.J.G.; Feyissa, A.H. Protein extraction from yellow mealworm (Tenebrio molitor) assisted by pulsed electric fields: Effect on foaming properties. LWT 2024, 213, 117041. [Google Scholar] [CrossRef]
- Teixeira, C.S.S.; Sá, B.C.; Villa, C.; Costa, J.; Mafra, I.; Ferreira, I.M.P.L.; Faria, M.A.; Tavares, T.G. Uncovering the Potential Somatic Angiotensin-Converting Enzyme (sACE) Inhibitory Capacity of Peptides from Acheta domesticus: Insights from In Vitro Gastrointestinal Digestion. Foods 2024, 13, 3462. [Google Scholar] [CrossRef]
- Mohd, T.N.; Zulkifli, N.F.; Hamizah, A.N. Upcycling of food waste generated from the fresh market by utilising black soldier fly larvae: Influence on growth, bioconversion, and nutritional composition. J. Environ. Manag. 2023, 349, 119467. [Google Scholar]
- Cong, L.; Dean, D.; Liu, C.; Wang, K.; Hou, Y. The Commercial Application of Insect Protein in Food Products: A Product Audit Based on Online Resources. Foods 2024, 13, 3509. [Google Scholar] [CrossRef]
- Fan, R.; Wang, W.; Zhang, R.; Zhu, M.; Liu, W.; Liu, P. Impact of hydrophobically modified cellulose nanofiber on the stability of Pickering emulsion containing insect protein. J. Sci. Food. Agric. 2024, 105, 569–578. [Google Scholar] [CrossRef]
- Rongsheng, S.; Limin, M.; Guiying, W.; Mengfei, L.; Chengjun, L.; Lusheng, L. Influences of Partial Substitution of Fish Meal with Defatted Black Soldier Fly (Hermetia illucens) Larvae Meal in Diets on Growth Performance, Biochemical Parameters, and Body Composition of Juvenile Chinese Soft-Shelled Turtles (Pelodiscus sinensis). Aquacult. Nutr. 2022, 10, 4278137. [Google Scholar]
- Xiaohua, C.; Mengmeng, L.; Guiying, W.; Kuiming, W.; Rongsheng, S.; Ziyu, W.; Lusheng, L. Evaluation of the Low Inclusion of Full-Fatted Hermetia illucens Larvae Meal for Layer Chickens: Growth Performance, Nutrient Digestibility, and Gut Health. Front. Vet. Sci. 2020, 7, 585843. [Google Scholar]
- Zechao, H.; Handong, L.; Sha, L.; Rongrong, X.; Jian, S.; Hong, J. Assessment of black soldier fly (Hermetia illucens) larvae meal as a potential substitute for soybean meal on growth performance and flesh quality of grass carp Ctenopharyngodon idellus. Anim. Nutr. 2023, 14, 425–449. [Google Scholar]
- Dörper, A.; Berman, H.M.; Gort, G.; van Harn, J.; Dicke, M.; Veldkamp, T. Effects of different black soldier fly larvae products on slow-growing broiler performance and carcass characteristics. Poult. Sci. 2024, 103, 103481. [Google Scholar] [CrossRef]
- Martínez Marín, A.L.; Gariglio, M.; Pozzo, S.; Capucchio, M.T.; Ferrocino, I.; Biasato, I.; Schiavone, A. Effects of partially defatted larvae meal of Black Soldier Fly (Hermetia illucens) on caecal microbiota and volatile compounds of Muscovy ducks (Cairina moschata domestica). Ital. J. Anim. Sci. 2023, 22, 1151–1161. [Google Scholar] [CrossRef]
