The Roles of Enzymes as Dietary Additives in Ruminant Diets: A Meta-Analysis
Simple Summary
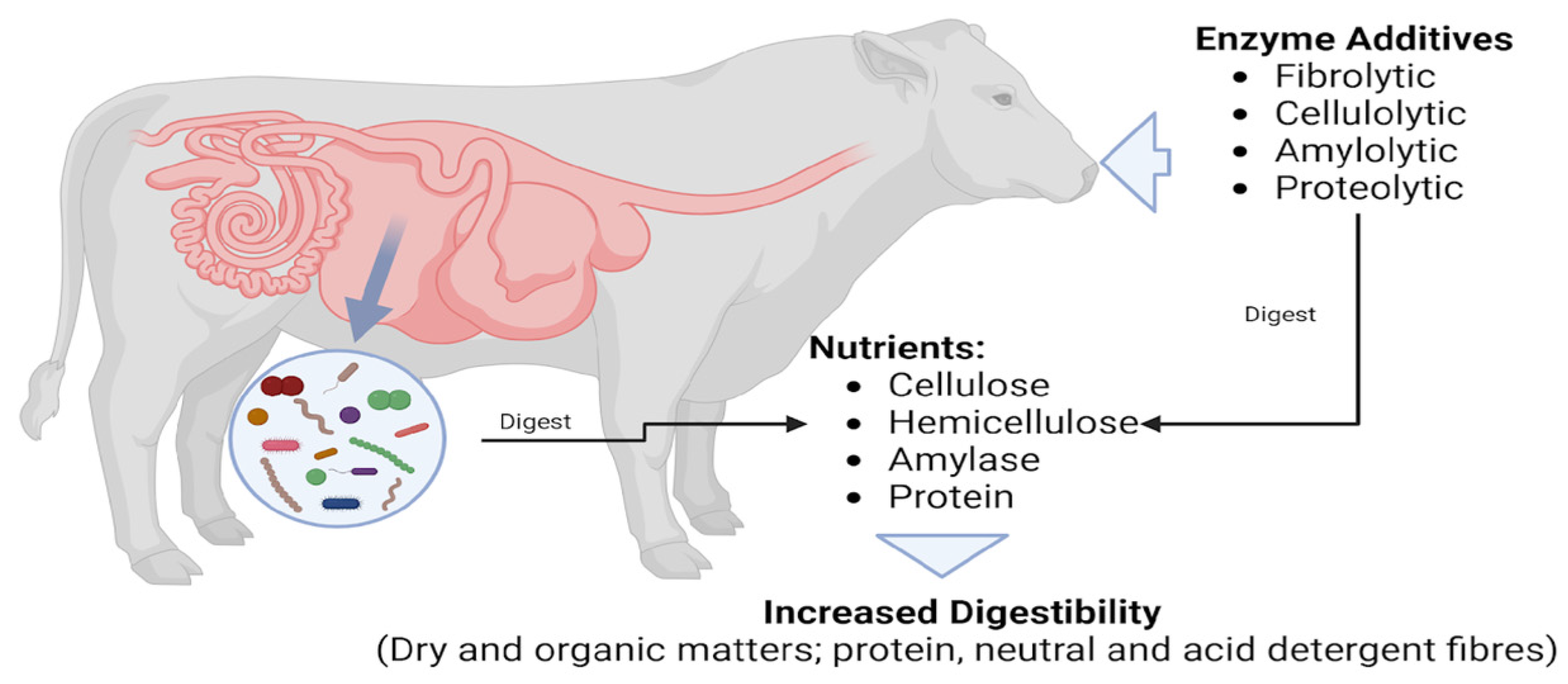
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DMI | Dry Matter Intake |
| OMI | Organic Matter Intake |
| DMD | Dry Matter Digestibility |
| OMD | Organic Matter Digestibility |
| CPD | Crude Protein Digestibility |
| ADFD | Acid Detergent Fibre Digestibility |
| NDFD | Neutral Detergent Fibre Digestibility |
| GP | Gas Production (hour) |
| VFA | Volatile Fatty Acid |
References
- Beauchemin, K.A.; Kreuzer, M.; O’Mara, F.; McAllister, T.A. Nutritional management for enteric methane abatement: A review. Aust. J. Exp. Agric. 2008, 48, 21–27. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Beauchemin, K.A.; Krehbiel, C.R.; Newbold, C.J. Enzymes, bacterial direct-fed microbials and yeast: Principles for use in ruminant nutrition. In Biology of Growing Animals; Elsevier: Amsterdam, The Netherlands, 2006; Volume 4, pp. 251–284. [Google Scholar]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Rahmatillah, R.S.; Ramdani, D.; Hernaman, I.; Jayanegara, A.; Yanza, Y.R. Exploring multiple impacts of dietary tea supplements on ruminants: A meta-analysis. Adv. Anim. Vet. Sci. 2024, 12, 1924–1931. [Google Scholar] [CrossRef]
- Wallace, B.C.; Lajeunesse, M.J.; Dietz, G.; Dahabreh, I.J.; Trikalinos, T.A.; Schmid, C.H.; Gurevitch, J. OpenMEE: Intuitive, open-source software for meta-analysis in ecology and evolutionary biology. Methods Ecol. Evol. 2017, 8, 941–947. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Galkanda-Arachchige, H.S.C.; Wilson, A.E.; Davis, D.A. Success of fishmeal replacement through poultry by-product meal in aquaculture feed formulations: A meta-analysis. Rev. Aquac. 2020, 12, 1624–1636. [Google Scholar] [CrossRef]
- Punekar, N.S. Enzymes: Catalysis, Kinetics and Mechanisms, 1st ed.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Richard, J.P. Enabling role of ligand-driven conformational changes in enzyme evolution. Biochemistry 2022, 61, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Mares, D.J.; Mrva, K.; Fincher, G.B. Enzyme activities. In Encyclopedia of Grain Science; Elsevier: Oxford, UK, 2004; pp. 357–365. [Google Scholar]
- Roque, B.M.; Reyes, G.C.; Tewoldebrhan, T.A.; Apphuamy, J.A.D.R.N.; Lee, J.-J.; Seo, S.; Kebreab, E. Exogenous β-mannanase supplementation improved immunological and metabolic responses in lactating dairy cows. J. Dairy Sci. 2019, 102, 4198–4204. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Puri, N.; Sharma, P.; Gupta, N. Mannanases: Microbial sources, production, properties and potential biotechnological applications. Appl. Microbiol. Biotechnol. 2012, 93, 1817–1830. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Treacher, R.J.; Nauman, G.A.; Smagala, A.M.; Endres, K.M.; Cohen, M.A. The effect of treating forages with fibrolytic enzymes on its nutritive value and lactation performance of dairy cows. J. Dairy Sci. 2000, 83, 115–122. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Salem, A.Z.M.; Gonzalez-Ronquillo, M.; Bórquez, J.L.; Gado, H.M.; Odongo, N.E.; Peñuelas, C.G. Effects of exogenous enzymes on in vitro gas production kinetics and ruminal fermentation of four fibrous feeds. Anim. Feed Sci. Technol. 2013, 179, 46–53. [Google Scholar] [CrossRef]
