Corticosteroid-Induced Sinus Bradycardia in a Dog with Systemic Lupus Erythematosus: A Case Report
Simple Summary
Abstract
1. Introduction
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SLE | Systemic lupus erythematosus |
| ANA | Antinuclear antibody |
| CT | Computed tomography |
| HCT | Hematocrit |
| CRP | C-reactive protein |
| IV | Intravenous |
| BID | Bis in die |
| SID | Semel in die |
| BP | Blood pressure |
Appendix A
| Hematology Variable | Day −50 | Day −43 | Day −35 | Day −15 | Reference Interval |
|---|---|---|---|---|---|
| Red Blood Cell (M/μL) | 7.6 | 6.81 | 6.39 | 6.6 | 5.65–8.87 |
| Hematocrit (%) | 43.3 | 39.8 | 37 | 37.8 | 37.3–61.7 |
| Hemoglobin (g/dL) | 14.9 | 13.8 | 12 | 12.6 | 13.1–20.5 |
| Mean Corpuscular Volume (fL) | 58 | 58.4 | 57.8 | 57.3 | 61.6–73.5 |
| Mean Corpuscular Hemoglobin (pg) | 19.9 | 20.3 | 18.9 | 19.1 | 21.2–25.9 |
| Mean Corpuscular Hemoglobin Concentration (g/dL) | 34.4 | 34.7 | 32.6 | 33.3 | 32.0–37.9 |
| Red Cell Distribution Width | 20.5 | 19.9 | 19.7 | 20.4 | 13.6–21.7 |
| Reticulocyte (K/μL) | 17.2 | 15.9 | 21.2 | 6.8 | 10.0–110.0 |
| White Blood Cell (K/μL) | 8.53 | 5.74 | 6.76 | 4.91 | 5.05–16.76 |
| Neutrophil (K/μL) | 1.87 | 2.27 | 0.89 | 0.74 | 2.95–11.64 |
| Lymphocyte (K/μL) | 1.73 | 2.16 | 3.48 | 2.04 | 1.05–5.10 |
| Monocyte (K/μL) | 4.33 | 1.14 | 1.84 | 1.9 | 0.16–1.12 |
| Eosinophil (K/μL) | 0.45 | 0.16 | 0.54 | 0.2 | 0.06–1.23 |
| Basophil (K/μL) | 0.14 | 0 | 0.01 | 0.03 | 0.00–0.10 |
| Platelet (K/μL) | 206 | 218 | 394 | 159 | 148–484 |
| Mean Platelet Volume (fL) | 14.5 | 13.6 | 12.7 | 13.1 | 8.7–13.2 |
| Platelet Distribution Width (fL) | 17 | 15.3 | 14.3 | 12.8 | 9.1–19.4 |
| Plateletcrit (%) | 0.3 | 0.3 | 0.5 | 0.21 | 0.14–0.46 |
| Biochemistry Variable | Day −50 | Day −43 | Day −35 | Day −15 | Reference Interval |
|---|---|---|---|---|---|
| C-Reactive Protein (mg/dL) | 9.6 | 4.3 | 6.2 | 9.2 | 0.0–1.0 |
| Glucose (mg/dL) | 88 | 77–150 | |||
| Creatinine (mg/dL) | 0.9 | 0.3–1.2 | |||
| Blood Urea Nitrogen (mg/dL) | 13 | 7–29 | |||
