Mineral Supplementation in Jade Perch (Scortum barcoo) Aquaponics with Lettuce: A Comparison with Hydroponics and RAS
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Experimental Design
2.2. Fish
2.3. Plants
2.4. Performance Parameters
2.5. Water Quality Monitoring
2.6. Statistical Analysis
3. Results
3.1. Water Quality Parameters
3.2. Fish and Plant Performance Parameters
4. Discussion
4.1. Water Quality and Fish Performance
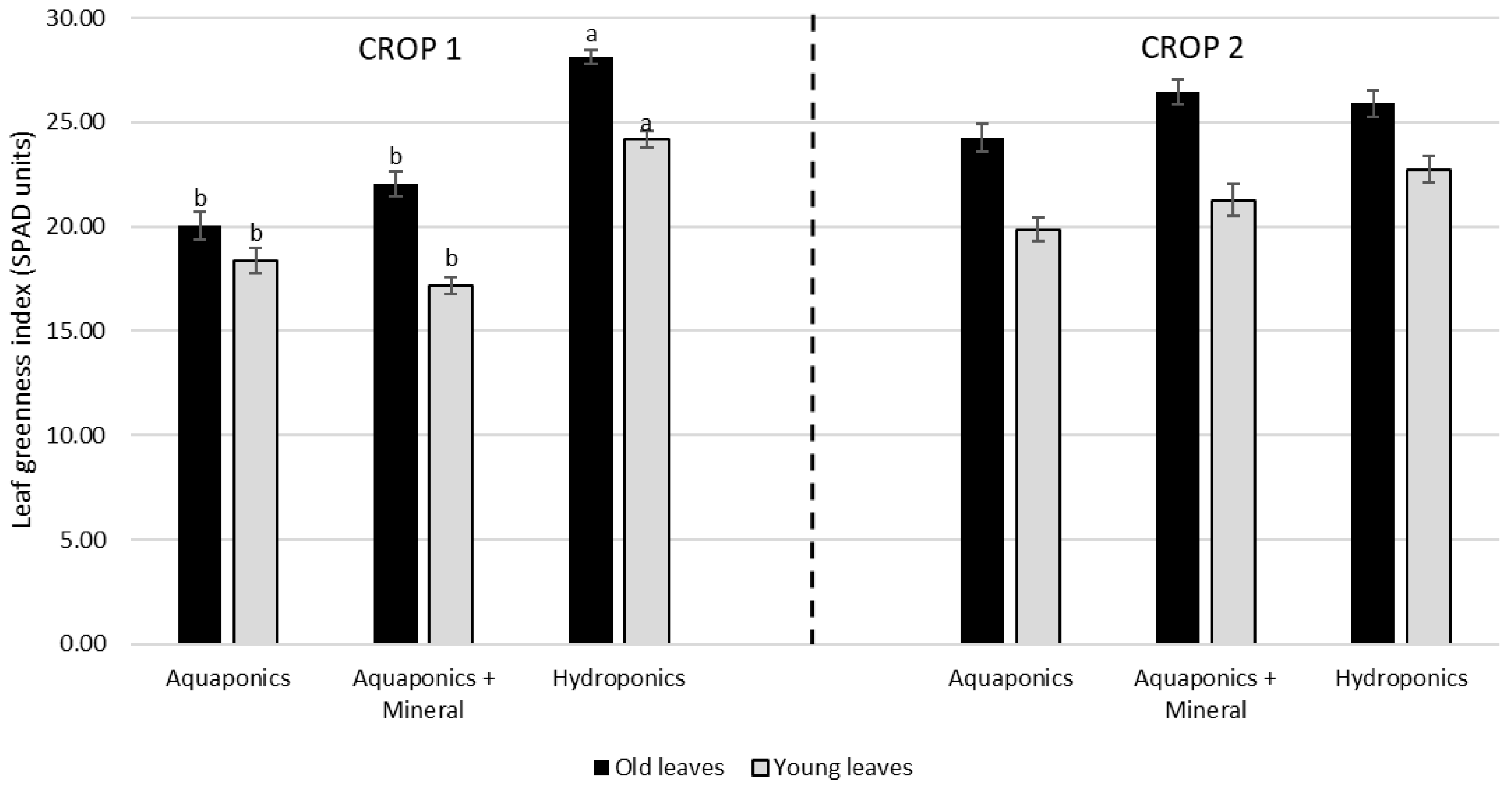
4.2. Nutrient Dynamics in Water, SPAD and Plant Performance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carlucci, D.; Nocella, G.; De Devitiis, B.; Viscecchia, R.; Bimbo, F.; Nardone, G. Consumer Purchasing Behaviour towards Fish and Seafood Products. Patterns and Insights from a Sample of International Studies. Appetite 2015, 84, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Brief: The State of World Fisheries and Aquaculture 2024. Blue Transformation in Action; FAO: Rome, Italy, 2024; pp. 1–40. [Google Scholar]
- Liu, X.; Wang, Y.; Liu, H.; Zhang, Y.; Zhou, Q.; Wen, X.; Guo, W.; Zhang, Z. A Systematic Review on Aquaculture Wastewater: Pollutants, Impacts, and Treatment Technology. Environ. Res. 2024, 262, 119793. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.; Fang, S.; Fanzo, J. A Global View of Aquaculture Policy. Food Policy 2023, 116, 102422. [Google Scholar] [CrossRef]
- Ottinger, M.; Clauss, K.; Kuenzer, C. Aquaculture: Relevance, Distribution, Impacts and Spatial Assessments—A Review. Ocean Coast. Manag. 2016, 119, 244–266. [Google Scholar] [CrossRef]
- Yep, B.; Zheng, Y. Aquaponic Trends and Challenges—A Review. J. Clean. Prod. 2019, 228, 1586–1599. [Google Scholar] [CrossRef]
- Forchino, A.A.; Lourguioui, H.; Brigolin, D.; Pastres, R. Aquaponics and Sustainability: The Comparison of Two Different Aquaponic Techniques Using the Life Cycle Assessment (LCA). Aquac. Eng. 2017, 77, 80–88. [Google Scholar] [CrossRef]
- Goddek, S.; Joyce, A.; Kotzen, B.; Burnell, G.M. Aquaponics Food Production Systems; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-15942-9. [Google Scholar]
- David, L.H.; Pinho, S.M.; Agostinho, F.; Costa, J.I.; Portella, M.C.; Keesman, K.J.; Garcia, F. Sustainability of Urban Aquaponics Farms: An Emergy Point of View. J. Clean. Prod. 2022, 331, 129896. [Google Scholar] [CrossRef]
- Mohanan, V.P.; Mahanta, N.R.; Kushwaha, A.; Goswami, L. Leveraging the Potential of Aquaponics for Urban Sustainability. In Emerging Trends to Approaching Zero Waste; Elsevier: Amsterdam, The Netherlands, 2022; pp. 59–78. [Google Scholar]