- Montalbán, A.; Madrid, J.; Hernández, F.; Schiavone, A.; Ruiz, E.; Sánchez, C.J.; Ayala, L.; Fiorilla, E.; Miró, S.M. The Influence of Alternative Diets and Whole Dry Black Soldier Fly Larvae (Hermetia illucens) on the Production Performance, Blood Status, and Egg Quality of Laying Hens. Animals 2024, 14, 2550. [Google Scholar] [CrossRef]
- Malla, N.; Roos, N.; Van der Heide, M.E.; Nørgaard, J.V. Effect of feeding meal of yellow and lesser mealworm and defatted black soldier fly larvae on growth performance and gut health of weaned piglets. Anim. Feed Sci. Techol. 2024, 309, 115917. [Google Scholar] [CrossRef]
- Zhao, J.; Ban, T.; Miyawaki, H.; Hirayasu, H.; Izumo, A.; Iwase, S.; Kasai, K.; Kawasaki, K. Long-Term Dietary Fish Meal Substitution with the Black Soldier Fly Larval Meal Modifies the Caecal Microbiota and Microbial Pathway in Laying Hens. Animals 2023, 13, 2629. [Google Scholar] [CrossRef]
- Patrycja, Z.; Beata, S.; Anna, A.; Kinga, S. Effects of Partial Replacement of Soybean Meal with Defatted Hermetia illucens Meal in the Diet of Laying Hens on Performance, Dietary Egg Quality, and Serum Biochemical and Redox Indices. Animals 2023, 13, 527. [Google Scholar] [CrossRef]
- Attivi, K.; Mlaga, K.G.; Agboka, K.; Tona, K.; Kouame, Y.A.E.; Lin, H. Effect of fish meal replacement by black Soldier Fly (Hermetia illucens) larvae meal on serum biochemical indices, thyroid hormone and zootechnical performance of laying chickens. J. Appl. Poult. Res. 2022, 31, 100275. [Google Scholar] [CrossRef]
- Huang, C.; Hernandez, C.E.; Wall, H.; Tahamtani, F.M.; Ivarsson, E.; Sun, L. Live black soldier fly (Hermetia illucens) larvae in feed for laying hens: Effects on hen gut microbiota and behavior. Poult. Sci. 2024, 103, 103429. [Google Scholar] [CrossRef] [PubMed]
- Lokaewmanee, K.; Suttibak, S.; Sukthanapirat, R.; Sriyoha, R.; Chanasakhatana, N.; Baotong, S.; Trithalen, U. Laying hen performance, feed economy, egg quality and yolk fatty acid profiles from laying hens fed live black soldier fly larvae. Czech. J. Anim. Sci. 2023, 68, 169–177. [Google Scholar] [CrossRef]
- Romero, C.; Cenalmor, J.C.; Chamorro, S.; Redondo, C. Effect of Different Dietary Doses of Black Soldier Fly Meal on Performance and Egg Quality in Free-Range Reared Laying Hens. Animals 2024, 1, 3340. [Google Scholar] [CrossRef] [PubMed]
- Mwaniki, Z.; Shoveller, A.K.; Huber, L.; Kiarie, E.G. Complete replacement of soybean meal with defatted black soldier fly larvae meal in Shaver White hens feeding program (28–43 wks of age): Impact on egg production, egg quality, organ weight, and apparent retention of components 1. Poult. Sci. 2020, 99, 959–965. [Google Scholar] [CrossRef]
- Patterson, P.H.; Acar, N.; Ferguson, A.D.; Trimble, L.D.; Sciubba, H.B.; Koutsos, E.A. The impact of dietary Black Soldier Fly larvae oil and meal on laying hen performance and egg quality. Poult. Sci. 2021, 100, 101272. [Google Scholar] [CrossRef]
- Khan, S.; Shi, X.; Cai, R.; Zhao, S.; Li, X.; Khan, I.M.; Yin, Z.; Lu, H.; Hilal, M.G.; Yi, R. Assessing the performance, egg quality, serum analysis, heavy metals and essential trace metals accumulation in laying hen eggs and tissues fed black soldier fly (Hermetia illucens) larvae meal. Poult. Sci. 2024, 103, 104315. [Google Scholar] [CrossRef]
