Low-Density Polyethylene Microplastics in the Rumen: Implications for Rumen Fermentation Dynamics and Utilization of Concentrate Feed
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Feed Samples
2.2. Microplastic Characteristics
2.3. In Vitro Rumen Fermentation
- Produced gas volume is expressed in mL.
- Recorded gas pressure is expressed in bar.
- Vf is the total volume of the incubation bottle expressed in mL.
- Vi is the volume of inoculum added to each bottle expressed in mL.
- Patm is the atmospheric pressure expressed in bar.
- GP is the net gas production expressed in mL/g dry matter of concentrate.
- t is the incubation time expressed in hours.
- B is the asymptotic gas production expressed in mL/g dry matter of concentrate.
- C is the constant gas production rate expressed in %/h.
- Lag is the onset time of gas production expressed in hours.
- T ½ is the time to half-maximal gas production expressed in hours.
- AGPR is the average gas production rate between the start of the incubation and time to half-maximal gas production expressed in mL/h.
- dOM is the degradable organic matter expressed in %.
- GP24 is the net gas production (mL) from 200 mg of concentrate after 24 h of incubation.
- CP is the crude protein of concentrate expressed in %.
- Ash is the ash of concentrate expressed in %.
- ME is the metabolizable energy expressed in MJ/kg dry matter of concentrate.
2.4. Statistical Analysis
- Yij is the observation.
- μ is the general mean.
- MPsi is the effect of the level of addition of MPs.
- Periodj is the effect of the incubation period.
- εij is the experimental residual error.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aardema, H.; Vethaak, A.D.; Kamstra, J.H.; Legler, J. Farm Animals as a Critical Link between Environmental and Human Health Impacts of Micro-and Nanoplastics. Microplast. Nanoplast. 2024, 4, 5. [Google Scholar] [CrossRef]
- Khan, A.; Qadeer, A.; Wajid, A.; Ullah, Q.; Rahman, S.U.; Ullah, K.; Safi, S.Z.; Ticha, L.; Skalickova, S.; Chilala, P.; et al. Microplastics in Animal Nutrition: Occurrence, Spread, and Hazard in Animals. J. Agric. Food Res. 2024, 17, 101258. [Google Scholar] [CrossRef]