- Kasozi, N.; Tandlich, R.; Fick, M.; Kaiser, H.; Wilhelmi, B. Iron Supplementation and Management in Aquaponic Systems: A Review. Aquac. Rep. 2019, 15, 100221. [Google Scholar] [CrossRef]
- Verma, A.K.; Chandrakant, M.H.; John, V.C.; Peter, R.M.; John, I.E. Aquaponics as an Integrated Agri-Aquaculture System (IAAS): Emerging Trends and Future Prospects. Technol. Forecast. Soc. Change 2023, 194, 122709. [Google Scholar] [CrossRef]
- Roosta, H.R.; Hamidpour, M. Effects of Foliar Application of Some Macro- and Micro-Nutrients on Tomato Plants in Aquaponic and Hydroponic Systems. Sci. Hortic. 2011, 129, 396–402. [Google Scholar] [CrossRef]
- Meena, L.L.; Verma, A.K.; Krishnani, K.K.; Hittinahalli, C.M.; Haridas, H.; John, V.C. Combined Foliar Application Effect of Iron and Potassium on Growth of Okra and Striped Catfish Using Media Bed Based Aquaponics. Aquaculture 2023, 569, 739398. [Google Scholar] [CrossRef]
- Meena, L.L.; Verma, A.K.; Krishnani, K.K.; Reang, D.; Chandrakant, M.H.; John, V.C. Effects of Foliar Application of Macronutrients (K, P) and Micronutrient (Fe) on the Growth of Okra (Abelmoschus esculentus (L.) Moench) and Pangasius (Pangasianodon hypophthalmus) in a Recirculating Aquaponic System. South Afr. J. Bot. 2023, 160, 384–393. [Google Scholar] [CrossRef]
- Delaide, B.; Goddek, S.; Gott, J.; Soyeurt, H.; Jijakli, M. Lettuce (Lactuca sativa L. Var. Sucrine) Growth Performance in Complemented Aquaponic Solution Outperforms Hydroponics. Water 2016, 8, 467. [Google Scholar] [CrossRef]
- Harika, N.; Verma, A.K.; Krishnani, K.K.; Hittinahalli, C.M.; Reddy, R.; Pai, M. Supplementation of Potassium in Aquaculture Wastewater and Its Effect on Growth Performance of Basil (Ocimum basilicum L) and Pangasius (Pangasianodon hypophthalmus) in NFT-Based Aquaponics. Sci. Hortic. 2024, 323, 112521. [Google Scholar] [CrossRef]
- John, V.C.; Verma, A.K.; Krishnani, K.K.; Chandrakant, M.H.; Bharti, V.S.; Varghese, T. Optimization of Potassium (K+) Supplementation for Growth Enhancement of Spinacia oleracea L. and Pangasianodon hypophthalmus (Sauvage, 1878) in an Aquaponic System. Agric. Water Manag. 2022, 261, 107339. [Google Scholar] [CrossRef]
- Robaina, L.; Pirhonen, J.; Mente, E.; Sánchez, J.; Goosen, N. Fish Diets in Aquaponics. In Aquaponics Food Production Systems; Springer International Publishing: Cham, Switzerland, 2019; pp. 333–352. [Google Scholar]