| Phosphorus (mg/dL) | 5.7 | 5.1–10.4 | |||
| Calcium (mg/dL) | 9.4 | 7.8–12.6 | |||
| Total Protein (g/dL) | 8 | 4.8–7.2 | |||
| Albumin (g/dL) | 2.8 | 2.1–3.6 | |||
| Globulin (g/dL) | 5.2 | 2.3–3.8 | |||
| Alanine Aminotransferase (U/L) | 26 | 8–75 | |||
| Alkaline Phosphatase (U/L) | 154 | 46–337 | |||
| Gamma-Glutamyl Transferase (U/L) | 0 | 0–2 | |||
| Total Bilirubin (mg/dL) | 0.2 | 0.0–0.8 | |||
| Cholesterol (mg/dL) | 135 | 100–400 | |||
| Amylase (U/L) | 1126 | 300–1300 | |||
| Lipase (U/L) | 223 | 100–1500 | |||
| Sodium (mmol/L) | 152 | 145–157 | |||
| Potassium (mmol/L) | 3.3 | 3.5–5.5 | |||
| Chloride (mmol/L) | 115 | 105–119 | |||
| Lactic Acid (mmol/L) | 1.9 | 0.50–2.50 |
| SNAP 4D× Plus | Results | Blood Parasitic PCRs | Results |
|---|---|---|---|
| Dirofilaria immitis | (–) | Babesia spp. | (–) |
| Anaplasma phagocytophilum | (–) | Babesia gibsoni | (–) |
| Anaplasma platys | (–) | Ehrlichia canis | (–) |
| Ehrlichia canis | (–) | Anaplasma platys | (–) |
| Ehrlichia ewingii | (–) | Mycoplasma hemofelis | (–) |
| Borrelia burgdorfer | (–) | Mycoplasma hemocanis | (–) |
| Leptospira spp. | (–) |
Appendix B
| Hematology Variable | Day 1 | Day 2 | Day 4 | Day 6 | Reference Interval |
|---|---|---|---|---|---|
| Red Blood Cell (M/μL) | 4.43 | 5.3 | 5.73 | 5.97 | 5.65–8.87 |
| Hematocrit (%) | 25.5 | 31.8 | 34.1 | 34.9 | 37.3–61.7 |
| Hemoglobin (g/dL) | 9.1 | 10.8 | 11.7 | 12.2 | 13.1–20.5 |
| Mean Corpuscular Volume (fL) | 57.6 | 60 | 59.5 | 58.5 | 61.6–73.5 |
| Mean Corpuscular Hemoglobin (pg) | 20.5 | 20.4 | 20.4 | 20.4 | 21.2–25.9 |
| Mean Corpuscular Hemoglobin Concentration (g/dL) | 35.7 | 34 | 34.3 | 35 | 32.0–37.9 |
| Red Cell Distribution Width | 18.4 | 18.3 | 18.7 | 19.6 | 13.6–21.7 |
| Reticulocyte (K/μL) | 6.6 | 7.4 | 10.9 | 8.4 | 10.0–110.0 |
| White Blood Cell (K/μL) | 14.03 | 37.36 | 35.6 | 31.83 | 5.05–16.76 |
| Neutrophil (K/μL) | 9.54 | 32.51 | 33.1 | 28.19 | 2.95–11.64 |
| Lymphocyte (K/μL) | 2.39 | 2.71 | 1.06 | 2.02 | 1.05–5.10 |