- Urli, S.; Corte Pause, F.; Crociati, M.; Baufeld, A.; Monaci, M.; Stradaioli, G. Impact of Microplastics and Nanoplastics on Livestock Health: An Emerging Risk for Reproductive Efficiency. Animals 2023, 13, 1132. [Google Scholar] [CrossRef] [PubMed]
- Maganti, S.S.; Akkina, R.C. Detection and Characterisation of Microplastics in Animal Feed. Online J. Anim. Feed Res. 2023, 13, 348–356. [Google Scholar] [CrossRef]
- Glorio Patrucco, S.; Rivoira, L.; Bruzzoniti, M.C.; Barbera, S.; Tassone, S. Development and Application of a Novel Extraction Protocol for the Monitoring of Microplastic Contamination in Widely Consumed Ruminant Feeds. Sci. Total Environ. 2024, 947, 174493. [Google Scholar] [CrossRef] [PubMed]
- Skilbeck, O.J. The Observation of Microplastics as Emerging Pollutants in UK Dairy Farms. Master’s Thesis, The University of Leeds, School of Geography, Leeds, UK, 2022. [Google Scholar]
- Dong, X.; Liu, X.; Hou, Q.; Wang, Z. From Natural Environment to Animal Tissues: A Review of Microplastics (Nanoplastics) Translocation and Hazards Studies. Sci. Total Environ. 2023, 855, 158686. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, K.L.; Lawson, P.; Emerson, B. Fate of Plastics in Cattle Digestive Systems. J. Agric. Saf. Health 2022, 28, 205–214. [Google Scholar] [CrossRef]
- Shelver, W.L.; McGarvey, A.M.; Billey, L.O. Disposition of [14 C]-Polystyrene Microplastics after Oral Administration to Lactating Sheep. Food Addit. Contam. Part A 2024, 41, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- González Puetate, I.R.; Martínez Cepeda, G.E.; Torres Lasso, P.; Chávez Toledo, K.N.; Guevara Enrique, G. Microplásticos En Materia Fecal de Rumiantes En Ecuador. Cienc. Vet. 2024, 26, 114–129. [Google Scholar] [CrossRef]
- Beriot, N.; Peek, J.; Zornoza, R.; Geissen, V.; Huerta Lwanga, E. Low Density-Microplastics Detected in Sheep Faeces and Soil: A Case Study from the Intensive Vegetable Farming in Southeast Spain. Sci. Total Environ. 2021, 755, 142653. [Google Scholar] [CrossRef]
- Wu, T.; Shu, X.; Wang, C.; Li, W.; Zhu, D.; Wang, J.; Zhang, Y.; Yang, X.; Wang, X. Microplastic Pollution of Threatened Terrestrial Wildlife in Nature Reserves of Qinling Mts., China. Glob. Ecol. Conserv. 2024, 51, e02865. [Google Scholar] [CrossRef]
