Maternal Ferrous Sucrose Supplementation Improves Reproductive Performance of Sows and Hepatic Iron Stores of Neonatal Piglets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Ferrous Sucrose Samples
2.3. Animals, Diets, and Experimental Design
2.4. Recording and Sample Collection
2.5. Analytical Methods
2.5.1. Determination of Hemoglobin and Serum Iron Relative Indices
2.5.2. Measurement of Mineral Element Contents in Serum and Tissue Samples of Liver and Placenta
2.5.3. Histological Examination
2.5.4. Real-Time Quantitative RT-PCR
2.6. Statistical Analysis
3. Results
3.1. Maternal Ferrous Sucrose Supplementation Had Little Effect on Blood Hemoglobin and Hematocrit in Sows
3.2. Maternal Ferrous Sucrose Supplementation Improved Serum Iron Level in Sows and Offspring
3.3. Maternal Ferrous Sucrose Supplementation Increased Litter Weight and Average Weight of Neonatal Piglets
3.4. Maternal Ferrous Sucrose Supplementation Tended to Increase Duodenal Villus Height of Neonatal Piglets
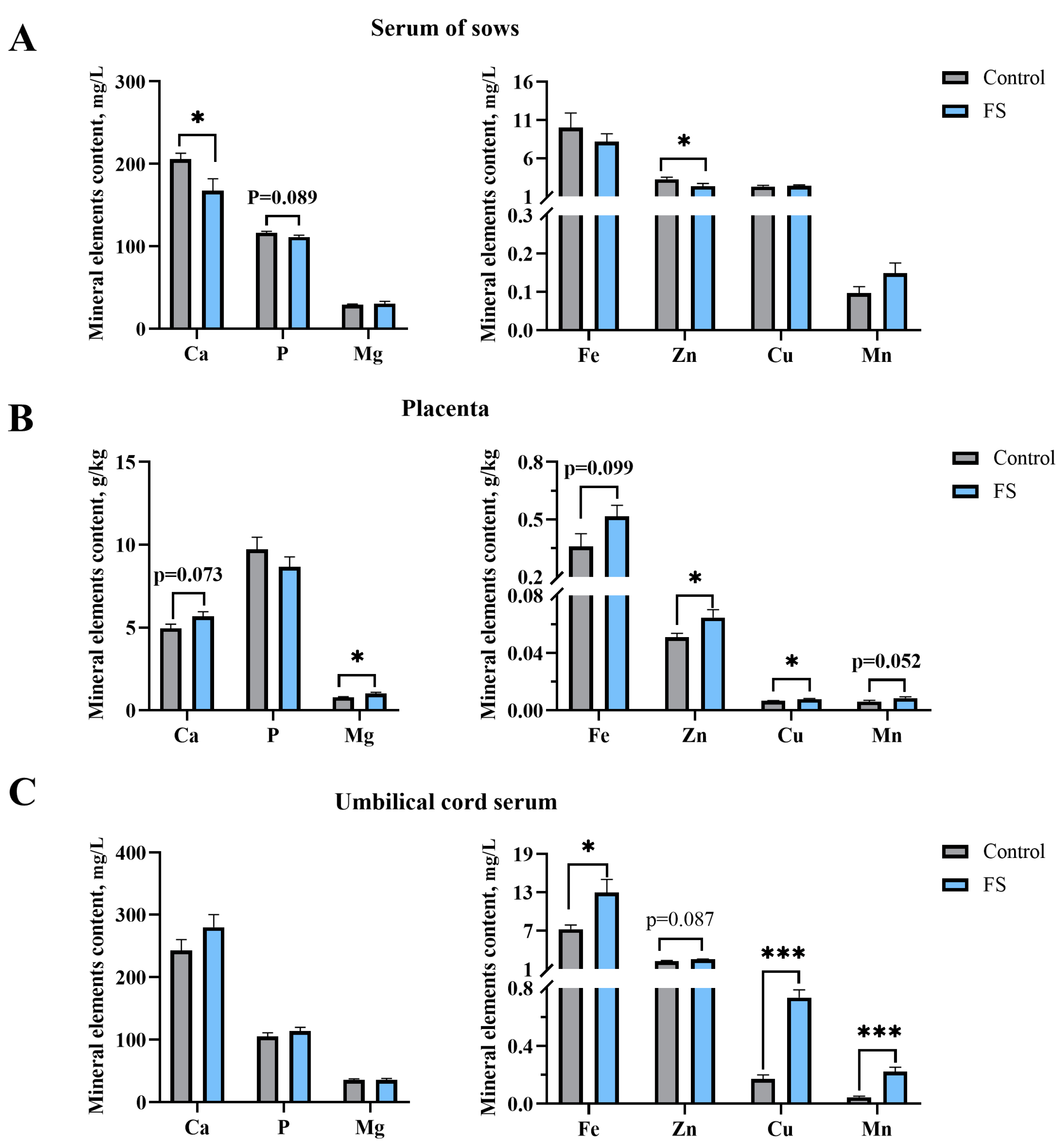
3.5. Maternal Ferrous Sucrose Supplementation Reduced Serum Calcium and Zinc in Sows
3.6. Maternal Ferrous Sucrose Supplementation Enhanced the Contents of Trace Elements in the Placenta and Umbilical Cord Serum
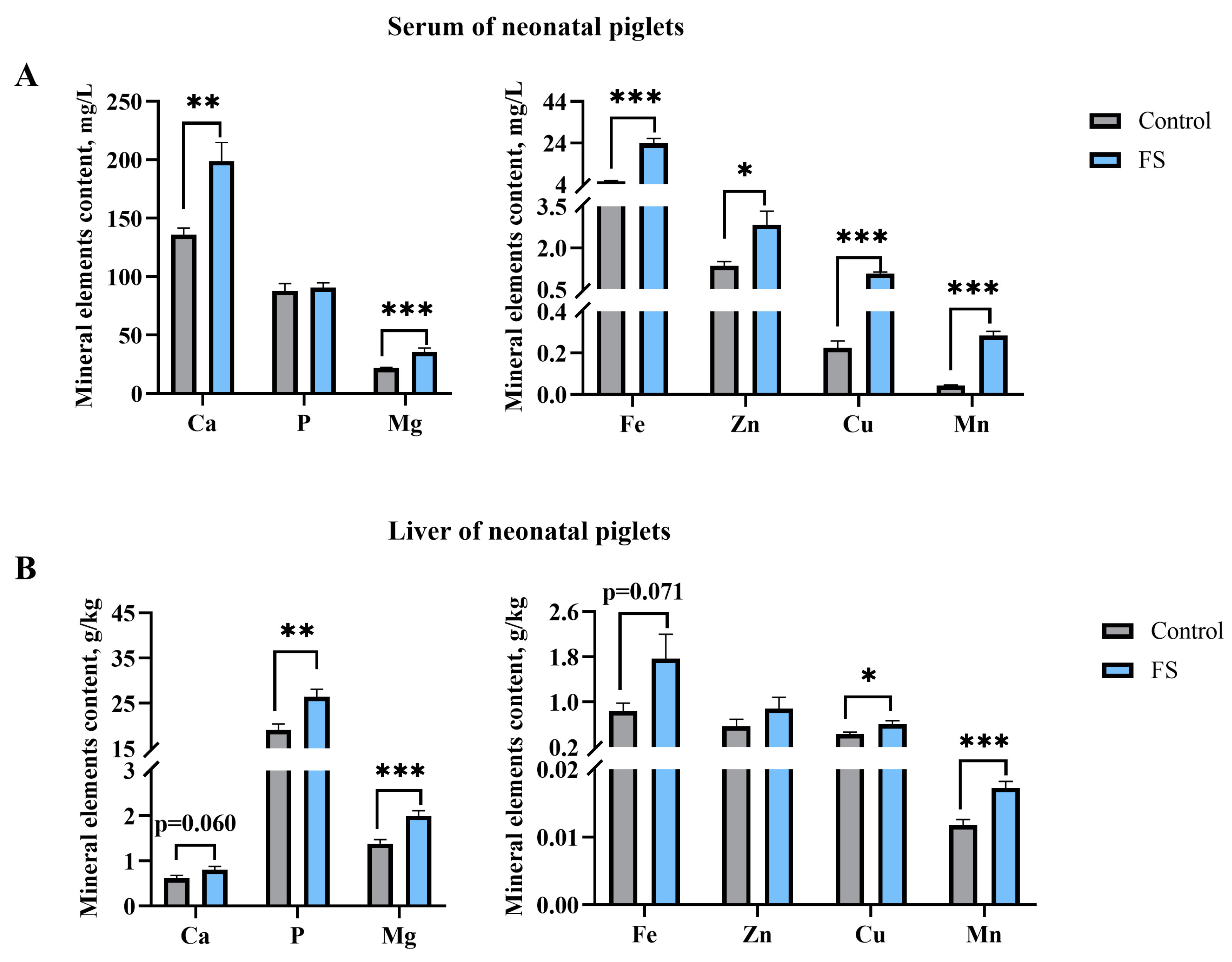
3.7. Maternal Ferrous Sucrose Supplementation Improved Circulating Iron and Hepatic Iron Stores of Neonatal Piglets
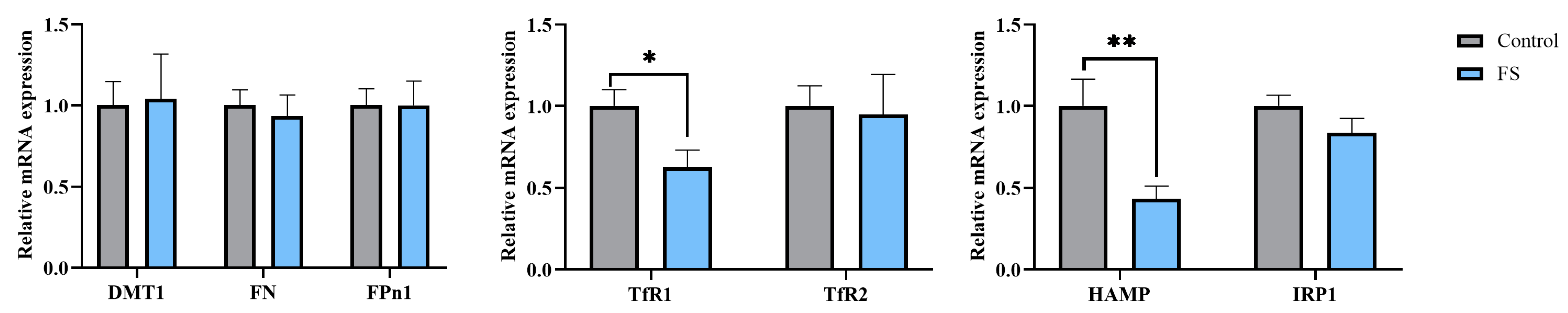
3.8. Maternal Ferrous Sucrose Supplementation Inhibited mRNA Expression of Transferrin Receptor 1 and Hepcidin in the Placenta
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lieu, P.T.; Heiskala, M.; Peterson, P.A.; Yang, Y. The roles of iron in health and disease. Mol. Aspects Med. 2001, 22, 1–87. [Google Scholar] [CrossRef] [PubMed]
- Galy, B.; Conrad, M.; Muckenthaler, M. Mechanisms controlling cellular and systemic iron homeostasis. Nat. Rev. Mol. Cell Biol. 2024, 25, 133–155. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.L. Iron biology in immune function, muscle metabolism and neuronal functioning. J. Nutr. 2001, 131, 568S–579S; discussion 580S. [Google Scholar] [CrossRef]
- Mu, Q.; Chen, L.; Gao, X.; Shen, S.; Sheng, W.; Min, J.; Wang, F. The role of iron homeostasis in remodeling immune function and regulating inflammatory disease. Sci. Bull. 2021, 66, 1806–1816. [Google Scholar] [CrossRef]
- Koenig, M.D.; Tussing-Humphreys, L.; Day, J.; Cadwell, B.; Nemeth, E. Hepcidin and iron homeostasis during pregnancy. Nutrients 2014, 6, 3062–3083. [Google Scholar] [CrossRef]