| Monocyte (K/μL) | 1.9 | 2.08 | 1.4 | 1.46 | 0.16–1.12 |
| Eosinophil (K/μL) | 0.19 | 0.01 | 0 | 0.01 | 0.06–1.23 |
| Basophil (K/μL) | 0.01 | 0.05 | 0 | 0.15 | 0.00–0.10 |
| Platelet (K/μL) | 80.55 | 101.25 | 211 | 310 | 148–484 |
| Mean Platelet Volume (fL) | 16.2 | 16.6 | 14.1 | 13.2 | 8.7–13.2 |
| Platelet Distribution Width (fL) | NT | NT | NT | 14.1 | 9.1–19.4 |
| Plateletcrit (%) | 0.11 | 0.13 | 0.3 | 0.41 | 0.14–0.46 |
| Biochemistry Variable | Day 1 | Day 2 | Day 6 | Reference Interval |
|---|---|---|---|---|
| Glucose (mg/dL) | 95 | 98 | 103 | 80.9–121.1 |
| Creatinine (mg/dL) | 0.5 | 0.6 | 0.5–1.4 | |
| Blood Urea Nitrogen (mg/dL) | 14 | 17 | 8.5–28.7 | |
| Phosphorus (mg/dL) | 4.1 | 3.8 | 2–4.2 | |
| Calcium (mg/dL) | 9.4 | 9.4 | 9.1–11.2 | |
| Total Protein (g/dL) | 6.9 | 6.9 | 6.6 | 4.7–7.6 |
| Albumin (g/dL) | 2 | 2.1 | 2.4 | 2.8–3.8 |
| Globulin (g/dL) | 4.9 | 4.8 | 4.2 | 1.9–4.4 |
| Albumin/Globulin Ratio | 0.4 | 0.4 | 0.6 | 0.7–2.3 |
| Aspartate Aminotransferase (U/L) | 32 | 25 | 14–52.5 | |
| Alanine Aminotransferase (U/L) | 36 | 136 | 20–83.5 | |
| Alkaline Phosphatase (U/L) | 172 | 291 | 10.6–111.2 | |
| Total Bilirubin (mg/dL) | 0.13 | 0.13 | 0.1–0.2 | |
| Sodium (mmol/L) | 142 | 145 | 143.8–157.5 | |
| Potassium (mmol/L) | 3.7 | 4.4 | 3.9–5.6 | |
| Chloride (mmol/L) | 110 | 105 | 105.8–117 | |
| Magnesium (mg/dL) | 1.6 | 1.5 | 1.6–5.5 | |
| Total Carbon Dioxide (mmol/L) | 25 | 30 | 18.7–32.2 | |
| Cholesterol (mg/dL) | 213 | 141 | 112.4–385.2 | |
| Anion Gap | 10.7 | 14.4 | 8.4–24.3 | |
| Osmolality | 302 | 311 | 305–338.4 | |
| C-Reactive Protein (mg/dL) | 8.8 | 9.4 | 0–1.0 | |
| Cardiac Troponin I (ng/mL) | 14.8 | 1.82 | 0–1.0 |
| Urinalysis Variable | D1 | Reference |
|---|---|---|
| Collection method | Midstream voided | |
| Color | Amber | Yellow |
| Turbidity | Turbid | Clear |
| Specific gravity (Rm) | 1.055 | 1.015–1.045 |
| Urine Protein-to-Creatinine Ratio | 0.11 | <0.5 |
| Glucose (mg/dL) | - | Normal |
| Bilirubin (mg/dL) | 1 (1+) | Negative |
