Morphology-Based In-Ovo Sexing of Chick Embryos Utilizing a Low-Cost Imaging Apparatus and Machine Learning
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
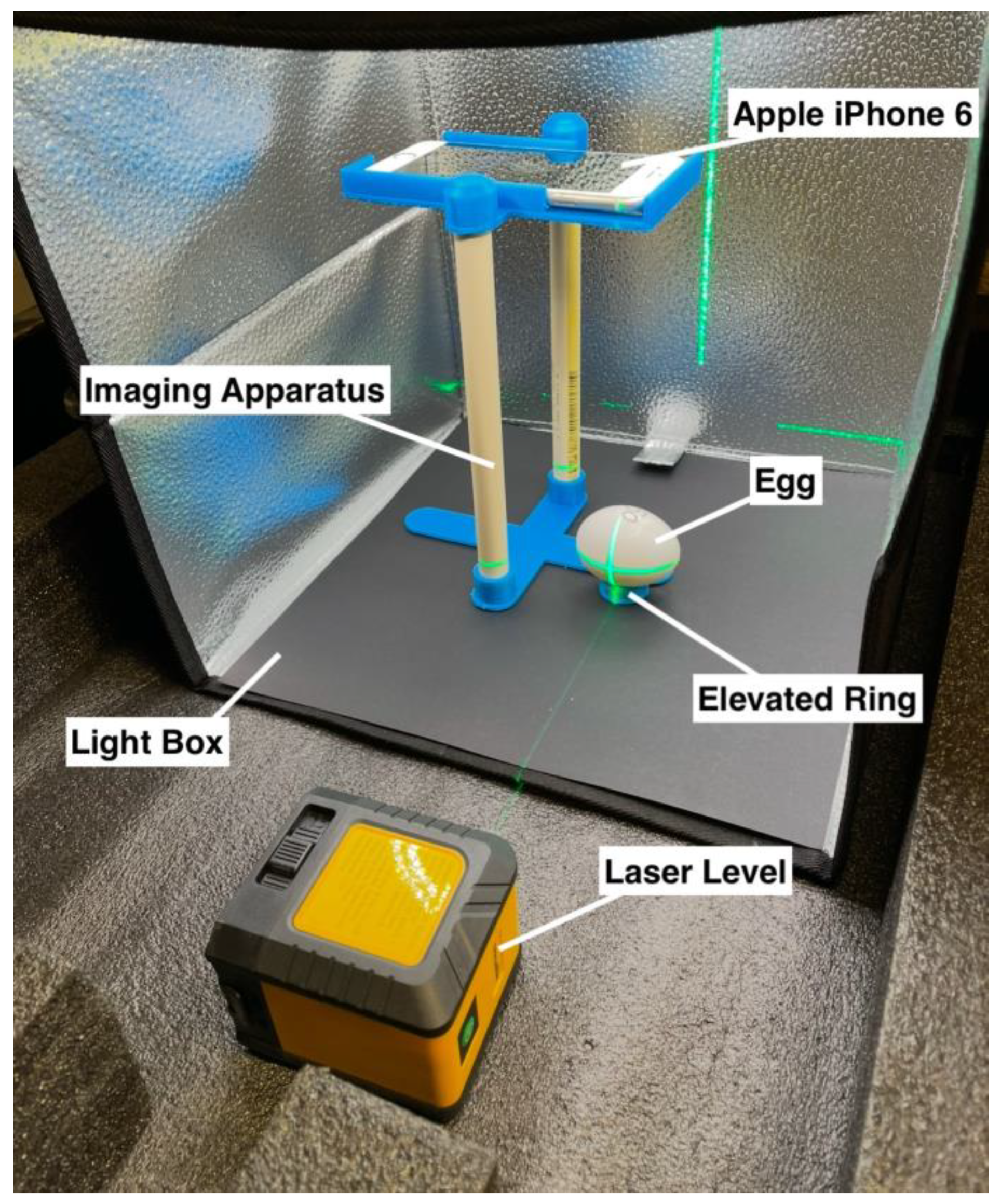
2.1. Construction of Imaging Apparatus
2.2. Comparison of Imaging Apparatus to 3D Scanner
2.3. Chicken Egg Incubation, Imaging, and Sexing