- Bothwell, T.H. Iron requirements in pregnancy and strategies to meet them. Am. J. Clin. Nutr. 2000, 72, 257s–263s. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.W.; Redmond, H.E.; Livingston, C.W. Iron Deficiency Anemia as a Cause of Stillbirths in Swine. J. Am. Vet. Med. Assoc. 1965, 147, 746–748. [Google Scholar]
- Venn, J.A.; McCance, R.A.; Widdowson, E.M. Iron metabolism in piglet anaemia. J. Comp. Pathol. Ther. 1947, 57, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Mazgaj, R.; Lipinski, P.; Starzynski, R.R. Iron Supplementation of Pregnant Sows to Prevent Iron Deficiency Anemia in Piglets: A Procedure of Questionable Effectiveness. Int. J. Mol. Sci. 2024, 25, 4106. [Google Scholar] [CrossRef] [PubMed]
- Mazgaj, R.; Szudzik, M.; Lipinski, P.; Jonczy, A.; Smuda, E.; Kamyczek, M.; Cieslak, B.; Swinkels, D.; Lenartowicz, M.; Starzynski, R.R. Effect of Oral Supplementation of Healthy Pregnant Sows with Sucrosomial Ferric Pyrophosphate on Maternal Iron Status and Hepatic Iron Stores in Newborn Piglets. Animals 2020, 10, 1113. [Google Scholar] [CrossRef]
- Peters, J.C.; Mahan, D.C. Effects of neonatal iron status, iron injections at birth, and weaning in young pigs from sows fed either organic or inorganic trace minerals. J. Anim. Sci. 2008, 86, 2261–2269. [Google Scholar] [CrossRef]
- Pond, W.G.; Maner, J.H.; Lowrey, R.S.; Loosli, J.K. Parenteral Iron Administration to Sows during Gestation or Lactation. J. Anim. Sci. 1961, 20, 747–750. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 11th ed.; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Arredondo, M.A.; Casas, G.A.; Stein, H.H. Increasing levels of microbial phytase increases the digestibility of energy and minerals in diets fed to pigs. Anim. Feed Sci. Technol. 2019, 248, 27–36. [Google Scholar] [CrossRef]
- She, Y.; Sparks, J.C.; Stein, H.H. Effects of increasing concentrations of an Escherichia coli phytase on the apparent ileal digestibility of amino acids and the apparent total tract digestibility of energy and nutrients in corn-soybean meal diets fed to growing pigs. J. Anim. Sci. 2018, 96, 2804–2816. [Google Scholar] [CrossRef]
- Bhattarai, S.; Framstad, T.; Nielsen, J.P. Iron treatment of pregnant sows in a Danish herd without iron deficiency anemia did not improve sow and piglet hematology or stillbirth rate. Acta Vet. Scand. 2019, 61, 60. [Google Scholar] [CrossRef]