| Ketone (mg/dL) | - | Negative |
| pH | 6.0 | 6.0–7.5 |
| Protein (mg/dL) | 30 (1+) | Negative |
| UBG (EU/dL) | 1 (1+) | Normal |
| Nitrite (/μL) | NA | Negative |
| Occult Blood (/μL) | - | Negative |
| Leukocyte esterase (/μL) | 100 (2+) | Negative |
| Red Blood Cells per High Power Field (/HPF) | 0–3 | <5 |
| White Blood Cells (/HPF) | 2–5 | <5 |
| Epithelial cells | - | None to few |
| Squamous (/HPF) | - | |
| Transitional (/HPF) | - | |
| Renal tubular (/HPF) | - | |
| Cast | None | |
| Cellular per Low Power Field (/LPF) | - | |
| Granular (/LPF) | - | |
| Waxy (/LPF) | - | |
| Hyaline cast (/LPF) | - | None |
| Crystal (/HPF) | - | None |
| Sperm (/HPF) | - | None |
| Microorganisms | - | None |
References
- Fournel, C.; Chabanne, L.; Caux, C.; Faure, J.R.; Rigal, D.; Magnol, J.P.; Monier, J.C. Canine systemic lupus erythematosus. I: A study of 75 cases. Lupus 1992, 1, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Grindem, C.B.; Johnson, K.H. Systemic lupus erythematosus: Literature review and report of 42 new canine cases. J. Am. Anim. Hosp. Assoc. 1983, 19, 489–503. [Google Scholar]
- Scott, D.W.; Walton, D.K.; Manning, T.O.; Smith, C.A.; Lewis, R.M. Canine lupus erythematosus. I. Systemic lupus erythematosus. J. Am. Anim. Hosp. Assoc. 1983, 19, 461–479. [Google Scholar]
- Smee, N.M.; Harkin, K.R.; Wilkerson, M.J. Measurement of serum antinuclear antibody titer in dogs with and without systemic lupus erythematosus: 120 cases (1997–2005). J. Am. Vet. Med. Assoc. 2007, 230, 1180–1183. [Google Scholar] [CrossRef]
- Kim, D.H.; Han, H.J.; Kim, J.H. Successful management of systemic lupus erythematosus with levamisole in a Dachshund dog. Korean J. Vet. Res. 2021, 61, e1. [Google Scholar] [CrossRef]
- Viviano, K.R. Glucocorticoids, Cyclosporine, Azathioprine, Chlorambucil, and Mycophenolate in Dogs and Cats: Clinical Uses, Pharmacology, and Side Effects. Vet. Clin. N. Am. Small Anim. Pract. 2022, 52, 797–817. [Google Scholar] [CrossRef]