2.4. Chicken Egg Morphology Measurement
2.5. Morphology-Based Machine Learning Analysis
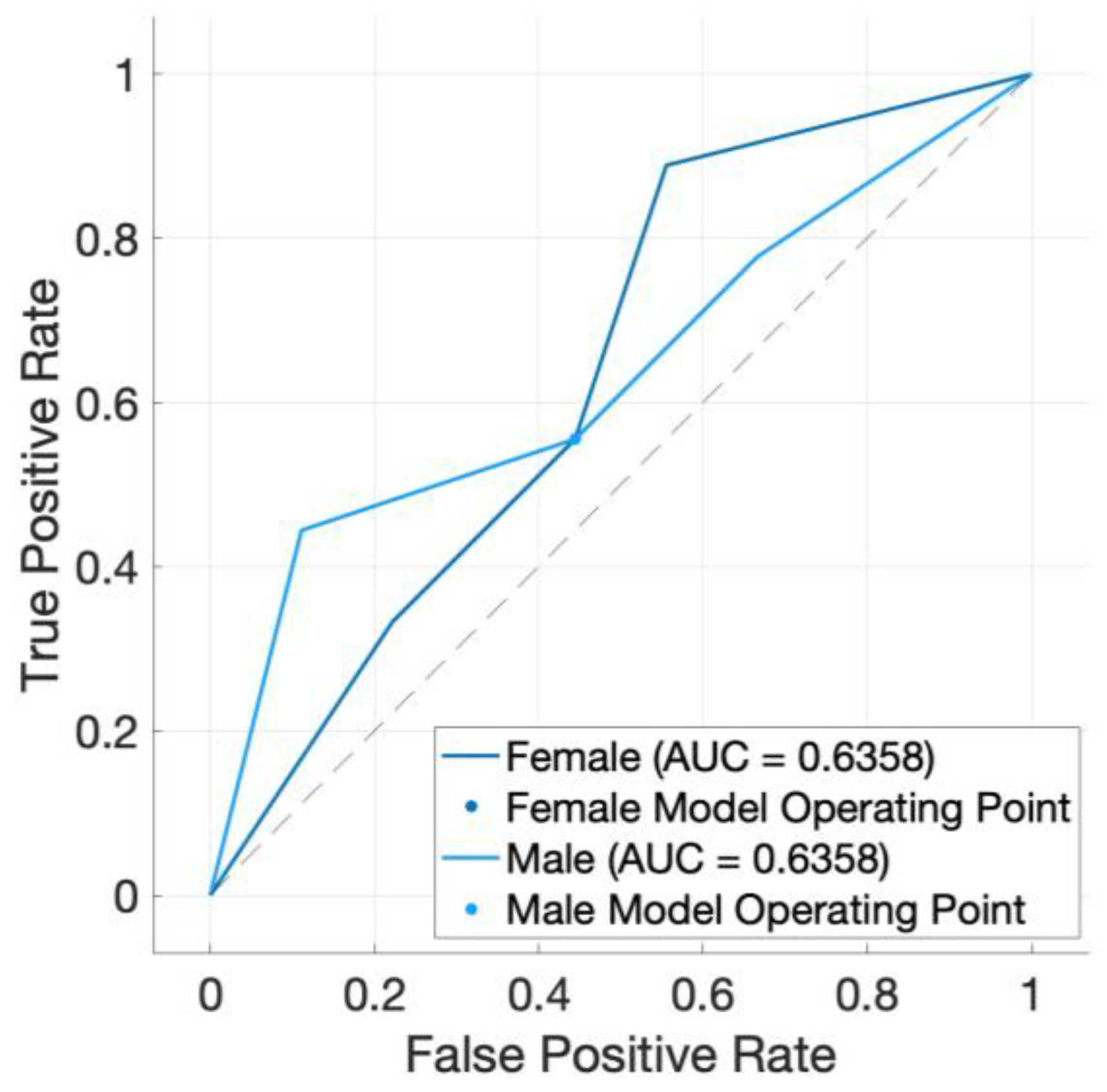