- Wan, D.; Zhang, Y.M.; Wu, X.; Lin, X.; Shu, X.G.; Zhou, X.H.; Du, H.T.; Xing, W.G.; Liu, H.N.; Li, L.; et al. Maternal dietary supplementation with ferrous N-carbamylglycinate chelate affects sow reproductive performance and iron status of neonatal piglets. Animal 2018, 12, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, W.; Dong, D.; Jiang, S.; Yang, Z.; Wang, Y. Effect of different sources and levels of iron in the diet of sows on iron status in neonatal pigs. Anim. Nutr. 2018, 4, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Buffler, M.; Becker, C.; Windisch, W.M. Effects of different iron supply to pregnant sows (Sus scrofa domestica L.) on reproductive performance as well as iron status of new-born piglets. Arch. Anim. Nutr. 2017, 71, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Wang, H.K.; Li, L.X.; Ma, Y.X. The strategies for the supplementation of vitamins and trace minerals in pig production: Surveying major producers in China. Anim. Biosci. 2021, 34, 1350–1364. [Google Scholar] [CrossRef]
- Zhao, P.; Upadhaya, S.D.; Li, J.; Kim, I. Comparison effects of dietary iron dextran and bacterial-iron supplementation on growth performance, fecal microbial flora, and blood profiles in sows and their litters. Anim. Sci. J. 2015, 86, 937–942. [Google Scholar] [CrossRef]
- Poveda, C.; Pereira, D.I.A.; Lewis, M.; Walton, G.E. The Impact of Low-Level Iron Supplements on the Faecal Microbiota of Irritable Bowel Syndrome and Healthy Donors Using In Vitro Batch Cultures. Nutrients 2020, 12, 3819. [Google Scholar] [CrossRef] [PubMed]
- Paesano, R.; Torcia, F.; Berlutti, F.; Pacifici, E.; Ebano, V.; Moscarini, M.; Valenti, P. Oral administration of lactoferrin increases hemoglobin and total serum iron in pregnant women. Biochem. Cell Biol. 2006, 84, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Yee, J.; Besarab, A. Iron sucrose: The oldest iron therapy becomes new. Am. J. Kidney Dis. 2002, 40, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. BOARD-INVITED REVIEW: Intrauterine growth retardation: Implications for the animal sciences. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef]
- Gao, L.; Lin, X.; Xie, C.; Zhang, T.; Wu, X.; Yin, Y. The time of Calcium Feeding Affects the Productive Performance of Sows. Animals 2019, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Matte, J.J.; Audet, I. Maternal perinatal transfer of vitamins and trace elements to piglets. Animal 2020, 14, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Gao, L.M.; Liu, G.Y.; Tai, W.J.; Xie, C.Y.; Wu, X. Effects of Maternal Dietary Polysaccharide Iron Supplement on Mineral Elements and Iron Level of Neonatal Piglets. Biol. Trace Elem. Res. 2024, 202, 2588–2597. [Google Scholar] [CrossRef]