- Siqwepu, O.; Salie, K.; Goosen, N. Evaluation of Chelated Iron and Iron Sulfate in the Diet of African Catfish, Clarias gariepinus to Enhance Iron Excretion for Application in Integrated Aquaponics Systems. J. World Aquac. Soc. 2020, 51, 1034–1053. [Google Scholar] [CrossRef]
- Luo, X.; Rauan, A.; Xing, J.; Sun, J.; Wu, W.; Ji, H. Influence of Dietary Se Supplementation on Aquaponic System: Focusing on the Growth Performance, Ornamental Features and Health Status of Koi Carp (Cyprinus carpio Var. Koi), Production of Lettuce (Lactuca Sativa) and Water Quality. Aquac. Res. 2021, 52, 505–517. [Google Scholar] [CrossRef]
- Tsoumalakou, E.; Mente, E.; Kormas, K.A.; Katsoulas, N.; Vlahos, N.; Kapsis, P.; Levizou, E. Precise Monitoring of Lettuce Functional Responses to Minimal Nutrient Supplementation Identifies Aquaponic System’s Nutrient Limitations and Their Time-Course. Agriculture 2022, 12, 1278. [Google Scholar] [CrossRef]
- Gao, X.; Xu, Y.; Shan, J.; Jiang, J.; Zhang, H.; Ni, Q.; Zhang, Y. Effects of Different Stocking Density Start-up Conditions on Water Nitrogen and Phosphorus Use Efficiency, Production, and Microbial Composition in Aquaponics Systems. Aquaculture 2024, 585, 740696. [Google Scholar] [CrossRef]
- Gomon, M.F. Scortum barcoo in Fishes of Australia. Available online: https://fishesofaustralia.net.au/home/species/701#summary (accessed on 17 January 2025).
- Gao, S.; Huang, W.; Peng, S.; Zhou, J.; Zhan, H.; Lu, T.; Liang, W.; Li, J.; Zhang, Y.; Li, W.; et al. Molecular Characterization and Expression Analysis of Nine Toll like Receptor (TLR) Genes in Scortum barcoo under Streptococcus agalactiae Infection. Int. J. Biol. Macromol. 2024, 254, 127667. [Google Scholar] [CrossRef]
- Lu, Y.; Li, R.; Xia, L.; Cheng, J.; Xia, H.; Zhan, Q.; Yu, D.; You, X.; Gu, R.; Xu, J.; et al. A Chromosome-Level Genome Assembly of the Jade Perch (Scortum barcoo). Sci. Data 2022, 9, 408. [Google Scholar] [CrossRef] [PubMed]
- Kayansamruaj, P.; Rangsichol, A.; Dong, H.T.; Rodkhum, C.; Maita, M.; Katagiri, T.; Pirarat, N. Outbreaks of Ulcerative Disease Associated with Ranavirus Infection in Barcoo Grunter, Scortum barcoo (McCulloch & Waite). J. Fish Dis. 2017, 40, 1341–1350. [Google Scholar] [CrossRef]
- Department of Agriculture and Fisheries. Ross Lobegeiger Report to Farmers: Aquaculture Production Summary for Queensland 2021–2022. Available online: https://www.publications.qld.gov.au/ckan-publications-attachments-prod/resources/71d81237-8f35-45b1-bfee-46cb04792ded/2021-22-aquaculture-production-summary-report.pdf (accessed on 17 January 2025).