- Tatta, E.R.; Imchen, M.; Moopantakath, J.; Kumavath, R. Bioprospecting of microbial enzymes: Current trends in industry and healthcare. Appl. Microbiol. Biotechnol. 2022, 106, 1813–1835. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Rode, L.M.; Sewalt, V.J.H. Fibrolytic enzymes increase fibre digestibility and growth rate of steers fed dry forages. Can. J. Anim. Sci. 2003, 83, 521–528. [Google Scholar]
- McAllister, T.A.; Hristov, A.N.; Beauchemin, K.A.; Rode, L.M.; Cheng, K.J. Enzymes in ruminant diets. In Enzymes in Farm Animal Nutrition; CABI: Wallingford, UK, 2001; pp. 273–298. [Google Scholar]
- Rode, L.M.; Yang, W.Z.; Beauchemin, K.A. Fibrolytic enzyme supplements for dairy cows in early lactation. J. Dairy Sci. 1999, 82, 2121–2126. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Zhou, M.; Holtshausen, L.; Alexander, T.W.; McAllister, T.A.; Guan, L.L.; Beauchemin, K.A. A fibrolytic enzyme additive for lactating Holstein cow diets: Ruminal fermentation, rumen microbial populations, and enteric methane emissions. J. Dairy Sci. 2012, 95, 1419–1427. [Google Scholar] [CrossRef]
- Salem, A.Z.M.; Gado, H.M.; Colombatto, D.; Elghandour, M.M.Y. Effects of exogenous enzymes on nutrient digestibility, ruminal fermentation and growth performance in beef steers. Livest. Sci. 2013, 154, 69–73. [Google Scholar] [CrossRef]
- Refat, B.; Christensen, D.A.; McKinnon, J.J.; Yang, W.; Beattie, A.D.; McAllister, T.A.; Eun, J.-S.; Abdel-Rahman, G.A.; Yu, P. Effect of fibrolytic enzymes on lactational performance, feeding behavior, and digestibility in high-producing dairy cows fed a barley silage-based diet. J. Dairy Sci. 2018, 101, 7971–7979. [Google Scholar] [CrossRef]
- Chen, K.H.; Huber, J.T.; Simas, J.; Theurer, C.B.; Yu, P.; Chan, S.C.; Santos, F.; Wu, Z.; Swingle, R.S.; DePeters, E.J. Effect of enzyme treatment or steam-flaking of sorghum grain on lactation and digestion in dairy cows. J. Dairy Sci. 1995, 78, 1721–1727. [Google Scholar] [CrossRef]
- Klingerman, C.M.; Hu, W.; McDonell, E.E.; Der Bedrosian, M.C.; Kung, L., Jr. An evaluation of exogenous enzymes with amylolytic activity for dairy cows. J. Dairy Sci. 2009, 92, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Eun, J.S.; Beauchemin, K.A. Effects of exogenous fibrolytic enzymes on the structural carbohydrate composition of alfalfa hay and corn silage. Anim. Feed Sci. Technol. 2007, 140, 164–182. [Google Scholar]