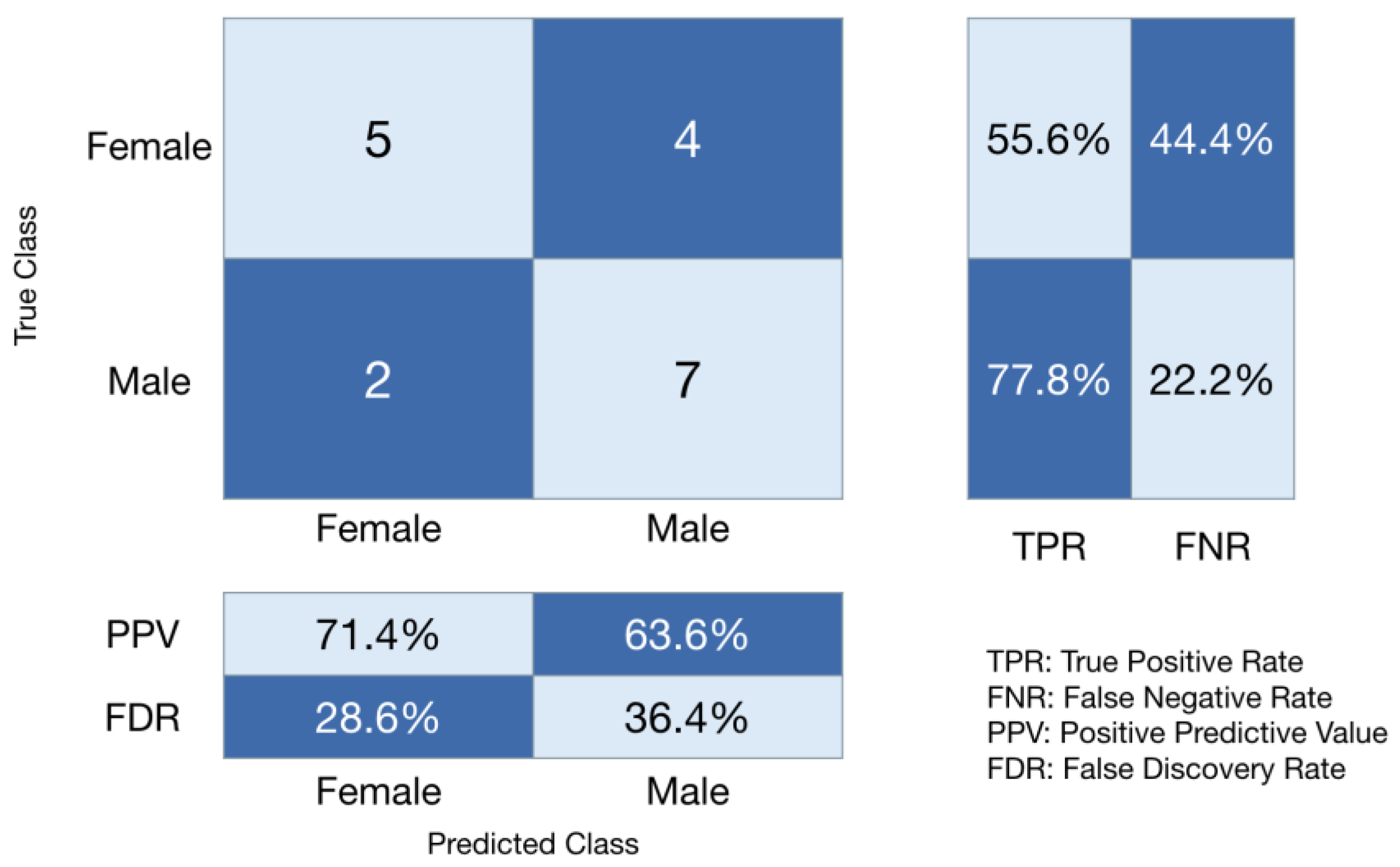
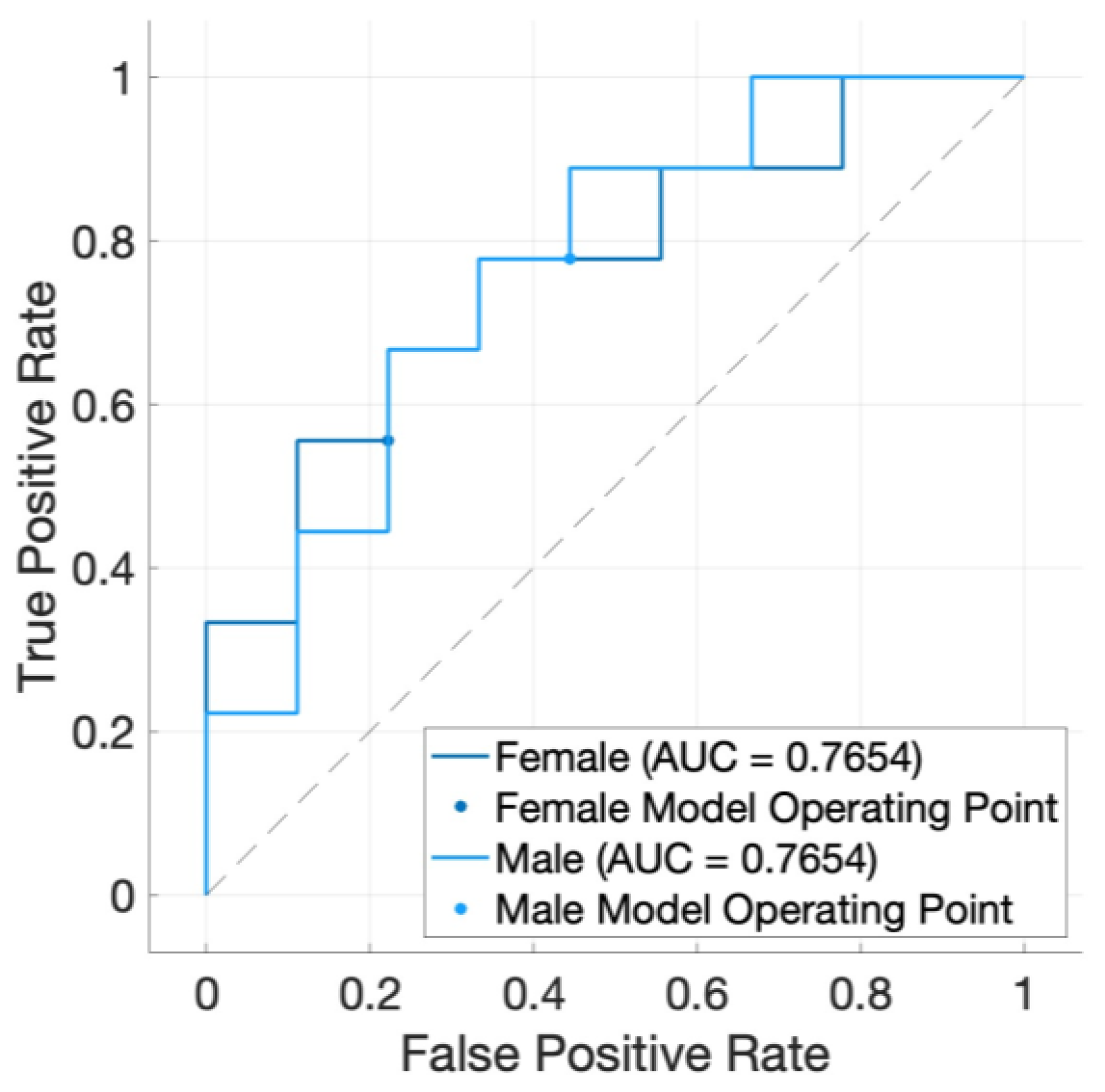
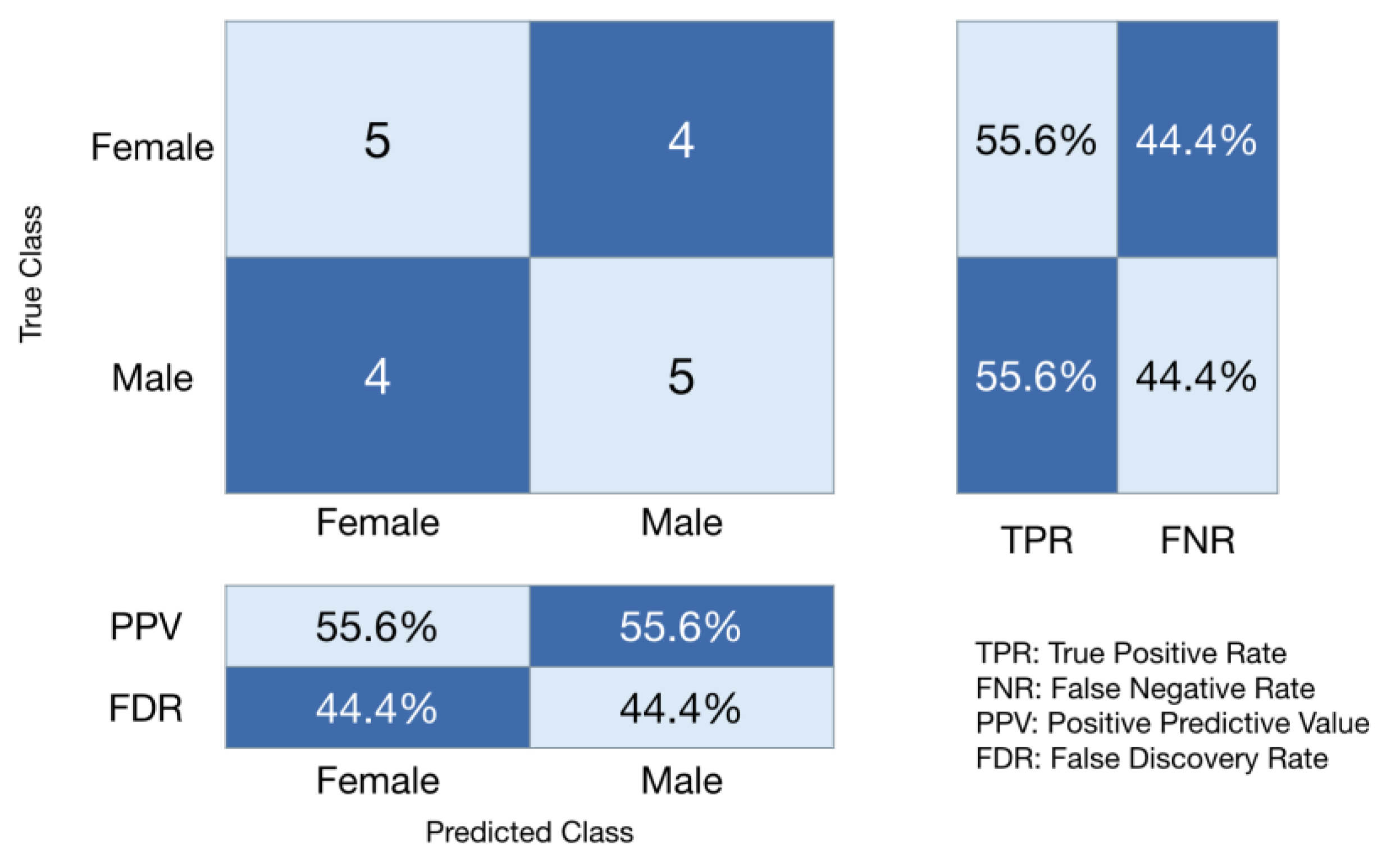
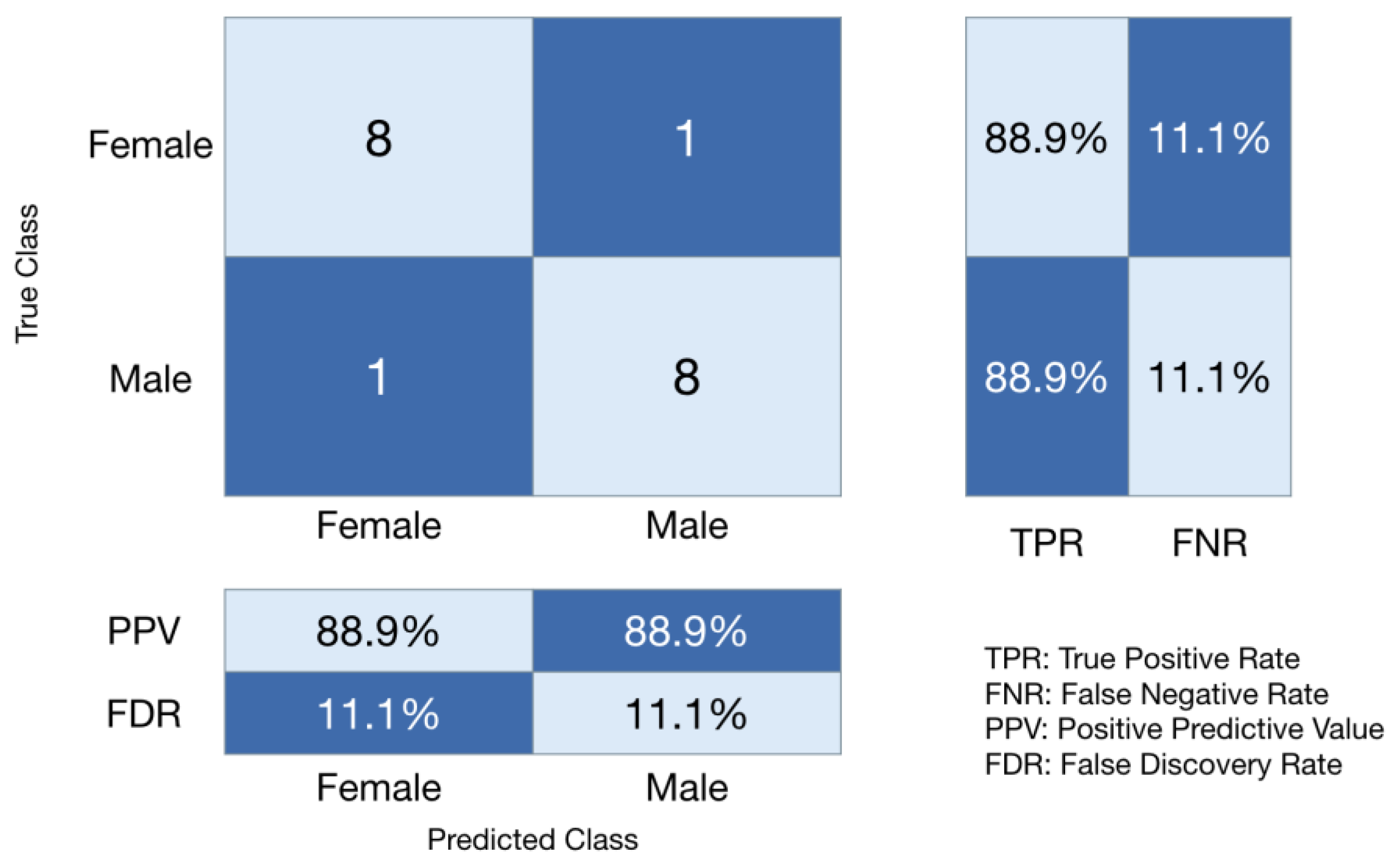