- Álvarez-Méndez, S.J.; Díaz-Peña, F.J.; Gómez-Escabia, S.; González-Sálamo, J.; Hernández-Borges, J. Tracking Anthropogenic Microparticles in Wildlife of an Alpine Insular Environment. J. Hazard. Mater. 2024, 465, 133291. [Google Scholar] [CrossRef]
- Teampanpong, J.; Duengkae, P. Using Feces to Indicate Plastic Pollution in Terrestrial Vertebrate Species in Western Thailand. PeerJ 2024, 12, e17596. [Google Scholar] [CrossRef] [PubMed]
- Borreani, G.; Tabacco, E. Plastics in Animal Production. In A Guide to the Manufacture, Performance, and Potential of Plastics in Agriculture; Elsevier: Amsterdam, The Netherlands, 2017; pp. 145–185. ISBN 978-0-08-102170-5. [Google Scholar]
- Varó, I.; Osorio, K.; Estensoro, I.; Naya-Català, F.; Sitjà-Bobadilla, A.; Navarro, J.C.; Pérez-Sánchez, J.; Torreblanca, A.; Piazzon, M.C. Effect of Virgin Low Density Polyethylene Microplastic Ingestion on Intestinal Histopathology and Microbiota of Gilthead Sea Bream. Aquaculture 2021, 545, 737245. [Google Scholar] [CrossRef]
- Mahmood, M.; Hussain, S.M.; Sarker, P.K.; Ali, S.; Arif, M.S.; Nazish, N.; Riaz, D.; Ahmad, N.; Paray, B.A.; Naeem, A. Toxicological Assessment of Dietary Exposure of Polyethylene Microplastics on Growth, Nutrient Digestibility, Carcass and Gut Histology of Nile Tilapia (Oreochromis Niloticus) Fingerlings. Ecotoxicology 2024, 33, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Colpaert, R.; Petit Dit Grézériat, L.; Louzon, M.; De Vaufleury, A.; Gimbert, F. Polyethylene Microplastic Toxicity to the Terrestrial Snail Cantareus Aspersus: Size Matters. Environ. Sci. Pollut. Res. 2022, 29, 29258–29267. [Google Scholar] [CrossRef]
- Wang, J.; Tian, H.; Shi, Y.; Yang, Y.; Yu, F.; Cao, H.; Gao, L.; Liu, M. The Enhancement in Toxic Potency of Oxidized Functionalized Polyethylene-Microplastics in Mice Gut and Caco-2 Cells. Sci. Total Environ. 2023, 903, 166057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qin, K.; Liu, C. Low-Density Polyethylene Enhances the Disturbance of Microbiome and Antibiotic Resistance Genes Transfer in Soil-Earthworm System Induced by Pyraclostrobin. J. Hazard. Mater. 2024, 465, 133459. [Google Scholar] [CrossRef] [PubMed]