- Pech-Cervantes, A.A.; Ogunade, I.M.; Jiang, Y.; Irfan, M.; Arriola, K.G.; Amaro, F.X.; Gonzalez, C.F.; DiLorenzo, N.; Bromfield, J.J.; Vyas, D.; et al. An expansin-like protein expands forage cell walls and synergistically increases hydrolysis, digestibility and fermentation of livestock feeds by fibrolytic enzymes. PLoS ONE 2019, 14, e0224381. [Google Scholar] [CrossRef]
- Reddish, M.A.; Kung, L., Jr. The effect of feeding a dry enzyme mixture with fibrolytic activity on the performance of lactating cows and digestibility of a diet for sheep. J. Dairy Sci. 2007, 90, 4724–4729. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, R.; Gado, H.; El-Sayed, H.; Abd El Mawla, S. Usage of treated rice straw with exogenous anaerobic bacterial enzymes (ZAD) for Ossimi sheep. Ann. Agric. Sci. 2012, 57, 183–190. [Google Scholar] [CrossRef]
- Ribeiro, G.O., Jr.; Gonçalves, L.C.; Pereira, L.G.R.; Chaves, A.V.; Wang, Y.; Beauchemin, K.A.; McAllister, T.A. Effect of fibrolytic enzymes added to an Andropogon gayanus grass silage–concentrate diet on rumen fermentation in batch cultures and the artificial rumen (Rusitec). Animal 2015, 9, 1153–1162. [Google Scholar] [CrossRef]
- Basmaeil, S.M.; Suliman, G.M.; Al Garadi, M.A.; Al-Badwi, M.A.; Abdelrahman, M.M.; Al-Harbi, F.S.; Swelum, A.A. Effects of increasing levels of lasalocid supplementation on growth performance, serum biochemistry, ruminal fermentation profile, in vitro nutrient digestibility, and gas production of growing goats. Front. Vet. Sci. 2023, 10, 1181426. [Google Scholar] [CrossRef]
- Nogoy, K.M.C.; Lee, J.I.; Yu, J.; Sang, J.I.; Hong, H.K.; Ji, Y.G.; Li, X.Z.; Choi, S.H. Supplementing Proteolytic Enzymes Increased the In Vitro Nutrient Effective Degradability and Fermentation Characteristics of Pineapple Waste Silage. Fermentation 2023, 9, 218. [Google Scholar] [CrossRef]
- Salem, A.Z.; Buendía-Rodríguez, G.; Elghandour, M.M.; Berasain, M.A.M.; Jiménez, F.J.P.; Pliego, A.B.; Rodríguez, M.A. Effects of cellulase and xylanase enzymes mixed with increasing doses of Salix babylonica extract on in vitro rumen gas production kinetics of a mixture of corn silage with concentrate. J. Integr. Agric. 2015, 14, 131–139. [Google Scholar] [CrossRef]
- Sufyan, A.; Khan, N.A.; Akbar, A.; Tang, S.; Tan, Z. Scaling-up fungal pretreatment of lignocellulose biomass: Impact on nutritional value, ruminal degradability, methane production, and performance of lactating dairy cows. Livest. Sci. 2024, 285, 105499. [Google Scholar] [CrossRef]
- Romero, J.J.; Macias, E.G.; Ma, Z.X.; Martins, R.M.; Staples, C.R.; Beauchemin, K.A.; Adesogan, A.T. Improving the performance of dairy cattle with a xylanase-rich exogenous enzyme preparation. J. Dairy Sci. 2016, 99, 3486–3496. [Google Scholar] [CrossRef] [PubMed]
- Tewoldebrhan, T.A.; Appuhamy, J.A.D.R.N.; Lee, J.-J.; Niu, M.; Seo, S.; Jeong, S.; Kebreab, E. Exogenous β-mannanase improves feed conversion efficiency and reduces somatic cell count in dairy cattle. J. Dairy Sci. 2017, 100, 244–252. [Google Scholar] [CrossRef]