- Pérez-Alenza, D.; Melián, C. Hyperadrenocorticism in dogs. In Textbook of Veterinary Internal Medicine, 8th ed.; Ettinger, S.J., Feldman, E.C., Côté, E., Eds.; Elsevier: St. Louis, MO, USA, 2017; pp. 1795–1811. [Google Scholar]
- Stroeder, J.; Evans, C.; Mansell, H. Corticosteroid-induced bradycardia: Case report and review of the literature. Can. Pharm. J. 2015, 148, 235–240. [Google Scholar] [CrossRef]
- Nagakura, A.; Morikawa, Y.; Sakakibara, H.; Miura, M. Bradycardia associated with prednisolone in children with severe Kawasaki disease. J. Pediatr. 2017, 185, 106–111.e1. [Google Scholar] [CrossRef]
- Vasheghani-Farahani, A.; Sahraian, M.A.; Darabi, L.; Aghsaie, A.; Minagar, A. Incidence of various cardiac arrhythmias and conduction disturbances due to high-dose intravenous methylprednisolone in patients with multiple sclerosis. J. Neurol. Sci. 2011, 309, 75–78. [Google Scholar] [CrossRef]
- Akikusa, J.D.; Feldman, B.M.; Gross, G.J.; Silverman, E.D.; Schneider, R. Sinus bradycardia after intravenous pulse methylprednisolone. Pediatrics 2007, 119, e778–e782. [Google Scholar] [CrossRef]
- Gugten, A.V.D.; Bierings, M.; Frenkel, J. Glucocorticoid-associated bradycardia. J. Pediatr. Hematol. Oncol. 2008, 30, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Beyler, O.; Demir, C. Pulse methylprednisolone-induced sinus bradycardia: A case report. Exp. Clin. Transplant. 2023, 21, 921–924. [Google Scholar] [PubMed]
- Sodero, A.; Squitieri, M.; Mazzeo, S.; Pasca, M.; Matà, S.; Pieri, F.; Bessi, V.; Sorbi, S. Acute symptomatic sinus bradycardia in high-dose methylprednisolone therapy in a woman with inflammatory myelitis: A case report and review of the literature. Clin. Med. Insights Case Rep. 2019, 12, 1179547619831026. [Google Scholar] [CrossRef]
- Üsküdar Cansu, D.; Bodakçi, E.; Korkmaz, C. Dose-dependent bradycardia as a rare side effect of corticosteroids: A case report and review of the literature. Clin. Rheumatol. 2018, 38, 2337–2343. [Google Scholar] [CrossRef]