- Business Queensland. Jade Perch Aquaculture. Available online: https://www.business.qld.gov.au/industries/farms-fishing-forestry/fisheries/aquaculture/species/jade-perch (accessed on 17 January 2025).
- Australian Bureau of Meteorology. Daily Global Solar Exposure: Bribie Island Alert. Available online: http://www.bom.gov.au/jsp/ncc/cdio/weatherData/av?p_nccObsCode=193&p_display_type=dailyDataFile&p_startYear=2022&p_c=-335851107&p_stn_num=040978 (accessed on 17 January 2025).
- Rakocy, J.E.; Losordo, T.M.; Masser, M.P. Recirculating Aquaculture Tank Production Systems: Aquaponics: Integrating Fish and Plant Culture; SRAC Publications: Leland, MS, USA, 2006; pp. 1–16. [Google Scholar]
- Fimbres-Acedo, Y.E.; Servín-Villegas, R.; Garza-Torres, R.; Endo, M.; Fitzsimmons, K.M.; Emerenciano, M.G.; Magallón-Servín, P.; López-Vela, M.; Magallón-Barajas, F.J. Hydroponic horticulture using residual waters from Oreochromis niloticus aquaculture with biofloc technology in photoautotrophic conditions with Chlorella microalgae. Aquac Res. 2020, 51, 4340–4360. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hall International: Upper Saddle River, NJ, USA, 1999; pp. 1–944. ISBN 9780131008465. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd ed.; W. H. Freeman: New York, NY, USA, 1995; pp. 1–880. ISBN 978-0716724117. [Google Scholar]
- El-Sayed, A.F.M. Tilapia Culture, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–348. ISBN 9780128165096. [Google Scholar]
- Pinho, S.M.; Molinari, D.; de Mello, G.L.; Fitzsimmons, K.M.; Coelho Emerenciano, M.G. Effluent from a Biofloc Technology (BFT) Tilapia Culture on the Aquaponics Production of Different Lettuce Varieties. Ecol. Eng. 2017, 103, 146–153. [Google Scholar] [CrossRef]
- Pinho, S.M.; de Mello, G.L.; Fitzsimmons, K.M.; Emerenciano, M.G.C. Integrated Production of Fish (Pacu Piaractus mesopotamicus and Red Tilapia Oreochromis sp.) with Two Varieties of Garnish (Scallion and Parsley) in Aquaponics System. Aquac. Int. 2018, 26, 99–112. [Google Scholar] [CrossRef]
- Tyson, R.V.; Simonne, E.H.; White, J.M.; Lamb, E.M. Reconciling Water Quality Parameters Impacting Nitrification in Aquaponics: The PH Levels. Proc. Fla. State Hortic. Soc. 2004, 117, 79–83. [Google Scholar]
- Ani, J.S.; Manyala, J.O.; Masese, F.O.; Fitzsimmons, K. Effect of Stocking Density on Growth Performance of Monosex Nile Tilapia (Oreochromis niloticus) in the Aquaponic System Integrated with Lettuce (Lactuca sativa). Aquac. Fish. 2022, 7, 328–335. [Google Scholar] [CrossRef]
- Baßmann, B.; Harbach, H.; Weißbach, S.; Palm, H.W. Effect of Plant Density in Coupled Aquaponics on the Welfare Status of African Catfish, Clarias gariepinus. J. World Aquac. Soc. 2020, 51, 183–199. [Google Scholar] [CrossRef]
- Villarroel, M.; Miranda-de la Lama, G.C.; Escobar-Álvarez, R.; Moratiel, R. Fish Welfare in Urban Aquaponics: Effects of Fertilizer for Lettuce (Lactuca sativa L.) on Some Physiological Stress Indicators in Nile Tilapia (Oreochromis niloticus L.). Water 2022, 14, 935. [Google Scholar] [CrossRef]
- Rafiee, G.R.; Ros Saad, C.; Kamarudin, M.S.; Ismail, M.R.; Sijam, K. Effects of Supplementary Nutrient in an Aquaponic System for Production of Ornamental Red Tilapia (Oreochromis sp.) and Lettuce (Lactuca sativa Var Longifolia). J. Surv. Fish. Sci. 2019, 5, 65–75. [Google Scholar] [CrossRef]
- McCauley, A.; Jones, C.; Jacobsen, J. Plant Nutrient Functions and Deficiency and Toxicity Symptoms. Available online: https://store.msuextension.org/publications/AgandNaturalResources/4449-9.pdf (accessed on 25 November 2024).