- Mahmoud, A.E.; Morel, P.C.H.; Potter, M.A.; Ravindran, V. The apparent metabolisable energy and ileal amino digestibility of black soldier fly (Hermetia illucens) larvae meal for broiler chickens. Br. Poult. Sci. 2023, 64, 562–567. [Google Scholar] [CrossRef]
- De Marco, M.; Martínez, S.; Hernandez, F.; Madrid, J.; Gai, F.; Rotolo, L.; Belforti, M.; Bergero, D.; Katz, H.; Dabbou, S. Nutritional value of two insect larval meals (Tenebrio molitor and Hermetia illucens) for broiler chickens: Apparent nutrient digestibility, apparent ileal amino acid digestibility and apparent metabolizable energy. Anim. Feed. Sci. Technol. 2015, 209, 211–218. [Google Scholar] [CrossRef]
- Schiavone, A.; De Marco, M.; Martínez, S.; Dabbou, S.; Renna, M.; Madrid, J.; Hernandez, F.; Rotolo, L.; Costa, P.; Gai, F. Nutritional value of a partially defatted and a highly defatted black soldier fly larvae (Hermetia illucens L.) meal for broiler chickens: Apparent nutrient digestibility, apparent metabolizable energy and apparent ileal amino acid digestibility. J. Anim. Sci. Biotechnol. 2017, 8, 897–905. [Google Scholar] [CrossRef]
- Xin, Y.; Xu, M.; Chen, L.; Wang, G.; Lu, W.; Liu, Z.; Shang, R.; Li, Y.; Wang, Z.; Sun, H. Effects of Different Defatting Methods of Black Soldier Fly (Hermetia illucens) Larvae Meal on the Metabolic Energy and Nutrient Digestibility in Young Laying Hens. Animals 2024, 14, 2521. [Google Scholar] [CrossRef]
- Uni, Z.; Ferket, R.P. Methods for early nutrition and their potential. World’s Poult. Sci. J. 2004, 60, 101–111. [Google Scholar] [CrossRef]
- Alagawany, M.; El-Hack, M.E.A.; Farag, M.R.; Ruchi Tiwari, R.T.; Swati Sachan, S.S.; Kumaragurubaran Karthik, K.K.; Kuldeep Dhama, K.D. Positive and negative impacts of dietary protein levels in laying hens. Asian J. Anim. Sci. 2016, 10, 165–174. [Google Scholar] [CrossRef][Green Version]
- Fan, K.; Hongfei, H.; Chen, Y.; Fenfen, L.; Dongping, H.; Jingcheng, Z. Process optimization and quality comparison of black soldier fly larvae oils extracted by different methods. China Oils. Fats 2021, 46, 15–20. [Google Scholar]
- GB/T 6432-2018; Determination of Crude Protein in Feeds-Kjeldahl Method. State Administration for Market Regulation, China National Standardization Administration Committee: Beijing, China, 2018.
- GB/T 6433-2006; Determination of Crude Fat in Feeds. State Administration for Market Regulation, China National Standardization Administration Committee: Beijing, China, 2006.
- GB/T 6436-2018; Determination of Calcium in Feeds. State Administration for Market Regulation, China National Standardization Administration Committee: Beijing, China, 2018.
- GB/T 6437-2018; Determination of Phosphorus in Feeds-Spectrophotometry. State Administration for Market Regulation, China National Standardization Administration Committee: Beijing, China, 2018.