- Al Shibli, A.; Al Attrach, I.; Hamdan, M.A. Bradycardia following oral corticosteroid use: Case report and literature review. Arab J. Nephrol. Transplant. 2012, 5, 47–49. [Google Scholar]
- Sharma, S.; Abdelmoity, S.; Ilyas, M. Bradycardia: A rare but significant side effect of oral steroids in patients with epileptic spasms. Pediatr. Neurol. 2020, 112, 2–4. [Google Scholar] [CrossRef]
- Khandelwal, K.; Madathala, R.R.; Chennaiahgari, N.; Yousuffuddin, M. Steroid-induced sinus bradycardia. Cureus 2021, 13, e15065. [Google Scholar] [CrossRef]
- Ueda, N.; Yoshikawa, T.; Chihara, M.; Kawaguchi, S.; Niinomi, Y.; Yasaki, T. Atrial fibrillation following methylprednisolone pulse therapy. Pediatr. Nephrol. 1988, 2, 29–31. [Google Scholar] [CrossRef]
- Guillén, E.L.; Ruíz, A.M.; Bugallo, J.B. Hypotension, bradycardia, and asystole after high-dose intravenous methylprednisolone in a monitored patient. Am. J. Kidney Dis. 1998, 32, E4. [Google Scholar] [CrossRef]
- Kundu, A.; Fitzgibbons, T.P. Acute symptomatic sinus bradycardia in a woman treated with pulse dose steroids for multiple sclerosis: A case report. J. Med. Case Rep. 2015, 9, 216. [Google Scholar] [CrossRef]
- Ohshima, M.; Kawahata, K.; Kanda, H.; Yamamoto, K. Sinus bradycardia after intravenous pulse methylprednisolone therapy in patients with systemic lupus erythematosus. Mod. Rheumatol. 2019, 29, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Osuagwu, F.; Jahnke, B. Intravenous methylprednisolone-induced nocturnal sinus bradycardia in a multiple sclerosis patient. Prim. Care Companion CNS Disord. 2016, 18, 10.4088/PCC.15l01830. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taylor, M.R.; Gaco, D. Symptomatic sinus bradycardia after a treatment course of high-dose oral prednisone. J. Emerg. Med. 2013, 45, e55–e58. [Google Scholar] [CrossRef]
- Tvede, N.; Nielsen, L.P.; Andersen, V. Bradycardia after high-dose intravenous methylprednisolone therapy. Scand. J. Rheumatol. 1986, 15, 302–304. [Google Scholar] [CrossRef]