- Deng, Q.; Wang, Y.; Wang, X.; Wang, Q.; Yi, Z.; Xia, J.; Hu, Y.; Zhang, Y.; Wang, J.; Wang, L.; et al. Effects of dietary iron level on growth performance, hematological status, and intestinal function in growing-finishing pigs. J. Anim. Sci. 2021, 99, skab002. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Wu, Y.Y.; Wang, J.L.; Dong, Z.L.; Yu, Q.; Xia, S.S.; Liu, C.X.; Wang, H.H.; Wu, X. Enteromorpha prolifera polysaccharide-Fe (III) complex promotes intestinal development as a new iron supplement. Sci. China Life Sci. 2025, 68, 219–231. [Google Scholar] [CrossRef]
- Fu, Y.C.; Li, E.K.; Casey, T.M.; Johnson, T.A.; Adeola, O.; Ajuwon, K.M. Impact of maternal live yeast supplementation to sows on intestinal inflammatory cytokine expression and tight junction proteins in suckling and weanling piglets. J. Anim. Sci. 2024, 102, skae008. [Google Scholar] [CrossRef]
- Xie, C.; Wu, X.; Long, C.; Wang, Q.; Fan, Z.; Li, S.; Yin, Y. Chitosan oligosaccharide affects antioxidant defense capacity and placental amino acids transport of sows. Bmc Vet. Res. 2016, 12, 243. [Google Scholar] [CrossRef]
- Gao, L.M.; Liu, G.Y.; Wang, H.L.; Wassie, T.; Wu, X. Maternal pyrimidine nucleoside supplementation regulates fatty acid, amino acid and glucose metabolism of neonatal piglets. Anim. Nutr. 2022, 11, 309–321. [Google Scholar] [CrossRef]
- Mao, K.; Liu, L.; Mo, T.; Pan, H.C.; Liu, H. Preparation, Characterization, and Antioxidant Activity of an Isomaltooligosaccharide-Iron Complex (IIC). J. Carbohy. Chem. 2015, 34, 430–443. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Wassie, T.; Wu, Y.Y.; Wu, X. Advances on novel iron saccharide-iron (III) complexes as nutritional supplements. Crit. Rev. Food Sci. 2024, 64, 10239–10255. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.M.; Cheng, C.L.; Zhao, H.T.; Jing, J.; Gong, N.; Lu, W.H. In vivo anti-radiation activities of the Ulva pertusa polysaccharides and polysaccharide-iron(III) complex. Int. J. Biol. Macromol. 2013, 60, 341–346. [Google Scholar] [CrossRef]
- Jia, N.; Qiao, H.R.; Zhu, W.; Zhu, M.H.; Meng, Q.H.; Lu, Q.; Zu, Y.G. Antioxidant, immunomodulatory, oxidative stress inhibitory and iron supplementation effect of polysaccharide-iron (III) complex on iron-deficiency anemia mouse model. Int. J. Biol. Macromol. 