- Dabbou, S.; Gai, F.; Biasato, I.; Capucchio, M.T.; Biasibetti, E.; Dezzutto, D.; Meneguz, M.; Plachà, I.; Gasco, L.; Schiavone, A. Black soldier fly defatted meal as a dietary protein source for broiler chickens: Effects on growth performance, blood traits, gut morphology and histological features. J. Anim. Sci. Biotechnol. 2018, 9, 891–900. [Google Scholar] [CrossRef]
- Khan, S.; Shi, X.; Cai, R.; Shuai, Z.; Mao, W.; Khan, I.M.; Swelum, A.A.; Guo, J. Effect of black soldier fly (Hermetia illucens) larvae meal and oil on the performance, biochemical profile, intestinal health and gut microbial dynamics in laying hens. Poult. Sci. 2024, 103, 104460. [Google Scholar] [CrossRef]
- Lemme, A.; Klüber, P. Rethinking Amino Acid Nutrition of Black Soldier Fly Larvae (Hermetia illucens) Based on Insights from an Amino Acid Reduction Trial. Insects 2024, 15, 862. [Google Scholar] [CrossRef]
- Ibrar, A.; Fatma, İ.; Roshan, R.; Umair, A.; Eren, K.; Usman, A. A Review of Black Soldier Fly (Hermetia illucens) as a Potential Alternative Protein Source in Broiler Diets. Ann. Anim. Sci. 2023, 23, 939–949. [Google Scholar]
- Chang, S.; Song, M.; Lee, J.; Oh, H.; Song, D.; An, J.; Cho, H.; Park, S.; Jeon, K.; Lee, B. Effect of black soldier fly larvae as substitutes for fishmeal in broiler diet. J. Anim. Sci. Technol. 2023, 65, 1290–1307. [Google Scholar] [CrossRef]
- Wynants, E.; Frooninckx, L.; Van Miert, S.; Geeraerd, A.; Claes, J.; Van Campenhout, L. Risks related to the presence of Salmonella sp. during rearing of mealworms (Tenebrio molitor) for food or feed: Survival in the feeding substrate and transmission to the larvae. Food Cont. 2019, 100, 227–234. [Google Scholar] [CrossRef]
- Li, X.; Rahimnejad, S.; Wang, L.; Lu, K.; Song, K.; Zhang, C. Substituting fish meal with housefly (Musca domestica) maggot meal in diets for bullfrog Rana (Lithobates) catesbeiana: Effects on growth, digestive enzymes activity, antioxidant capacity and gut health. Aquaculture 2018, 499, 295–305. [Google Scholar] [CrossRef]
- Pozzo, L.; Cavallarin, L.; Antoniazzi, S.; Guerre, P.; Biasibetti, E.; Capucchio, M.T.; Schiavone, A. Feeding a diet contaminated with ochratoxin A for broiler chickens at the maximum level recommended by the EU for poultry feeds (0.1 mg/kg). 2. Effects on meat quality, oxidative stress, residues and histological traits. J. Anim. Phys. Anim. Nutr. 2013, 97, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yin, Y.; Nyachoti, C.M.; Huang, R.; Li, T.; Yang, C.; Yang, X.; Gong, J.; Peng, J.; Qi, D. Effect of dietary supplementation of chitosan and galacto-mannan-oligosaccharide on serum parameters and the insulin-like growth factor-I mRNA expression in early-weaned piglets. Domest. Anim. Endocrinol. 2005, 28, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.; Tianshuo, Z.; Xin, L.; Shan, W. Isolation and enrichment of Boldenone and its main metabolite in urinary samples to further determine the 13C/12C ratios by gas chromatography/combustion/isotope ratio mass spectrometry. J. Chromatogr. A 2023, 1707, 464297. [Google Scholar]