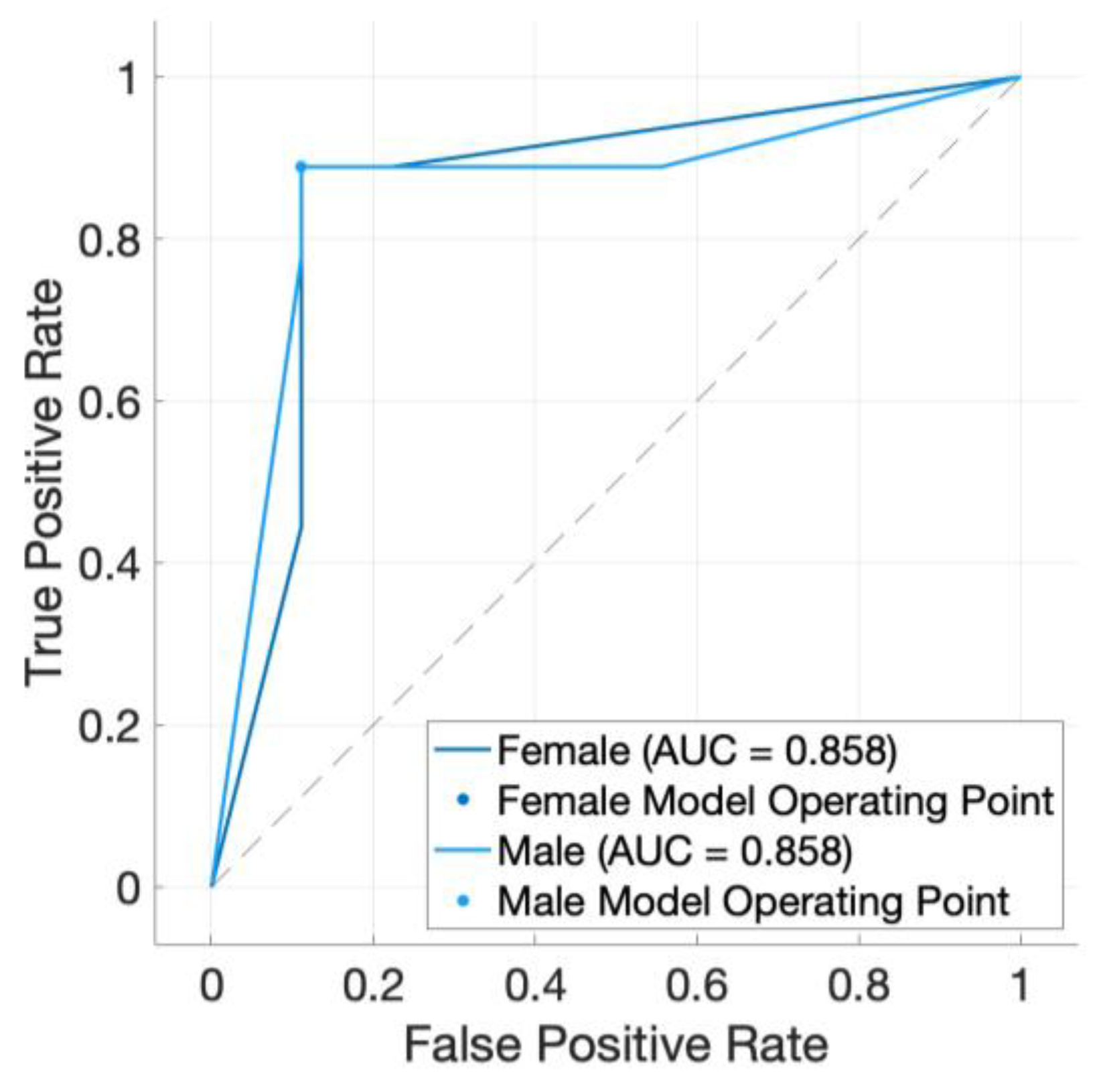
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- Brice-Saddler, M. France Says Its poultry Industry Will Stop Shredding Male Chicks Alive by 2022. Washington Post. Available online: https://www.washingtonpost.com/science/2020/01/29/shredding-chicks-ban/ (accessed on 5 December 2024).