2019, 132, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Meng, Y.B.; Liu, Y.; Meng, Q.H.; Zhang, Z.D.; Li, J.; Lu, Q. A novel iron supplements preparation from polysaccharide and assessment of antioxidant, lymphocyte proliferation and complement fixing activities. Int. J. Biol. Macromol. 2018, 108, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.L.; Shi, F.F.; Li, L.; Xu, J.X.; Chen, M.; Wu, L.; Hong, J.L.; Qian, M.; Bai, W.D.; Liu, B.; et al. Preparation of a novel Grifola frondosa polysaccharide-chromium (III) complex and its hypoglycemic and hypolipidemic activities in high fat diet and streptozotocin-induced diabetic mice. Int. J. Biol. Macromol. 2019, 131, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Dong, Z.L.; Tang, W.J.; Zhou, J.; Guo, L.; Gong, C.Y.; Liu, G.; Wan, D.; Yin, Y.L. Dietary iron regulates intestinal goblet cell function and alleviates invasion in mice. Sci. China Life Sci. 2023, 66, 2006–2019. [Google Scholar] [CrossRef]
- Xing, X.K.; Zhang, C.Y.; Ji, P.; Yang, J.; Li, Q.H.; Pan, H.B.; An, Q.C. Effects of Different Iron Supplements on Reproductive Performance and Antioxidant Capacity of Pregnant Sows as Well as Iron Content and Antioxidant Gene Expression in Newborn Piglets. Animals 2023, 13, 517. [Google Scholar] [CrossRef]
- Rochette, L.; Gudjoncik, A.; Guenancia, C.; Zeller, M.; Cottin, Y.; Vergely, C. The iron-regulatory hormone hepcidin: A possible therapeutic target? Pharmacol. Ther. 2015, 146, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Bayeva, M.; Chang, H.C.; Wu, R.X.; Ardehali, H. When less is more: Novel mechanisms of iron conservation. Trends Endocrinol. Metab. 2013, 24, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Fleming, M.D. The placenta: The forgotten essential organ of iron transport. Nutr. Rev. 2016, 74, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Ferretti, F.; Branchi, F.; Tomba, C.; Lombardo, V.; Scricciolo, A.; Doneda, L.; Roncoroni, L. Sucrosomial Iron Supplementation in Anemic Patients with Celiac Disease Not Tolerating Oral Ferrous Sulfate: A Prospective Study. Nutrients 2018, 10, 330. [Google Scholar] [CrossRef]
- Sangkhae, V.; Nemeth, E. Placental iron transport: The mechanism and regulatory circuits. Free Radic. Biol. Med. 2019, 133, 254–261. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Hepcidin and Iron in Health and Disease. Annu. Rev. Med. 2023, 74, 261–277. [Google Scholar] [CrossRef]
- van Santen, S.; Kroot, J.J.C.; Zijderveld, G.; Wiegerinck, E.T.; Spaanderman, M.E.A.; Swinkels, D.W. The iron regulatory hormone hepcidin is decreased in pregnancy: A prospective longitudinal study. Clin. Chem. Lab. Med. 2013, 51, 1395–1401. [Google Scholar] [CrossRef]