- Harfoot, C.G. Anatomy, physiology and microbiology of the ruminant digestive tract. In Lipid Metabolism in Ruminant Animals; Elsevier: Amsterdam, The Netherlands, 1981; pp. 1–19. ISBN 978-0-08-023789-3. [Google Scholar]
- Dehority, B.A. Gastrointestinal Tracts of Herbivores, Particularly the Ruminant: Anatomy, Physiology and Microbial Digestion of Plants. J. Appl. Anim. Res. 2002, 21, 145–160. [Google Scholar] [CrossRef]
- Yáñez-Ruiz, D.R.; Bannink, A.; Dijkstra, J.; Kebreab, E.; Morgavi, D.P.; O’Kiely, P.; Reynolds, C.K.; Schwarm, A.; Shingfield, K.J.; Yu, Z.; et al. Design, Implementation and Interpretation of in Vitro Batch Culture Experiments to Assess Enteric Methane Mitigation in Ruminants—A Review. Anim. Feed Sci. Technol. 2016, 216, 1–18. [Google Scholar] [CrossRef]
- AOAC, Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 2000.
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A Simple Gas Production Method Using a Pressure Transducer to Determine the Fermentation Kinetics of Ruminant Feeds. Anim. Feed. Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Muizelaar, W.; Bani, B.; Kuhla, B.; Tapio, I.; Yáñez-Ruiz, D.; van Gastelen, D. Rumen Fluid Sampling via Oral Stomach Tubing Method. In Methods in Cattle Physiology and Behaviour Research: Recommendations from the SmartCow Consortium; Publisso: Cologne, Germany, 2020. [Google Scholar]
- Menke, K.H.; Steingass, H. Estimation of the Energetic Feed Value Obtained from Chemical Analysis and in Vitro Gas Production Using Rumen Fluid. Anim. Res. Dev. 1988, 28, 91–97. [Google Scholar]
- Colkesen, M.; Kamalak, A.; Canbolat, O.; Gurbuz, Y.; Ozkan, C.O. Effect of Cultivar and Formaldehyde Treatment of Barley Grain on Rumen Fermentation Characteristics Using in Vitro Gas Production. South Afr. J. Anim. Sci. 2005, 35, 206–212. [Google Scholar] [CrossRef][Green Version]
- Ozkan, C.O.; Kamalak, A.; Atalay, A.I.; Tatliyer, A.; Kaya, E. Effect of Peppermint (Mentha Piperita) Essential Oil on Rumen Microbial Fermentation of Barley Grain. J. Appl. Anim. Res. 2015, 43, 287–290. [Google Scholar] [CrossRef]