- Mattson, N.; Merrill, T. Symptoms of Common Nutrient Deficiencies in Hydroponic Lettuce. Available online: https://www.e-gro.org/pdf/Mattson_Lettuce_2015_9.pdf (accessed on 25 November 2024).
- Ayipio, E.; Wells, D.E.; McQuilling, A.; Wilson, A.E. Comparisons between Aquaponic and Conventional Hydroponic Crop Yields: A Meta-Analysis. Sustainability 2019, 11, 6511. [Google Scholar] [CrossRef]
- Jones, J.B., Jr. Complete Guide for Growing Plants Hydroponically; CRC Press: Boca Raton, FL, USA, 2014; pp. 1–221. ISBN 9781439876695. [Google Scholar]
- Goddek, S.; Delaide, B.; Mankasingh, U.; Ragnarsdottir, K.; Jijakli, H.; Thorarinsdottir, R. Challenges of Sustainable and Commercial Aquaponics. Sustainability 2015, 7, 4199–4224. [Google Scholar] [CrossRef]
- Pinho, S.M.; Lima, J.P.; David, L.H.; Oliveira, M.S.; Goddek, S.; Carneiro, D.J.; Keesman, K.J.; Portella, M.C. Decoupled FLOCponics Systems as an Alternative Approach to Reduce the Protein Level of Tilapia Juveniles’ Diet in Integrated Agri-Aquaculture Production. Aquaculture 2021, 543, 736932. [Google Scholar] [CrossRef]
- Lenz, G.L.; Durigon, E.G.; Lapa, K.R.; Emerenciano, M.G.C. Produção de Alface (Lactuca sativa) Em Efluentes de Um Cultivo de Tilápias Mantidas Em Sistema BFT Em Baixa Salinidade. Bol. Inst. Pesca 2017, 43, 614–630. [Google Scholar] [CrossRef]
- Beauchamp, W.R.; Pickens, J.M.; Sibley, J.L.; Chappell, J.A.; Martin, N.R.; Newby, A.F. Salt Level in a Simulated Aquaponic System and Effects on Bibb Lettuce. Int. J. Veg. Sci. 2018, 24, 122–136. [Google Scholar] [CrossRef]
- Cerozi, B.S.; Fitzsimmons, K. Phosphorus Dynamics Modeling and Mass Balance in an Aquaponics System. Agric. Syst. 2017, 153, 94–100. [Google Scholar] [CrossRef]



| Parameter | Aquaponics | Aquaponics + Mineral | RAS | Hydroponics | |
|---|---|---|---|---|---|
| Temperature AM (°C) | Mean ± SD | 25.77 ± 0.94 | 26.11 ± 0.78 | 25.73 ± 0.76 | 26.10 ± 2.26 |
| Min–max | 23.60–28.50 | 23.80–28.40 | 23.50–28.30 | 22.11–28.20 | |
| Temperature PM (°C) | Mean ± SD | 26.25 ± 1.38 | 26.58 ± 1.41 | 26.14 ± 1.46 | 26.45 ± 1.43 |
| Min–max | 22.10–29.70 | 22.80–30.00 | 21.70–30.40 | 22.10–32.70 | |
| Dissolved oxygen (mg L–1) | Mean ± SD | 7.25 ± 0.66 | 7.37 ± 0.26 | 7.35 ± 0.69 | 7.87 ± 0.20 |
| Min–max | 5.82–8.06 | 6.60–8.02 | 5.66–8.05 | 7.07–8.30 | |
| pH | Mean ± SD | 7.28 ± 0.47 | 7.15 ± 0.48 | 7.65 ± 0.32 | 6.86 ± 0.55 |
| Min–max | 6.49–8.41 | 6.33–8.37 | 6.77–8.39 | 6.30–7.53 | |