- Sangkhae, V.; Fisher, A.L.; Wong, S.; Koenig, M.D.; Tussing-Humphreys, L.; Chu, A.; Lelic, M.; Ganz, T.; Nemeth, E. Effects of maternal iron status on placental and fetal iron homeostasis. J. Clin. Investig. 2020, 130, 625–640. [Google Scholar] [CrossRef]
- Rehu, M.; Punnonen, K.; Ostland, V.; Heinonen, S.; Westerman, M.; Pulkki, K.; Sankilampi, U. Maternal serum hepcidin is low at term and independent of cord blood iron status. Eur. J. Haematol. 2010, 85, 345–352. [Google Scholar] [CrossRef]
- Sangkhae, V.; Fisher, A.L.; Chua, K.J.; Ruchala, P.; Ganz, T.; Nemeth, E. Maternal hepcidin determines embryo iron homeostasis in mice. Blood 2020, 136, 2206–2216. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Wang, R.; Deng, Z.; Zhou, J.; Li, N.; Li, W.; Zheng, L. Structural characterization and calcium absorption-promoting effect of sucrose-calcium chelate in Caco-2 monolayer cells and mice. J. Food Sci. 2024, 89, 1773–1790. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, R.; Deng, Z.; Zhou, J.; Li, W.; Du, Q.; Zheng, L. Structural Characterization of Zinc-Sucrose Complex and Its Ability to Promote Zinc Absorption in Caco-2 Monolayer Cells and Mice. J. Agric. Food Chem. 2023, 71, 12094–12104. [Google Scholar] [CrossRef] [PubMed]
- Ashmead, H.D. Comparative Intestinal-Absorption and Subsequent Metabolism of Metal Amino-Acid Chelates and Inorganic Metal-Salts. In Biological Trace Element Research; American Chemical Society Publications: Washington, DC, USA, 1991; Volume 445, pp. 306–319. [Google Scholar]
- Liao, Z.C.; Guan, W.T.; Chen, F.; Hou, D.X.; Wang, C.X.; Lv, Y.T.; Qiao, H.Z.; Chen, J.; Han, J.H. Ferrous bisglycinate increased iron transportation through DMT1 and PepT1 in pig intestinal epithelial cells compared with ferrous sulphate. J. Anim. Feed Sci. 2014, 23, 153–159. [Google Scholar] [CrossRef]
- Mahan, D.C.; Watts, M.R.; St-Pierre, N. Macro- and micromineral composition of fetal pigs and their accretion rates during fetal development. J. Anim. Sci. 2009, 87, 2823–2832. [Google Scholar] [CrossRef]
- Sampath, V.; Sureshkumar, S.; Seok, W.J.; Kim, I.H. Role and functions of micro and macro-minerals in swine nutrition: A short review. J. Anim. Sci. Technol. 2023, 65, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.L.; Trakooljul, N.; Liu, H.C.; Moeser, A.J.; Spears, J.W. Iron Transporters Are Differentially Regulated by Dietary Iron, and Modifications Are Associated with Changes in Manganese Metabolism in Young Pigs. J. Nutr. 2009, 139, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T.; Sandstead, H.H.; Prasad, A.S.; Newberne, P.M.; Fraker, P.J. Zinc—Health-Effects and Research Priorities for the 1990s. Environ. Health Perspect. 1994, 102, 5–46. [Google Scholar]