- Marinov, M.; Fuessel, M.U.; Unterrainer, A.F. Bradycardia after dexamethasone for postoperative nausea and vomiting prophylaxis during induction of anaesthesia. Br. J. Anaesth. 2013, 111, 1025–1026. [Google Scholar] [CrossRef][Green Version]
- Miqdad, M.A.; Mohamad, A.; Ali, F.; Mourad, A.R.; Alamri, A. Methylprednisolone-induced symptomatic sinus bradycardia in a multiple sclerosis patient: A case report. Cureus 2022, 14, e21443. [Google Scholar] [CrossRef]
- Nemec Svete, A.; Verk, B.; Čebulj-Kadunc, N.; Salobir, J.; Rezar, V.; Domanjko Petrič, A. Inflammation and its association with oxidative stress in dogs with heart failure. BMC Vet. Res. 2021, 17, 176. [Google Scholar] [CrossRef]
- Ali, S.; Zehra, A.; Khalid, M.U.; Hassan, M.; Shah, S.I.A. Role of C-reactive protein in disease progression, diagnosis and management. Discoveries 2023, 11, e179. [Google Scholar] [CrossRef]
- Ghosh, A.; Annigeri, S.; Nair, A. Low-dose steroid-induced bradyarrhythmias and treatment refractory hypokalaemia: A case report. Cardiol. Young 2021, 31, 651–653. [Google Scholar] [CrossRef]
- Zkib, J.; Sattout, R.; Faour, S.; Haddad, S.; Bassut, R.; Swed, W.; Hritani, S.; Mansouer, M.; Ghabally, M. Corticosteroid-induced bradycardia following high-dose methylprednisolone administration: A case report. Ann. Med. Surg. 2024, 86, 6300–6302. [Google Scholar] [CrossRef]
- Tripathy, S.K.; Das, S.; Malik, A. Bradycardia after pulse methylprednisolone therapy in a child—Uncommon side effect of a frequently used drug: A case report. J. Fam. Med. Prim. Care 2023, 12, 1006–1008. [Google Scholar] [CrossRef] [PubMed]
- Capilupi, M.J.; Kerath, S.M.; Becker, L.B. Vagus nerve stimulation and the cardiovascular system. Cold Spring Harb. Perspect. Med. 2020, 10, a034173. [Google Scholar]
- Han, Y.; Shao, M.; Yang, H.; Sun, H.; Sang, W.; Wang, L.; Wang, L.; Yang, S.; Jian, Y.; Tang, B.; et al. Safety and efficacy of cardioneuroablation for vagal bradycardia in a single-arm prospective study. Sci. Rep. 2024, 14, 5926. [Google Scholar] [CrossRef]