- Oh, J.; Harper, M.; Melgar, A.; Compart, D.M.P.; Hristov, A.N. Effects of Saccharomyces cerevisiae-based direct-fed microbial and exogenous enzyme products on enteric methane emission and productivity in lactating dairy cows. J. Dairy Sci. 2019, 102, 6065–6075. [Google Scholar] [CrossRef] [PubMed]
- Zilio, E.M.; Del Valle, T.A.; Ghizzi, L.G.; Takiya, C.S.; Dias, M.S.; Nunes, A.T.; Rennó, F.P. Effects of exogenous fibrolytic and amylolytic enzymes on ruminal fermentation and performance of mid-lactation dairy cows. J. Dairy Sci. 2019, 102, 4179–4189. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.F.; Oh, J.; Harper, M.; Melgar, A.; Räisänen, S.E.; Chen, X.; Nedelkov, K.; Karnezos, T.P.; Hristov, A.N. Effects of an exogenous enzyme preparation extracted from a mixed culture of Aspergillus spp. on lactational performance, metabolism, and digestibility in primiparous and multiparous cows. J. Dairy Sci. 2022, 105, 7344–7353. [Google Scholar] [CrossRef]
- Vargas-Rodriguez, C.F.; Engstrom, M.; Azem, E.; Bradford, B.J. Effects of dietary amylase and sucrose on productivity of cows fed low-starch diets. J. Dairy Sci. 2014, 97, 4464–4470. [Google Scholar] [CrossRef]
- Jarrett, J.P.; Wilson, J.W.; Ray, P.P.; Knowlton, K.F. The effects of forage particle length and exogenous phytase inclusion on phosphorus digestion and absorption in lactating cows. J. Dairy Sci. 2014, 97, 411–418. [Google Scholar] [CrossRef]
- Lewis, G.E.; Sanchez, W.K.; Hunt, C.W.; Guy, M.A.; Pritchard, G.T.; Swanson, B.I.; Treacher, R.J. Effect of direct-fed fibrolytic enzymes on the lactational performance of dairy cows. J. Dairy Sci. 1999, 82, 611–617. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Yang, W.Z.; Rode, L.M. Effects of grain source and enzyme additive on site and extent of nutrient digestion in dairy cows. J. Dairy Sci. 1999, 82, 378–390. [Google Scholar] [CrossRef]
- Zheng, W.; Schingoethe, D.J.; Stegeman, G.A.; Hippen, A.R.; Treacher, R.J. Determination of when during the lactation cycle to start feeding a cellulase and xylanase enzyme mixture to dairy cows. J. Dairy Sci. 2000, 83, 2319–2325. [Google Scholar] [CrossRef]
- Toseti, L.B. Efeitos de Diferentes Aditivos e Fontes de Volumosos na Dieta de Bovinos Confinados. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2020. [Google Scholar]
- Arriola, K.G.; Kim, S.C.; Staples, C.R.; Adesogan, A.T. Effect of fibrolytic enzyme application to low- and high-concentrate diets on the performance of lactating dairy cattle. J. Dairy Sci. 2011, 94, 832–841. [Google Scholar] [CrossRef]
- Yang, H.J.; Xie, C.Y. Assessment of fibrolytic activities of 18 commercial enzyme products and their abilities to degrade the cell wall fraction of corn stalks in in vitro enzymatic and ruminal batch cultures. Anim. Feed Sci. Technol. 2010, 159, 110–121. [Google Scholar] [CrossRef]