- Chiu, C.; Cheng, M.; Chiang, M.; Wang, C.; Tsai, M.; Lin, G. Metabolomic Analysis Reveals Distinct Profiles in the Plasma and Urine Associated with IgE Reactions in Childhood Asthma. J. Clin. Med. 2020, 9, 887. [Google Scholar] [CrossRef]
- Marta, G.; Sihem, D.; Ilaria, B.; Teresa, C.M.; Elena, C.; Fuensanta, H.; Josefa, M.; Silvia, M.; Francesco, G.; Christian, C. Nutritional effects of the dietary inclusion of partially defatted Hermetia illucens larva meal in Muscovy duck. J. Anim. Sci. Biotechnol. 2019, 10, 37. [Google Scholar]
- Rafael, R. Protein tyrosine nitration: Biochemical mechanisms and structural basis of functional effects. Acc. Chem. Res. 2013, 46, 550. [Google Scholar]
- Li, S.; Ji, H.; Zhang, B.; Zhou, J.; Yu, H. Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp (Cyprinus carpio var. Jian): Growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological structure. Aquaculture 2017, 477, 62–70. [Google Scholar] [CrossRef]
- Secci, G.; Bovera, F.; Nizza, S.; Baronti, N.; Gasco, L.; Conte, G.; Serra, A.; Bonelli, A.; Parisi, G. Quality of eggs from Lohmann Brown Classic laying hens fed black soldier fly meal as substitute for soya bean. Animal 2019, 13, 2110. [Google Scholar] [CrossRef]
- Cullere, M.; Tasoniero, G.; Giaccone, V.; Acuti, G.; Marangon, A.; Dalle Zotte, A. Black soldier fly as dietary protein source for broiler quails: Meat proximate composition, fatty acid and amino acid profile, oxidative status and sensory traits. Animal 2018, 12, 640–647. [Google Scholar] [CrossRef]
- Cutrignelli, M.I.; Messina, M.; Tulli, F.; Randazzo, B.; Olivotto, I.; Gasco, L.; Loponte, R.; Bovera, F. Evaluation of an insect meal of the Black Soldier Fly (Hermetia illucens) as soybean substitute: Intestinal morphometry, enzymatic and microbial activity in laying hens. Res. Vet. Sci. 2018, 117, 209–215. [Google Scholar] [CrossRef]
- Bovera, F.; Loponte, R.; Pero, M.E.; Cutrignelli, M.I.; Calabrò, S.; Musco, N.; Vassalotti, G.; Panettieri, V.; Lombardi, P.; Piccolo, G. Laying performance, blood profiles, nutrient digestibility and inner organs traits of hens fed an insect meal from Hermetia illucens larvae. Res. Vet. Sci. 2018, 120, 86–93. [Google Scholar] [CrossRef]
- Suzuki, M.; Fujimoto, W.; Goto, M.; Morimatsu, M.; Syuto, B.; Iwanaga, T. Cellular Expression of Gut Chitinase mRNA in the Gastrointestinal Tract of Mice and Chickens. J. Histochem. Cytochem. 2002, 50, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Eri, T.; Akinori, K.; Azusa, K.; Hiromasa, M.; Ryo, M.; Yusuke, H.; Satoshi, W.; Misa, O.; Masayoshi, S.; Yasusato, S. Chitin digestibility is dependent on feeding behaviors, which determine acidic chitinase mRNA levels in mammalian and poultry stomachs. Sci. Rep. 2018, 8, 1461. [Google Scholar]