- Acierno, M.J.; Brown, S.; Coleman, A.E.; Jepson, R.E.; Papich, M.; Stepien, R.L.; Syme, H.M. ACVIM consensus statement: Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J. Vet. Intern. Med. 2018, 32, 1803–1822. [Google Scholar] [CrossRef]
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef]
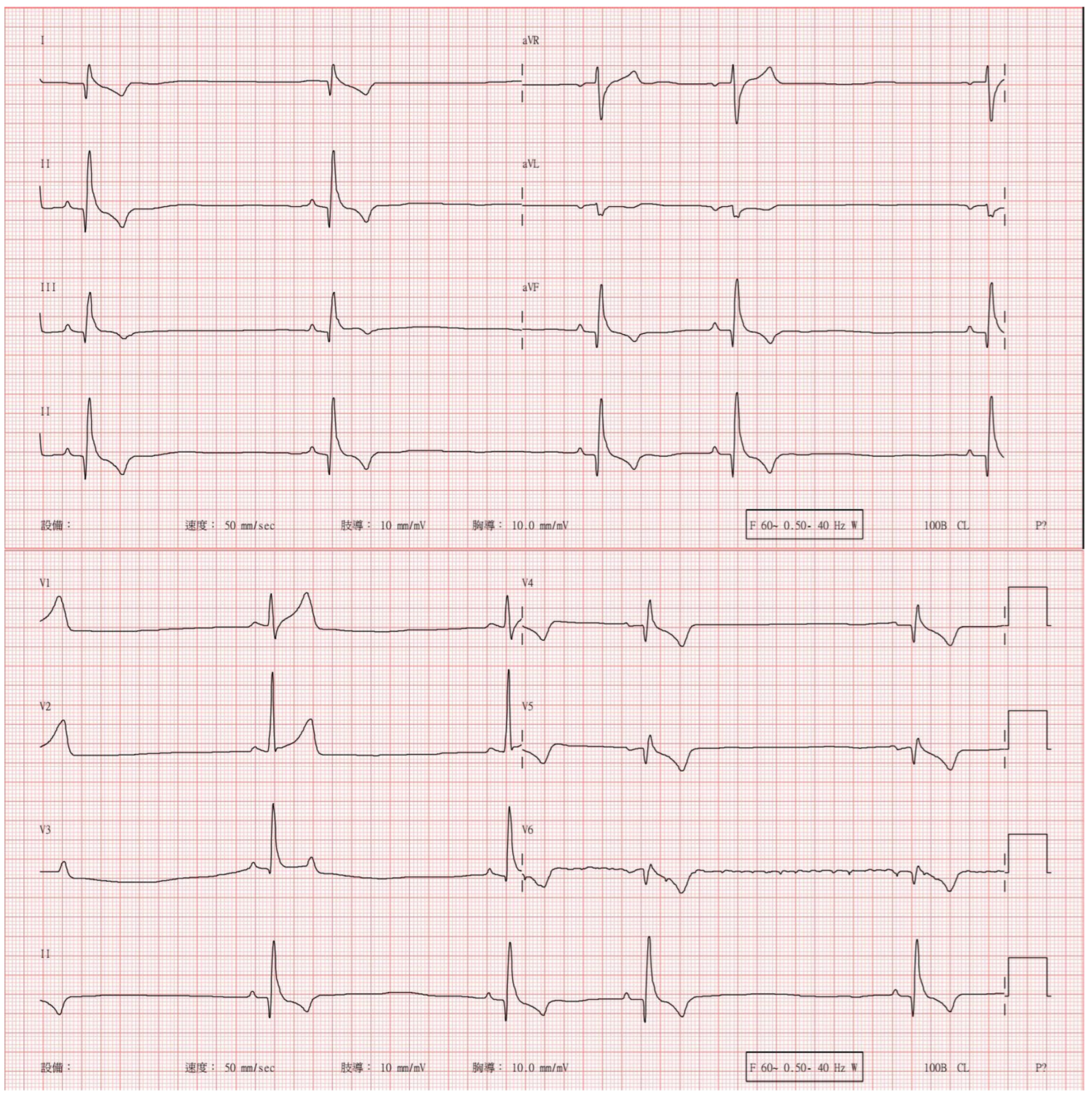
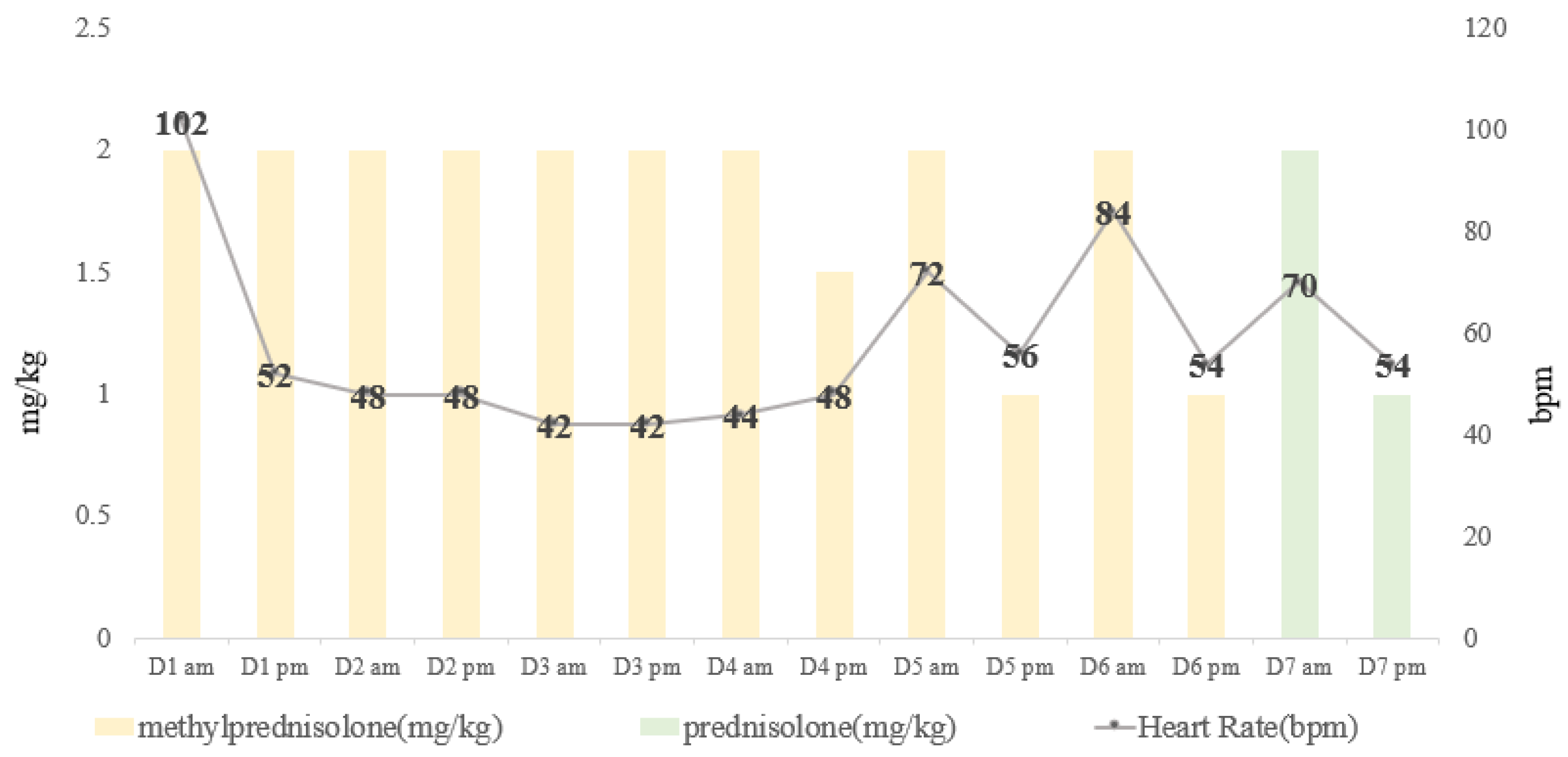
| Day | Heart Rate (Beats/min) | Respiratory Rate (Breaths/min) | Blood Pressure [SYS/DIA (MAP)] | Body Temperature (°C) | |
|---|---|---|---|---|---|
| 1 | am | 114 | 36 | 129/63 (87) | 38.5 |
| pm | 52 | 32 | 141/75 (84) | 37.9 | |
| 2 | am | 48 | 30 | 137/87 (99) | 37.8 |
| pm | 48 | 28 | 147/98 (114) | 37.7 | |
| 3 | am | 42 | 32 | 150/73 (101) | 37.8 |
| pm | 42 | 36 | 140/91 (107) | 37.7 | |
| 4 | am | 44 | 36 | 161/84 (110) | 37.8 |
| pm | 48 | 44 | 161/80 (107) | 37.9 | |
| 5 | am | 72 | 36 | 142/81 (101) | 38.0 |
| pm | 56 | 64 | 152/82 (105) | 37.9 | |
| 6 | am | 84 | 42 | 143/81 (103) | 38.0 |
| pm | 54 | 48 | 153/93 (113) | 38.0 | |
| 7 | am | 70 | 48 | 126/72 (90) | 38.4 |
| pm | 54 | 54 | 142/85 (104) | 38.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsou, F.-C.; Lim, E.-W.; Jann, P.-G.; Liu, P.-C. Corticosteroid-Induced Sinus Bradycardia in a Dog with Systemic Lupus Erythematosus: A Case Report. Animals 2025, 15, 375. https://doi.org/10.3390/ani15030375
Tsou F-C, Lim E-W, Jann P-G, Liu P-C. Corticosteroid-Induced Sinus Bradycardia in a Dog with Systemic Lupus Erythematosus: A Case Report. Animals. 2025; 15(3):375. https://doi.org/10.3390/ani15030375
Chicago/Turabian StyleTsou, Fang-Chi, Eng-Wen Lim, Pin-Guang Jann, and Pin-Chen Liu. 2025. "Corticosteroid-Induced Sinus Bradycardia in a Dog with Systemic Lupus Erythematosus: A Case Report" Animals 15, no. 3: 375. https://doi.org/10.3390/ani15030375
APA StyleTsou, F.-C., Lim, E.-W., Jann, P.-G., & Liu, P.-C. (2025). Corticosteroid-Induced Sinus Bradycardia in a Dog with Systemic Lupus Erythematosus: A Case Report. Animals, 15(3), 375. https://doi.org/10.3390/ani15030375