- Bala, P.; Malik, R.; Srinivas, B. Effect of fortifying concentrate supplement with fibrolytic enzymes on nutrient utilization, milk yield and composition in lactating goats. Anim. Sci. J. 2009, 80, 265–272. [Google Scholar] [CrossRef]
- Silvestre, T.; Fetter, M.; Räisänen, S.E.; Lage, C.F.A.; Stefenoni, H.; Melgar, A.; Hristov, A.N. Performance of dairy cows fed normal- or reduced-starch diets supplemented with an exogenous enzyme preparation. J. Dairy Sci. 2022, 105, 2288–2300. [Google Scholar] [CrossRef]
- Simon, A.L.; Copetti, P.M.; Lago, R.V.; Vitt, M.G.; Nascimento, A.L.; Silva, L.E.L.; Da Silva, A.S. Inclusion of exogenous enzymes in feedlot cattle diets: Impacts on physiology, rumen fermentation, digestibility and fatty acid profile in rumen and meat. Biotechnol. Rep. 2024, 41, e00824. [Google Scholar] [CrossRef] [PubMed]
- Fróes, R.; Bezerra, L.; Missasse, J.; Castro, D.; Barbosa, A.; Arce-Cordero, J.; Oliveira, R. Effects of yeast and exogenous fibrolytic enzyme additives on lamb performance and feed efficiency. Trop. Anim. Health Prod. 2024, 56, 235. [Google Scholar] [CrossRef] [PubMed]
- Van den Bossche, T.; Goossens, K.; Ampe, B.; Tamassia, L.F.M.; De Boever, J.L.; Vandaele, L. Effect of supplementing an α-amylase enzyme or a blend of essential oil components on the performance, nutrient digestibility, and nitrogen balance of dairy cows. J. Dairy Sci. 2024, 107, 4509–4523. [Google Scholar] [CrossRef]
- Bureenok, S.; Pitiwittayakul, N.; Saenmahayak, B.; Saithi, S.; Yuangklang, C.; Cai, Y.; Schonewille, J.T. Effects of fibrolytic enzyme supplementation on feed intake, digestibility and rumen fermentation characteristics in goats fed with Leucaena silage. Small Rumin. Res. 2024, 231, 107200. [Google Scholar] [CrossRef]
- Cueva, S.F.; Wasson, D.E.; Martins, L.F.; Räisänen, S.E.; Silvestre, T.; Hristov, A.N. Lactational performance, ruminal fermentation, and enteric gas emission of dairy cows fed an amylase-enabled corn silage in diets with different starch concentrations. J. Dairy Sci. 2024, 107, 4426–4448. [Google Scholar] [CrossRef]
- Krogstad, K.C.; Bradford, B.J. The effects of feeding α-amylase-enhanced corn silage with different dietary starch concentrations to lactating dairy cows on milk production, nutrient digestibility, and blood metabolites. J. Dairy Sci. 2023, 106, 4666–4681. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Guo, G.; Huo, W.; Xia, C.Q.; Chen, L.; Liu, Q. Effects of folic acid and riboflavin on growth performance, nutrient digestion and rumen fermentation in Angus bulls. Br. J. Nutr. 2023, 129, 1–9. [Google Scholar] [CrossRef]
- Pan, S.; Wang, D.; Lin, Y.; Cheng, M.; Zhu, F.; Guo, Y. Effects of ginger straw silage with enzymes on growth performance, digestion and metabolism, meat quality and rumen microflora diversity of Laiwu black goat. Animals 2024, 14, 2040. [Google Scholar] [CrossRef]