- Baião, N.C.; Lara, L. Oil and fat in broiler nutrition. Rev. Bras. Ciênc. Avíco 2005, 7, 129–141. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed—A review. J. Insects Food Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Asmaz, E.D.; Teker, H.T.; Sertkaya, Z.T.; Ceylani, T.; Genç, A.İ. Effect of middle-age plasma therapy on ileum morphology, immune defense (IgA) and cell proliferation (Ki-67) of female aged rats. Histochem. Cell. Biol. 2024, 163, 17. [Google Scholar] [CrossRef]
- Blaise, C. Secretory immunoglobulin A: Well beyond immune exclusion at mucosal surfaces. Immunopharmacol. Immunotoxicol. 2009, 31, 174–179. [Google Scholar]
- Lee, J.; Kim, Y.; Park, Y.; Yang, Y.; Jung, B.; Lee, B. Black soldier fly (Hermetia illucens) larvae enhances immune activities and increases survivability of broiler chicks against experimental infection of Salmonella Gallinarum. J. Vet. Med. Sci. 2018, 80, 736–740. [Google Scholar] [CrossRef]

| Items (%) | Inclusion Levels 1, % | |||
|---|---|---|---|---|
| 0 | 3 | 6 | 9 | |
| Ingredients | ||||
| Corn | 65.01 | 67.66 | 67.55 | 67.21 |
| Soybean meal | 26.37 | 22.30 | 22.63 | 20.07 |
| Corn gluten meal | 3.00 | 3.00 | 0.44 | 0.00 |
| Defatted BSFM | 0.00 | 3.00 | 6.00 | 9.00 |
| Soybean oil | 1.35 | 0.00 | 0.00 | 0.00 |
| Limestone | 1.40 | 1.09 | 0.54 | 0.83 |
| Dicalcium phosphate | 1.50 | 1.58 | 1.64 | 1.76 |
| L-Lysine·HCl | 0.27 | 0.28 | 0.10 | 0.04 |
| DL-Methionine | 0.10 | 0.09 | 0.10 | 0.09 |
| Vitamin and mineral premix | 1.00 | 1.00 | 1.00 | 1.00 |
| Total | 100.0 | 100.0 | 100.0 | 100.0 |
| Nutrient levels * | ||||
| Metabolizable energy, MJ/kg | 12.13 | 12.13 | 12.13 | 12.13 |
| Crude protein, % | 19.00 | 19.00 | 19.00 | 19.00 |
| Calcium, % | 0.87 | 0.98 | 1.00 | 1.30 |
| Non-phytate phosphorus, % | 0.41 | 0.42 | 0.42 | 0.43 |
| Lysine, % | 1.05 | 1.05 | 1.05 | 1.05 |
| Methionine, % | 0.41 | 0.41 | 0.41 | 0.41 |
| Items 1 | Groups 2 | |||
|---|---|---|---|---|
| G0 | G3 | G6 | G9 | |
| 1 to 21 days of age | ||||
| ADG/(g/d) | 6.20 ± 0.12 a | 6.68 ± 0.12 b | 6.59 ± 0.15 ab | 6.21 ± 0.10 a |
| ADFI/(g/d) | 18.92 ± 0.22 | 18.95 ± 0.41 | 19.01 ± 0.22 | 19.01 ± 0.19 |
| FCR | 3.05 ± 0.01 a | 2.84 ± 0.02 b | 2.88 ± 0.01 ab | 3.06 ± 0.02 a |
| TL/(mm) | 45.81 ± 0.38 | 45.90 ± 0.52 | 45.96 ± 0.62 | 45.89 ± 0.84 |
| 22 to 42 days of age | ||||
| ADG/(g/d) | 14.17 ± 0.06 a | 14.93 ± 0.09 b | 14.48 ± 0.10 ab | 14.49 ± 0.09 ab |
| ADFI/(g/d) | 26.56 ± 0.36 | 27.05 ± 0.41 | 26.29 ± 0.48 | 26.34 ± 0.45 |
| FCR | 1.87 ± 0.03 | 1.81 ± 0.02 | 1.82 ± 0.04 | 1.82 ± 0.02 |
| TL/(mm) | 23.43 ± 1.99 | 24.49 ± 1.47 | 24.24 ± 1.30 | 24.13 ± 1.16 |
| 1 to 42 days of age | ||||
| ADG/(g/d) | 10.19 ± 0.15 a | 10.81 ± 0.11 b | 10.54 ± 0.10 ab | 10.35 ± 0.09 ab |
| ADFI/(g/d) | 22.74 ± 0.46 | 23.00 ± 0.27 | 22.65 ± 0.49 | 22.68 ± 0.42 |
| FCR | 2.23 ± 0.03 | 2.13 ± 0.02 | 2.15 ± 0.03 | 2.19 ± 0.02 |
| TL/(mm) | 69.24 ± 1.23 | 70.39 ± 1.05 | 70.20 ± 1.00 | 70.02 ± 1.12 |
| SR/(%) | 92.85 ± 1.31 | 93.13 ± 1.58 | 94.14 ± 2.07 | 93.52 ± 1.63 |
| Items 1 | Groups 2 | |||
|---|---|---|---|---|
| G0 | G3 | G6 | G9 | |
| TP/(g/L) | 41.12 ± 2.01 a | 49.34 ± 1.41 b | 51.87 ± 2.40 b | 43.85 ± 2.84 a |
| ALB/(g/L) | 16.01 ± 0.33 | 18.92 ± 0.57 | 18.89 ± 1.33 | 16.51 ± 1.04 |