| Electrical conductivity (µS cm–1) | Mean ± SD | 1922.04 ± 727.59 | 2234.83 ± 833.27 | 2229.95 ± 893.07 | 1218.85 ± 165.89 |
| Min–max | 348.60–3274.00 | 431.70–3693.00 | 296.00–3797.00 | 8.26–1404.00 | |
| Turbidity (FNU) | Mean ± SD | 1.77 ± 1.21 | 1.87 ± 1.42 | 1.17 ± 0.73 | 0.12 ± 0.23 |
| Min–max | 0.01–5.68 | 0.03–8.76 | 0.01–3.82 | 0.00–1.18 | |
| TAN (mg L–1) | Mean ± SD | 1.26 ± 1.29 | 1.63 ± 1.32 | 1.34 ± 1.72 | 2.94 ± 3.20 |
| Min–max | 0.10–4.60 | 0.04–4.00 | 0.04–8.20 | 0.08–12.00 | |
| NO2 (mg L–1) | Mean ± SD | 0.30 ± 0.24 | 0.28 ± 0.25 | 0.69 ± 0.40 | 0.22 ± 0.32 |
| Min–max | 0.00–0.99 | 0.00–0.93 | 0.01–1.40 | 0.00–1.15 | |
| NO3 (mg L–1) | Mean ± SD | 203.41 ± 184.45 | 242.67 ± 196.23 | 236.40 ± 185.63 | 182.13 ± 119.04 |
| Min–max | 0.59–650.00 | 0.74–590.00 | 2.09–620.00 | 6.00–500.00 | |
| PO4 (mg L–1) | Mean ± SD | 25.66 ± 14.43 | 35.57 ± 23.68 | 28.60 ± 18.06 | 51.83 ± 24.01 |
| Min–max | 0.10–56.00 | 0.15–91.00 | 0.14–69.00 | 13.00–110.00 | |
| Alkalinity (mg CaCO3 L–1) | Mean ± SD | 50.93 ± 21.53 | 47.92 ± 24.04 | 103.91 ± 54.65 | 50.50 ± 25.90 |
| Min–max | 15.00–95.00 | 10.00–85.00 | 10.00–215.00 | 10.00–100.00 | |
| K (mg L–1) | Mean ± SD | 5.26 ± 2.24 | 13.21 ± 2.74 | 9.68 ± 5.47 | 40.66 ± 3.55 |
| Min–max | 1.92–6.5 | 9.15–15.13 | 2.07–15.09 | 37.11–45.35 | |
| Ca (mg L–1) | Mean ± SD | 62.38 ± 47.49 | 76.08 ± 47.10 | 17.96 ± 1.39 | 45.69 ± 7.31 |
| Min–max | 16.21–127.83 | 21.93–135.44 | 16.9–19.86 | 37.61–54.88 | |
| Mg (mg L–1) | Mean ± SD | 10.74 ± 1.26 | 12.61 ± 1.67 | 11.58 ± 0.70 | 7.77 ± 0.66 |
| Min–max | 9.05–12.04 | 10.35–14.26 | 10.61–12.29 | 6.95–8.33 | |
| S (mg L–1) | Mean ± SD | 19.98 ± 5.06 | 23.51 ± 5.77 | 21.04 ± 5.29 | 33.41 ± 4.21 |
| Min–max | 13.39–25.6 | 16.10–30.01 | 14.58–27.49 | 27.71–36.94 | |
| Na (mg/L) | Mean ± SD | 152.14 ± 58.7 | 201.27 ± 102.4 | 256.65 ± 115.41 | 23.8 ± 12.43 |
| Min–max | 83.45–198.3 | 106.5–250.2 | 98.1–402.53 | 14.2–42.4 |
| Parameter | Aquaponics | Aquaponics + Mineral | RAS | p-Value |
|---|---|---|---|---|
| Final ind. weight (g) | 104.77 ± 0.90 | 105.45 ± 1.13 | 107.24 ± 1.08 | 0.523 |
| SGR (%/day) | 3.14 ± 0.02 | 3.15 ± 0.01 | 3.17 ± 0.01 | 0.653 |
| Final biomass (kg tank−1) | 3.25 ± 0.08 | 3.37 ± 0.02 | 3.40 ± 0.04 | 0.466 |
| Productivity (kg m−3) | 13.53 ± 0.33 | 14.06 ± 0.10 | 14.15 ± 0.16 | 0.460 |
| Survival (%) | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | - |
| FCR | 1.24 ± 0.02 | 1.23 ± 0.01 | 1.21 ± 0.01 | 0.546 |