- Kara, K.; Özkaya, S.; Erbaş, S.; Baytok, E. Effect of Dietary Formic Acid on the in Vitro Ruminal Fermentation Parameters of Barley-Based Concentrated Mix Feed of Beef Cattle. J. Appl. Anim. Res. 2018, 46, 178–183. [Google Scholar] [CrossRef]
- France, J.; Dijkstra, J.; Dhanoa, M.S.; Lopez, S.; Bannink, A. Estimating the Extent of Degradation of Ruminant Feeds from a Description of Their Gas Production Profiles Observed in Vitro: Derivation of Models and Other Mathematical Considerations. Br. J. Nutr. 2000, 83, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Burk, A.D. Laboratory Manual for Classification and Morphology of Rumen Ciliate Protozoa, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-351-07391-2. [Google Scholar]
- Mei, W.; Chen, G.; Bao, J.; Song, M.; Li, Y.; Luo, C. Interactions between Microplastics and Organic Compounds in Aquatic Environments: A Mini Review. Sci. Total Environ. 2020, 736, 139472. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Yue, T.; Xu, Y.; Zhao, J.; Xing, B. Microplastics Reduce Lipid Digestion in Simulated Human Gastrointestinal System. Environ. Sci. Technol. 2020, 54, 12285–12294. [Google Scholar] [CrossRef] [PubMed]
- Spanghero, M.; Braidot, M.; Fabro, C.; Romanzin, A. A Meta-Analysis on the Relationship between Rumen Fermentation Parameters and Protozoa Counts in in Vitro Batch Experiments. Anim. Feed Sci. Technol. 2022, 293, 115471. [Google Scholar] [CrossRef]
- Eichinger, E.; Amin, N.; Kuenz, S.K.; Ecke, F.; Zollfran, C.; Windisc, W.; Seifert, J.; Brugge, D. Microplastics and Their Effects on Rumen Microbial Activity Ex Vivo. In Proceedings of the 2nd Swiss Young Microbiologists Symposium, Tierspital, Zurich, 8 July 2022. [Google Scholar]
- Mathison, G.W.; Okine, E.K.; McAllister, T.A.; Dong, Y.; Galbraith, J.; Dmytruk, O.I.N. Reducing Methane Emissions from Ruminant Animals. J. Appl. Anim. Res. 1998, 14, 1–28. [Google Scholar] [CrossRef]
- Tassone, S.; Barbera, S.; Kaihara, H.; Glorio Patrucco, S.; Abid, K. First Evidence of the Effects of Polyethylene Terephthalate Microplastics on Ruminal Degradability and Gastro-Intestinal Digestibility of Mixed Hay. Animals 2024, 14, 2139. [Google Scholar] [CrossRef] [PubMed]