- Krautwald-Junghanns, M.-E.; Cramer, K.; Fischer, B.; Förster, A.; Galli, R.; Kremer, F.; Mapesa, E.U.; Meissner, S.; Preisinger, R.; Preusse, G.; et al. Current Approaches to Avoid the Culling of Day-Old Male Chicks in the Layer Industry, with Special Reference to Spectroscopic Methods. Poult. Sci. 2018, 97, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Falcon, W.P.; Naylor, R.L.; Shankar, N.D. Rethinking Global Food Demand for 2050. Popul. Dev. Rev. 2022, 48, 921–957. [Google Scholar] [CrossRef]
- Rollin, B.E. Veterinary Medical Ethics. Can. Vet. J. = La Rev. Vet. Can. 2021, 62, 11–12. [Google Scholar]
- de Haas, E.N.; Oliemans, E.; van Gerwen, M.A.A.M. The Need for an Alternative to Culling Day-Old Male Layer Chicks: A Survey on Awareness, Alternatives, and the Willingness to Pay for Alternatives in a Selected Population of Dutch Citizens. Front. Vet. Sci. 2021, 8, 662197. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, K. Suitability of Day-Old Chicks as Food for Captive Snakes. J. Anim. Physiol. Anim. Nutr. 2010, 94, e296–e307. [Google Scholar] [CrossRef] [PubMed]
- Gautron, J.; Réhault-Godbert, S.; Van de Braak, T.G.H.; Dunn, I.C. Review: What Are the Challenges Facing the Table Egg Industry in the next Decades and What Can Be Done to Address Them? Animal 2021, 15, 100282. [Google Scholar] [CrossRef] [PubMed]
- Kollmansperger, S.; Anders, M.; Werner, J.; Saller, A.M.; Weiss, L.; Süß, S.C.; Reiser, J.; Schneider, G.; Schusser, B.; Baumgartner, C.; et al. Nociception in Chicken Embryos, Part II: Embryonal Development of Electroencephalic Neuronal Activity in Ovo as a Prerequisite for Nociception. Animals 2023, 13, 2839. [Google Scholar] [CrossRef] [PubMed]
- Haase, A.; Schusser, B.M.; Molina-Romero, M.; Goméz, P.A.; Aigner, M.; Huber, S.; Joos, A. Automated Noninvasive Determining the Sex of an Embryo of and the Fertility of a Bird’s Egg. WO 2019092265 A1, 31 March 2021. [Google Scholar]
- McKay, J.C. Spectrophotometric Analysis of Embryonic Chick Feather Color. US 2014069336 A1, 10 November 2015. [Google Scholar]
- Bruins, W.S.; Stutterhim, W.M. Method and System for the Non-Destructive In Ovo Determination of Fowl Gender. WO 2017204636 A2, 4 January 2018. [Google Scholar]
- Weigel, M.C.; Hofmann-Peiker, K.; Kleine, M. Method for Gender Identification in Domestic Chicken. WO 2017076957 A1, 11 May 2017. [Google Scholar]
- Torrella, K. Save the Male Chicks. Vox. Available online: https://www.vox.com/future-perfect/2023/5/1/23700952/egg-industry-male-chick-culling-animal-welfare (accessed on 5 December 2024).
- Corion, M.; Santos, S.; De Ketelaere, B.; Spasic, D.; Hertog, M.; Lammertyn, J. Trends in in Ovo Sexing Technologies: Insights and Interpretation from Papers and Patents. J. Anim. Sci. Biotechnol. 2023, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Li, B.; Zhu, J.; Wang, H.; Zhao, Y.; Zhao, W. A Review of Key Techniques for in Ovo Sexing of Chicken Eggs. Agriculture 2023, 13, 677. [Google Scholar] [CrossRef]
- Kayadan, M.; Uzun, Y. High Accuracy Gender Determination Using the Egg Shape Index. Sci. Rep. 2023, 13, 504. [Google Scholar] [CrossRef] [PubMed]
- Mappatao, G. Duck Egg Sexing by Eccentricity Determination Using Image Processing. J. Telecommun. Electron. Comput. Eng. (JTEC) 2018, 10, 71–75. [Google Scholar]
- Dioses, J.L.; Medina, R.P.; Fajardo, A.C.; Hernandez, A.A.; Angelo, I. Performance of Egg Sexing Classification Models in Philippine Native Duck. In Proceedings of the 2021 IEEE 12th Control and System Graduate Research Colloquium (ICSGRC), Shah Alam, Malaysia, 7 August 2021; pp. 248–253. [Google Scholar] [CrossRef]
- Yilmaz-Dikmen, B.; Dikmen, S. A Morphometric Method of Sexing White Layer Eggs. Rev. Bras. De Ciência Avícola 2013, 15, 203–210. [Google Scholar] [CrossRef]