- Hill, C.H.; Matrone, G. Chemical parameters in the study of in vivo and in vitro interactions of transition elements. Fed. Proc. 1970, 29, 1474–1481. [Google Scholar]
- Humphries, W.R.; Phillippo, M.; Young, B.W.; Bremner, I. The influence of dietary iron and molybdenum on copper metabolism in calves. Br. J. Nutr. 1983, 49, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.H.; Bidlack, W.R. Interrelationship of dietary ascorbic acid and iron on the tissue distribution of ascorbic acid, iron and copper in female guinea pigs. J. Nutr. 1980, 110, 1398–1408. [Google Scholar] [CrossRef]
- Johnson, M.A.; Hove, S.S. Development of anemia in copper-deficient rats fed high levels of dietary iron and sucrose. J. Nutr. 1986, 116, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Hambidge, K.M.; Miller, L.V.; Westcott, J.E.; Sheng, X.; Krebs, N.F. Zinc bioavailability and homeostasis. Am. J. Clin. Nutr. 2010, 91, 1478S–1483S. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.M.; Fairweather-Tait, S.J. Effect of calcium supplementation on daily nonheme-iron absorption and long-term iron status. Am. J. Clin. Nutr. 1998, 68, 96–102. [Google Scholar] [CrossRef]
- Zhang, B.; Sui, F.; Wang, B.; Wang, Y.; Li, W. Dietary combined supplementation of iron and Bacillus subtilis enhances reproductive performance, eggshell quality, nutrient digestibility, antioxidant capacity, and hematopoietic function in breeder geese. Poult. Sci. 2020, 99, 6119–6127. [Google Scholar] [CrossRef]


| Item | Quantity |
|---|---|
| Ingredients, % | |
| Yellow corn | 65.025 |
| Soybean meal | 11.20 |
| Soybean hull | 6.00 |
| Steam fish meal | 5.00 |
| Soybean oil | 1.00 |
| Expanded flaxseed | 2.00 |
| Fine stone powder | 0.80 |
| Expanded soybean | 5.00 |
| Sucrose | 1.00 |
| L-threonine | 0.12 |
| DL-methionine | 0.06 |
| Tryptophan | 0.05 |
| L-lysine HCl | 0.225 |
| NaCl | 0.36 |
| NaHCO3 | 0.18 |
| CaHPO4 | 0.98 |
| Premix 1 | 1.00 |
| Total | 100 |
| Nutrient composition 2, % | |
| NE 3, MJ/kg | 12.58 |
| Crude protein | 16.58 |
| Crude fiber | 4.5 |
| Total calcium | 0.898 |
| Total phosphorus | 0.641 |
| Lysine | 1.04 |
| Threonine | 0.72 |
| Methionine | 0.22 |
| Valine | 1.05 |
| Isoleucine | 0.72 |
| Leucine | 1.46 |
| STTD phosphorus 3 | 0.38 |