- Salah, N.; Sauvant, D.; Archimède, H. Response of Growing Ruminants to Diet in Warm Climates: A Meta-Analysis. Animal 2015, 9, 822–830. [Google Scholar] [CrossRef]
- Ben Ettoumia, R.; Vernet, J.; Ortigues-Marty, I.; Kraiem, K.; Majdoub-Mathlouthi, L. Effects of Metabolizable Energy Intake on Post Weaning Lamb Growth Performance, Carcass Tissue Composition and Internal Fat Depend on Animal Characteristics: A Meta-Analysis. Meat Sci. 2022, 185, 108719. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; Calsamiglia, S.; Stern, M.D. Nitrogen Metabolism in the Rumen. J. Dairy Sci. 2005, 88, E9–E21. [Google Scholar] [CrossRef]
- Chang, X.; Li, Y.; Han, Y.; Fang, Y.; Xiang, H.; Zhao, Z.; Zhao, B.; Zhong, R. Polystyrene Exposure Induces Lamb Gastrointestinal Injury, Digestive Disorders and Inflammation, Decreasing Daily Gain, and Meat Quality. Ecotoxicol. Environ. Saf. 2024, 277, 116389. [Google Scholar] [CrossRef]
- Mahadappa, P.; Krishnaswamy, N.; Karunanidhi, M.; Bhanuprakash, A.; Bindhuja, B.; Dey, S. Effect of Plastic Foreign Body Impaction on Rumen Function and Heavy Metal Concentrations in Various Body Fluids and Tissues of Buffaloes. Ecotoxicol. Environ. Saf. 2020, 189, 109972. [Google Scholar] [CrossRef]
- Olson, K.C.; Cochran, R.C.; Jones, T.J.; Vanzant, E.S.; Titgemeyer, E.C.; Johnson, D.E. Effects of Ruminal Administration of Supplemental Degradable Intake Protein and Starch on Utilization of Low-Quality Warm-Season Grass Hay by Beef Steers. J. Anim. Sci. 1999, 77, 1016. [Google Scholar] [CrossRef]
- Pengpeng, W.; Tan, Z. Ammonia Assimilation in Rumen Bacteria: A Review. Anim. Biotechnol. 2013, 24, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Mikolayunas-Sandrock, C.; Armentano, L.E.; Thomas, D.L.; Berger, Y.M. Effect of Protein Degradability on Milk Production of Dairy Ewes. J. Dairy Sci. 2009, 92, 4507–4513. [Google Scholar] [CrossRef] [PubMed]
- Galyon, H.; Vibostok, S.; Duncan, J.; Ferreira, G.; Whittington, A.; Havens, K.; McDevitt, J.; Cockrum, R. Clearance of Biodegradable Polymer and Polyethylene Films from the Rumens of Holstein Bull Calves. Animals 2023, 13, 928. [Google Scholar] [CrossRef] [PubMed]
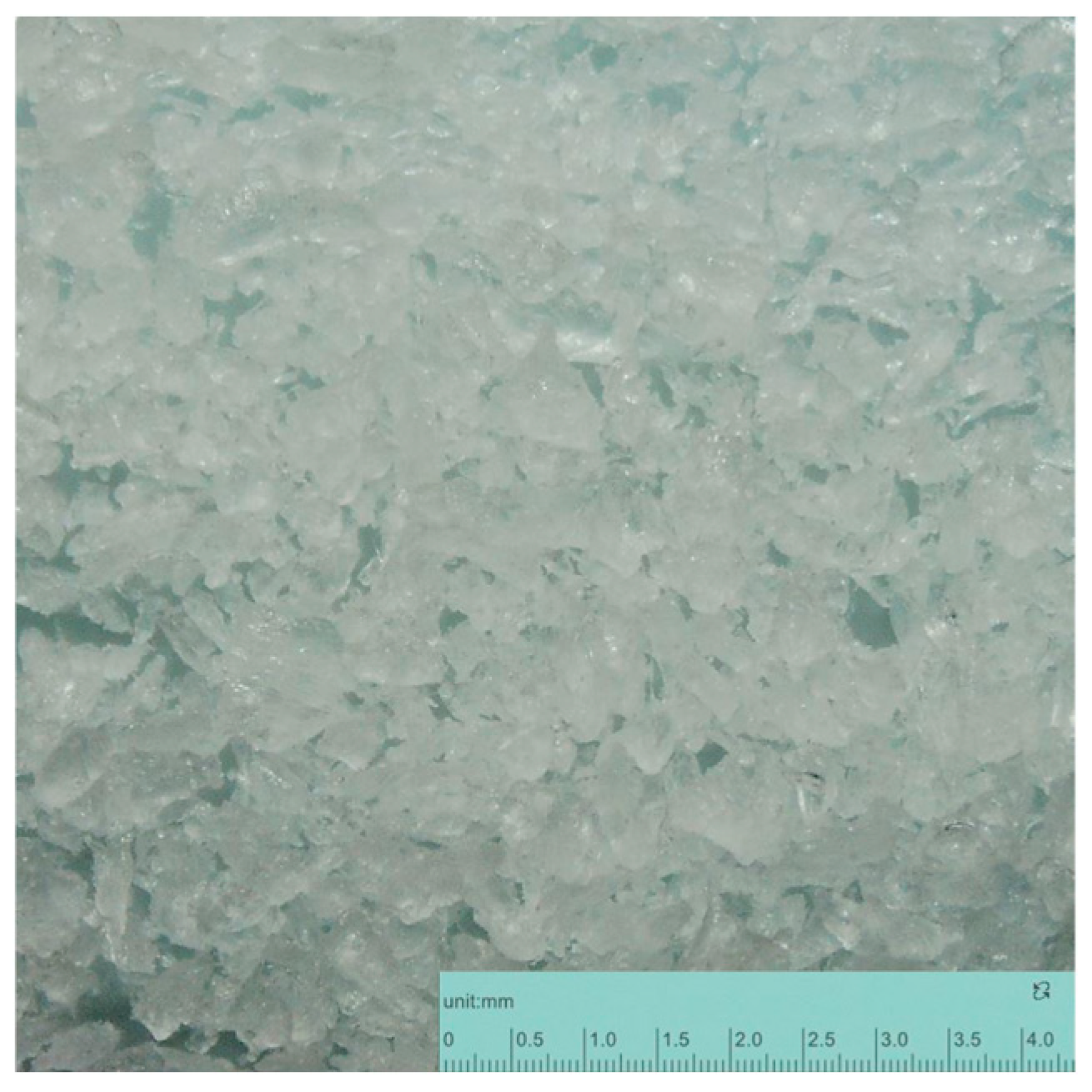
| Concentrate | |
|---|---|
| Dry matter 1 | 911 |
| Crude protein | 161 |