- Xue, Y.; Sun, H.; Guo, H.; Nie, C.; Nan, S.; Lu, Q.; Zhang, W. Effect of the supplementation of exogenous complex non-starch polysaccharidases on the growth performance, rumen fermentation and microflora of fattening sheep. Front. Vet. Sci. 2024, 11, 1396993. [Google Scholar] [CrossRef]
- Daniel, J.L.P.; Queiroz, O.C.M.; Arriola, K.G.; Staples, C.R.; Romero, J.J.; Shin, J.H.; Adesogan, A.T. Effects of maturity at ensiling of bermudagrass and fibrolytic enzyme application on the performance of early-lactation dairy cows. J. Dairy Sci. 2016, 99, 9716–9723. [Google Scholar] [CrossRef]
- Anadón, A.; Ares, I.; Martínez-Larrañaga, M.R.; Martínez, M.A. Enzymes in feed and animal health. Nutraceuticals Vet. Med. 2019, 303, 303–313. [Google Scholar]
- Nsereko, V.L.; Morgavi, D.P.; Rode, L.M.; Beauchemin, K.A.; McAllister, T.A. Effects of fungal enzyme preparations on hydrolysis and subsequent degradation of alfalfa hay fibre by mixed rumen microorganisms in vitro. Anim. Feed Sci. Technol. 2000, 88, 153–170. [Google Scholar] [CrossRef]
- Colombatto, D.; Mould, F.L.; Bhat, M.K.; Phipps, R.H.; Owen, E. Influence of fibrolytic enzymes on the hydrolysis and fermentation of pure cellulose and xylan by mixed ruminal microorganisms in vitro. J. Anim. Sci. 2003, 81, 1040–1050. [Google Scholar] [CrossRef]
- Smith, J.; Adams, H.; Turner, M. Enzyme additives and dry matter digestibility: A review of recent studies. Anim. Feed Sci. Technol. 2018, 34, 112–121. [Google Scholar]
- Johnson, R.; Lee, A.; Brown, T. Impact of enzyme supplementation on organic matter digestibility in livestock diets. J. Anim. Nutr. 2019, 45, 214–223. [Google Scholar]
- Williams, P.; Harris, J.; Patel, S. Fibrolytic enzymes in animal feed: Assessing the impact on fibre digestibility. Livest. Sci. 2020, 50, 78–85. [Google Scholar]
- Garcia, M.; Rodriguez, P.; Gomez, J. The effect of enzyme supplementation on milk yield in dairy cattle. Dairy Sci. Technol. 2021, 60, 134–142. [Google Scholar]
- Martinez, H.; Rivera, A.; Thompson, D. Enzyme additives and their impact on milk protein and fat content: A comprehensive review. J. Dairy Nutr. 2019, 48, 189–200. [Google Scholar]
- Johnson, A.; Kumar, V.; Lee, S. Lactose synthesis in the mammary gland: Regulatory mechanisms and dietary influences. J. Dairy Res. 2020, 55, 250–258. [Google Scholar]
- Nguyen, T.; Do, D.; Pham, H. Enhancing rumen fermentation through enzyme supplementation: A review. Livest. Res. Rural Dev. 2022, 34, 310–320. [Google Scholar]
- Kim, Y.H.; Nagata, R.; Ohtani, M. Effect of enzyme additives on volatile fatty acid production in the rumen. J. Anim. Sci. 2021, 99, 256–266. [Google Scholar]
- Sutton, J.D.; Beever, D.E.; Fisher, W.J. The significance of the acetate to propionate ratio in dairy cows. J. Dairy Sci. 2019, 88, 2424–2436. [Google Scholar]
- Bach, A.; Sola-Oriol, D.; Devant, M. Enzymes as feed additives: Their role in modifying rumen fermentation patterns. Anim. Feed Sci. Technol. 2020, 64, 45–52. [Google Scholar]