- Offen, D. Methods for Gender Determination of Avian Embryos in Unhatched Eggs and Means Thereof. WO 2017094015 A1, 8 June 2017. [Google Scholar]
- Rehman, S.A.; Akhter, S.; Khan, S.H.; Anjum, M.A. A Comparative Study on Quality, Proximate Composition and Cholesterol Content of Eggs and Meat in Fayoumi and Commercial White Leghorn Chickens. Cogent Food Agric. 2016, 2, 1195539. [Google Scholar] [CrossRef]
- Bansal, M.; Goyal, A.; Choudhary, A. A Comparative Analysis of K-Nearest Neighbor, Genetic, Support Vector Machine, Decision Tree, and Long Short Term Memory Algorithms in Machine Learning. Decis. Anal. J. 2022, 3, 100071. [Google Scholar] [CrossRef]
- Dongare, A.; Kharde, R.; Kachare, A. Introduction to Artificial Neural Network. Certif. Int. J. Eng. Innov. Technol. (IJEIT) 2008, 9001, 2277–3754. [Google Scholar]
- Lee, H.J.; Yoon, J.W.; Jung, K.M.; Kim, Y.M.; Park, J.S.; Lee, K.Y.; Park, K.J.; Hwang, Y.S.; Park, Y.H.; Rengaraj, D.; et al. Targeted Gene Insertion into Z Chromosome of Chicken Primordial Germ Cells for Avian Sexing Model Development. FASEB J. 2019, 33, 8519–8529. [Google Scholar] [CrossRef] [PubMed]
- Corion, M.; Portillo-Estrada, M.; Santos, S.; Everaert, N.; Lammertyn, J.; Hertog, M.; De Ketelaere, B. In Ovo Sexing of Chickens: Evaluating Volatile Organic Compounds Analysis Techniques and Daily Prediction Performance from the Onset of Incubation. Biosyst. Eng. 2024, 247, 109–118. [Google Scholar] [CrossRef]
- Galli, R.; Preusse, G.; Schnabel, C.; Bartels, T.; Cramer, K.; Krautwald-Junghanns, M.-E.; Koch, E.; Steiner, G. Sexing of Chicken Eggs by Fluorescence and Raman Spectroscopy through the Shell Membrane. PLoS ONE 2018, 13, e0192554. [Google Scholar] [CrossRef] [PubMed]
- Horkaew, P.; Kupittayanant, S.; Kupittayanant, P. Noninvasive in Ovo Sexing in Korat Chicken by Pattern Recognition of Its Embryologic Vasculature. J. Appl. Poult. Res. 2024, 33, 100424. [Google Scholar] [CrossRef]
| Morphological Feature | Description |
|---|---|
| Length | Maximum pixel distance between any two opposite points of the outline of an egg. |
| Width | Minimum pixel distance between any two opposite points of the outline of an egg. |
| Area | Number of pixels within the outline of an egg. |
| Eccentricity | Ratio of the pixel distance between the foci of the ellipse and its length [Equation (A1)]. |
| Extent | Ratio of pixels inside the outline of an egg and the total number of pixels in a bounding box around the outline of the egg [Equation (A2)]. |
| MATLAB Algorithms | Trial 1 | Trial 2 | Trial 3 | Mean |
|---|---|---|---|---|
| K-nearest neighbors (medium KNN) | 47.6% | 55.6% | 44.4% | 49.2% |
| Decision tree (boosted trees) | 83.8% | 55.6% | 72.2% | 70.5% |
| Support vector machine (cubic SVM) | 50.0% | 66.7% | 72.2% | 63.0% |
| Neural network (wide neural network) | 77.8% | 88.9% | 77.8% | 81.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Jacobs, L. Morphology-Based In-Ovo Sexing of Chick Embryos Utilizing a Low-Cost Imaging Apparatus and Machine Learning. Animals 2025, 15, 384. https://doi.org/10.3390/ani15030384
Zhang D, Jacobs L. Morphology-Based In-Ovo Sexing of Chick Embryos Utilizing a Low-Cost Imaging Apparatus and Machine Learning. Animals. 2025; 15(3):384. https://doi.org/10.3390/ani15030384
Chicago/Turabian StyleZhang, Daniel, and Leonie Jacobs. 2025. "Morphology-Based In-Ovo Sexing of Chick Embryos Utilizing a Low-Cost Imaging Apparatus and Machine Learning" Animals 15, no. 3: 384. https://doi.org/10.3390/ani15030384
APA StyleZhang, D., & Jacobs, L. (2025). Morphology-Based In-Ovo Sexing of Chick Embryos Utilizing a Low-Cost Imaging Apparatus and Machine Learning. Animals, 15(3), 384. https://doi.org/10.3390/ani15030384