| Ash | 41 |
| Neutral detergent fiber | 131 |
| Acid detergent fiber | 32 |
| Item | 0 | 3.3 | 6.6 | P-Period | P-MPs |
|---|---|---|---|---|---|
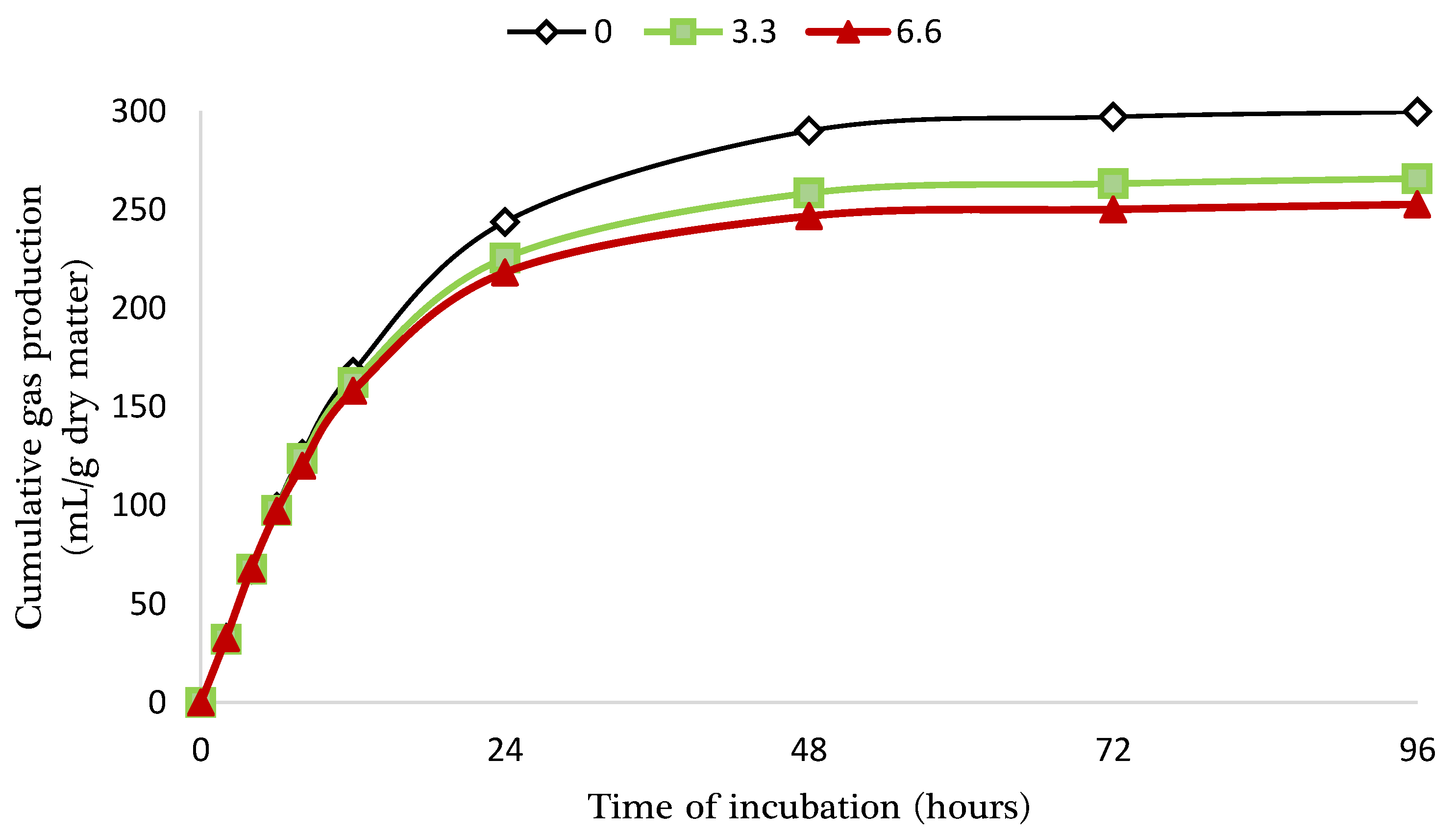
| B | 300.3 ± 10.47 a | 264.8 ± 9.34 b | 255.4 ± 7.67 b | NS | *** |
| C | 7.0 ± 0.23 b | 8.1 ± 0.11 a | 8.3 ± 0.10 a | NS | ** |
| Lag | 0.3 ± 0.07 | 0.3 ± 0.06 | 0.3 ± 0.04 | NS | NS |
| T ½ | 10.1 ± 0.39 a | 8.8 ± 0.07 b | 7.4 ± 0.06 c | NS | *** |
| AGPR | 29.6 ± 1.71 | 29.9 ± 0.99 | 29.6 ± 1.17 | NS | NS |
| Item | 0 | 3.3 | 6.6 | P-Period | P-MPs |
|---|---|---|---|---|---|
| dOM | 65.7 ± 1.61 a | 62.2 ± 1.41 b | 61.2 ± 1.33 b | NS | ** |
| ME | 8.9 ± 0.24 b | 8.4 ± 0.22 b | 8.2 ± 0.21 b | NS | ** |
| Item | 0 | 3.3 | 6.6 | P-Period | P-MPs |
|---|---|---|---|---|---|
| pH | 6.5 ± 0.34 | 6.6 ± 0.22 | 6.5 ± 0.30 | NS | NS |
| NH3-N | 24.9 ± 0.63 a | 22.1 ± 0.33 b | 22.1 ± 0.54 b | NS | ** |
| Protozoal counts | 4.4 ± 0.39 a | 3.5 ± 0.24 b | 3.4 ± 0.11 b | NS | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tassone, S.; Kaihara, H.; Barbera, S.; Glorio Patrucco, S.; Issaoui, R.; Abid, K. Low-Density Polyethylene Microplastics in the Rumen: Implications for Rumen Fermentation Dynamics and Utilization of Concentrate Feed. Animals 2025, 15, 297. https://doi.org/10.3390/ani15030297
Tassone S, Kaihara H, Barbera S, Glorio Patrucco S, Issaoui R, Abid K. Low-Density Polyethylene Microplastics in the Rumen: Implications for Rumen Fermentation Dynamics and Utilization of Concentrate Feed. Animals. 2025; 15(3):297. https://doi.org/10.3390/ani15030297
Chicago/Turabian StyleTassone, Sonia, Hatsumi Kaihara, Salvatore Barbera, Sara Glorio Patrucco, Rabeb Issaoui, and Khalil Abid. 2025. "Low-Density Polyethylene Microplastics in the Rumen: Implications for Rumen Fermentation Dynamics and Utilization of Concentrate Feed" Animals 15, no. 3: 297. https://doi.org/10.3390/ani15030297
APA StyleTassone, S., Kaihara, H., Barbera, S., Glorio Patrucco, S., Issaoui, R., & Abid, K. (2025). Low-Density Polyethylene Microplastics in the Rumen: Implications for Rumen Fermentation Dynamics and Utilization of Concentrate Feed. Animals, 15(3), 297. https://doi.org/10.3390/ani15030297