| Iron, mg/kg | 258.09 |
| Gene 1 | Nucleotide Sequence of Primers (5′–3′) | Size (bp) |
|---|---|---|
| FN | F: CGGGACAGAAGAGAATCCCC R: GTCCAAGAACCGGCGAAGTA | 166 |
| Fpn1 | F: TACCAACGGGGTACTTTGCC R: AGTGGGGAATGCAATTCAGGA | 217 |
| TfR1 | F: GGCTGTATTCTGCTCGTGGA R: AGCCAGAGCCCCAGAAGATA | 195 |
| DMT1 | F: GCAGGTGGTTGACGTCTGTA R: CACGCCCCCTTTGTAGATGT | 100 |
| TfR2 | F: GTGATGGAGACCCCCTTGTG R: GCCCATTATGAAAGGCGCTG | 161 |
| HAMP | F: ATCCCAGACAAGACAGCTCAC R: CCCACAGATTGCTTTGCGAC | 151 |
| IRP1 | F: GCGGCTCTTGACCAGATACA R: AGGGTCGTGCCTTCCTCTAT | 201 |
| β-actin | F: CTGCGGCATCCACGAAACT R: AGGGCCGTGATCTCCTTCTG | 132 |
| Period | Blood Parameters | Control | FS | p-Value |
|---|---|---|---|---|
| Before the study (day 95 of gestation) | Hemoglobin, g/L | 100.28 ± 3.44 | 103.47 ± 1.77 | 0.372 |
| Hematocrit, % | 29.50 ± 1.02 | 30.40 ± 0.53 | 0.397 | |
| After the study (parturition) | Hemoglobin, g/L | 108.45 ± 3.71 | 101.56 ± 1.52 | 0.112 |
| Hematocrit, % | 31.50 ± 1.28 | 29.81 ± 0.40 | 0.236 |
| Reproductive Performance | Control | FS | p-Value |
|---|---|---|---|
| Litter size, n | 13.56 ± 0.48 | 14.93 ± 0.73 | 0.113 |
| Number born alive, n | 13.28 ± 0.44 | 13.64 ± 0.58 | 0.613 |
| Number of stillbirths, n | 0.28 ± 0.11 | 0.71 ± 0.34 | 0.238 |
| IUGR 1, n | 0.83 ± 0.29 | 0.64 ± 0.20 | 0.618 |
| Birth litter weight, kg | 16.54 ± 0.52 | 19.97 ± 0.95 | 0.002 |
| Average weight, kg | 1.24 ± 0.04 | 1.36 ± 0.05 | 0.078 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, W.; Ma, X.; Liu, H.; Wang, Z.; Liu, C.; Xie, C. Maternal Ferrous Sucrose Supplementation Improves Reproductive Performance of Sows and Hepatic Iron Stores of Neonatal Piglets. Animals 2025, 15, 343. https://doi.org/10.3390/ani15030343
Tian W, Ma X, Liu H, Wang Z, Liu C, Xie C. Maternal Ferrous Sucrose Supplementation Improves Reproductive Performance of Sows and Hepatic Iron Stores of Neonatal Piglets. Animals. 2025; 15(3):343. https://doi.org/10.3390/ani15030343
Chicago/Turabian StyleTian, Wen, Xiaofan Ma, Hongwei Liu, Zhefeng Wang, Chunxue Liu, and Chunyan Xie. 2025. "Maternal Ferrous Sucrose Supplementation Improves Reproductive Performance of Sows and Hepatic Iron Stores of Neonatal Piglets" Animals 15, no. 3: 343. https://doi.org/10.3390/ani15030343
APA StyleTian, W., Ma, X., Liu, H., Wang, Z., Liu, C., & Xie, C. (2025). Maternal Ferrous Sucrose Supplementation Improves Reproductive Performance of Sows and Hepatic Iron Stores of Neonatal Piglets. Animals, 15(3), 343. https://doi.org/10.3390/ani15030343