| Crop One | Crop Two | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Aquaponics | Aquaponics + Mineral | Hydroponics | p-Value | Aquaponics | Aquaponics + Mineral | Hydroponics | p-Value |
| Total wet weight * (g) | 90.60 B ± 5.51 | 108.15 B ± 5.69 | 255.70 A ± 10.17 | <0.001 | 126.20 B ± 10.55 | 128.58 B ± 9.25 | 169.14 A ± 8.28 | <0.01 |
| Total length (cm) | 50.58 C ± 1.41 | 60.03 B ± 1.94 | 76.97 A ± 1.88 | <0.001 | 54.92 C ± 2.15 | 62.85 B ± 2.31 | 66.11 A ± 1.54 | <0.001 |
| Root length (cm) | 26.75 C ± 1.00 | 33.53 B ± 1.56 | 40.57 A ± 1.62 | <0.01 | 31.34 B ± 1.80 | 39.07 B ± 2.12 | 40.00 A ± 1.20 | <0.01 |
| Aerial length (cm) | 23.83 C ± 0.69 | 26.50 B ± 0.79 | 36.40 A ± 0.86 | 0.045 | 23.58 ± 0.84 | 23.78 ± 0.62 | 26.11 ± 0.96 | NS (0.370) |
| Number of leaves per plant (n) | 31.67 ± 2.37 | 36.03 ± 2.83 | 38.07 ± 1.88 | NS (0.453) | 29.50 ± 2.11 | 30.37 ± 1.96 | 30.77 ± 1.56 | NS (0.918) |
| Length of the largest leaf (cm) | 15.84 ± 0.57 | 17.08 ± 0.73 | 23.25 ± 0.95 | <0.001 | 19.83 ± 0.66 | 19.80 ± 0.68 | 21.02 ± 0.81 | NS (0.644) |
| Productivity * (kg m−2) | 1.95 B ± 0.22 | 2.33 B ± 0.19 | 5.50 A ± 0.09 | <0.001 | 2.31 ± 0.13 | 2.35 ± 0.44 | 3.09 ± 0.17 | NS (0.669) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emerenciano, M.G.C.; Slinger, J.; Koster, G.; Aland, J.; Lima, P.C.; Arsic, M.; O’Sullivan, C. Mineral Supplementation in Jade Perch (Scortum barcoo) Aquaponics with Lettuce: A Comparison with Hydroponics and RAS. Animals 2025, 15, 317. https://doi.org/10.3390/ani15030317
Emerenciano MGC, Slinger J, Koster G, Aland J, Lima PC, Arsic M, O’Sullivan C. Mineral Supplementation in Jade Perch (Scortum barcoo) Aquaponics with Lettuce: A Comparison with Hydroponics and RAS. Animals. 2025; 15(3):317. https://doi.org/10.3390/ani15030317
Chicago/Turabian StyleEmerenciano, Maurício Gustavo Coelho, Joel Slinger, George Koster, Jarvis Aland, Paula Camargo Lima, Maja Arsic, and Cathryn O’Sullivan. 2025. "Mineral Supplementation in Jade Perch (Scortum barcoo) Aquaponics with Lettuce: A Comparison with Hydroponics and RAS" Animals 15, no. 3: 317. https://doi.org/10.3390/ani15030317
APA StyleEmerenciano, M. G. C., Slinger, J., Koster, G., Aland, J., Lima, P. C., Arsic, M., & O’Sullivan, C. (2025). Mineral Supplementation in Jade Perch (Scortum barcoo) Aquaponics with Lettuce: A Comparison with Hydroponics and RAS. Animals, 15(3), 317. https://doi.org/10.3390/ani15030317