| GLO/(g/L) | 25.10 ± 1.08 a | 29.58 ± 0.80 b | 30.45 ± 2.11 b | 27.31 ± 1.45 ab |
| UN/(mmol/L) | 0.36 ± 0.01 a | 0.29 ± 0.01 b | 0.30 ± 0.01 ab | 0.33 ± 0.01 a |
| UA/(μmol/L) | 181.55 ± 13.61 | 162.58 ± 20.79 | 169.28 ± 25.61 | 175.20 ± 24.91 |
| TC/(mmol/L) | 4.04 ± 0.20 | 3.62 ± 0.25 | 3.65 ± 0.27 | 3.80 ± 0.24 |
| TG/(mmol/L) | 0.47 ± 0.14 a | 0.32 ± 0.03 b | 0.34 ± 0.02 b | 0.45 ± 0.21 a |
| GLU/(mmol/L) | 13.66 ± 0.37 | 13.71 ± 0.30 | 13.58 ± 0.32 | 13.60 ± 0.12 |
| ALT/(U/L) | 26.83 ± 1.22 | 24.95 ± 1.50 | 25.24 ± 0.25 | 26.63 ± 2.47 |
| AST/(U/L) | 181.72 ± 10.21 | 175.34 ± 5.04 | 168.23 ± 5.78 | 175.34 ± 14.21 |
| Items 1 | Groups 2 | |||
|---|---|---|---|---|
| G0 | G3 | G6 | G9 | |
| 21 days of age | ||||
| T-AOC (U/mL) | 7.53 ± 0.60 a | 7.72 ± 0.42 ab | 7.97 ± 0.32 b | 8.26 ± 0.32 b |
| GSH-Px (U/mL) | 411.49 ± 4.76 a | 465.81 ± 8.11 b | 470.45 ± 12.68 b | 473.15 ± 12.37 b |
| MDA (nmol/mL) | 8.02 ± 0.14 Aa | 6.25 ± 0.43 b | 5.47 ± 0.45 Bb | 5.34 ± 0.28 Bb |
| T-SOD (U/mL) | 138.26 ± 6.28 | 133.51 ± 4.43 | 140.56 ± 7.73 | 141.45 ± 8.20 |
| 42 days of age | ||||
| T-AOC (U/mL) | 7.87 ± 0.52 a | 8.28 ± 0.52 ab | 8.58 ± 0.39 b | 8.52 ± 0.40 b |
| GSH-Px (U/mL) | 414.05 ± 5.28 a | 478.53 ± 5.61 b | 481.88 ± 5.83 b | 478.90 ± 4.64 b |
| MDA (nmol/mL) | 8.11 ± 0.25 a | 6.34 ± 0.50 b | 6.26 ± 0.14 b | 6.10 ± 0.35 b |
| T-SOD (U/mL) | 149.14 ± 4.85 | 138.61 ± 5.73 | 149.76 ± 7.54 | 145.37 ± 6.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Chen, L.; Wang, G.; Zhao, Y.; Xin, Y.; Xu, M.; Wang, Y.; Song, H.; Fu, J.; Shang, R.; et al. Impact of Incorporating Defatted Black Soldier Fly Meal into Diet on Growth Performance, Serum Biochemical Parameters, Nutrient Digestibility, Morphology of the Intestinal Tract, and Immune Index of Brooding Laying Hens. Animals 2025, 15, 625. https://doi.org/10.3390/ani15050625
Li L, Chen L, Wang G, Zhao Y, Xin Y, Xu M, Wang Y, Song H, Fu J, Shang R, et al. Impact of Incorporating Defatted Black Soldier Fly Meal into Diet on Growth Performance, Serum Biochemical Parameters, Nutrient Digestibility, Morphology of the Intestinal Tract, and Immune Index of Brooding Laying Hens. Animals. 2025; 15(5):625. https://doi.org/10.3390/ani15050625
Chicago/Turabian StyleLi, Lusheng, Lifei Chen, Guiying Wang, Yinling Zhao, Yizhen Xin, Meng Xu, Yuxi Wang, Hanhan Song, Jiani Fu, Rongsheng Shang, and et al. 2025. "Impact of Incorporating Defatted Black Soldier Fly Meal into Diet on Growth Performance, Serum Biochemical Parameters, Nutrient Digestibility, Morphology of the Intestinal Tract, and Immune Index of Brooding Laying Hens" Animals 15, no. 5: 625. https://doi.org/10.3390/ani15050625
APA StyleLi, L., Chen, L., Wang, G., Zhao, Y., Xin, Y., Xu, M., Wang, Y., Song, H., Fu, J., Shang, R., & Zhang, J. (2025). Impact of Incorporating Defatted Black Soldier Fly Meal into Diet on Growth Performance, Serum Biochemical Parameters, Nutrient Digestibility, Morphology of the Intestinal Tract, and Immune Index of Brooding Laying Hens. Animals, 15(5), 625. https://doi.org/10.3390/ani15050625