| No. | References | Enzyme Type | Object | Test System | Response Variable |
|---|---|---|---|---|---|
| 1 | [22] | Xylanase and Cellulase | Dairy Cows | In vivo | ↑DMI, ↑OMI, ↑DMD, ↑OMD, ↑NDFD, ↓Milk Fat, ↑Milk Protein, ↑Milk lactose, ↓milk kg/d |
| 2 | [23] | Sorghum specific enzyme | Dairy Cows | In vivo | ↑DMI, ↓Milk Fat, ↑Milk Protein, ↑Milk lactose, ↑milk kg/d |
| 3 | [24] | Amylolytic enzyme | Dairy Cows | In vitro and in vivo | ↑DMI, ↑DMD, ↑CPD, ↑ADFD, ↑NDFD, ↑Milk Fat, ↑Milk Protein, ↓Milk lactose, ↑milk kg/d |
| 4 | [25] | Proteolytic enzyme | Dairy Cows | In vitro and in vivo | ↑OMI, ↑DMD, ↑OMD, ↑pH, ↓Milk Fat, ↑Milk Protein, ↑Milk lactose, ↑milk kg/d, ↓acetate, ↑propionate, ↓total VFA, A:P |
| 5 | [15] | Enzyme preparation mixture (ENZ) | Brown Swiss cows | In vitro | ↑DMD, ↑OMD, ↓pH, ↑GP12, ↑GP24, ↑GP48, ↑GP72 |
| 6 | [26] | Fibrolytic enzyme | In vitro | ↑DMD, ↑ADFD, ↑NDFD, ↓pH | |
| 7 | [27] | Cellulase and xylanase | Sheep | In vitro | ↓DMD, ↓ADFD, ↓NDFD |
| 8 | [28] | Exogenous anaerobic bacterial enzymes | Sheep | In vitro | ↑DMD, ↑OMD, ↑CPD, ↑ADFD, ↑NDFD |
| 9 | [21] | Cellulase and xylanase | Rumen | In vitro | ↑DMD, ↓OMD, ↓pH |
| 10 | [29] | Exogenous fibrolytic enzyme | Cows | In vitro | ↓pH |
| 11 | [30] | lasalocid | goat | In vitro | ↑DMD, ↑OMD, ↑pH |
| 12 | [31] | Proteolytic enzymes | Cow | In vitro | ↑DMD, ↑OMD, ↑ADFD, ↑NDFD |
| 13 | [32] | Cellulase and xylanase | Rumen | In vitro | ↓GP12, ↓GP24, ↓GP48, ↓GP72 |
| 14 | [33] | lignocellulose | Cow | In vitro, In vivo | ↑DMD, Total VFA, ↑Acetate, ↑Propionate, A:P ↓pH, ↑DMD, ↑OMD, ↑NDFD, ↑ADFD, ↑Milk Production, ↓Fat, ↓Protein, ↑Lactose |
| 15 | [34] | Xylanase-rich exogenous enzyme | Dairy cows | In vivo | ↓Milk Fat, ↓Milk Protein, ↓Milk lactose, ↑milk kg/d, ↓DMD |
| 16 | [35] | Β-manase | Dairy cows | In vivo | ↓Milk Fat, ~Milk Protein, ~Milk lactose, ~milk kg/d, ↓ADF, ↓NDF, ↓OMD, ↑DMD |
| 17 | [36] | Enzyme extract from Aspergillus oryzae & Aspergillus niger | Dairy cows | In vivo | ↓Milk Fat, ↑Milk Protein, ↓Milk lactose, ↑milk kg/d, ↑ADF, ↓NDF, ↓OMD, ↑total VFA, ↓DMD |
| 18 | [12] | Exogenous β-manase | Dairy cows | In vivo | ↑Milk Fat, ↓Milk Protein, ↑Milk lactose, ↓milk kg/d |
| 19 | [37] | Fibrolytic and amylolytic enzymes | Dairy cows | In vivo | ↓Milk Fat, ↓Milk Protein, ↓Milk lactose, ↓milk kg/d, acetate, propionate, total VFA, A:P |
| 20 | [38] | Exogenous enzyme from Aspergillus sp. | Dairy cows | In vivo | ↓Milk Fat, ↑Milk lactose, ↑milk kg/d |
| 21 | [39] | Amylase and sucrose | Dairy cows | In vivo | ↑Milk Fat, ↓Milk lactose, ↑milk kg/d |
| 22 | [40] | Exogenous phytase | Dairy cows | In vivo | ↓Milk Fat, ↓Milk Protein, ↑milk kg/d |
| 23 | [41] | Fibrolytic enzyme | Dairy cows | In vivo | ↓Milk Fat, ↓Milk Protein, ↑Milk lactose, ↑milk kg/d |
| 24 | [42] | Cellulase and xylanase | Dairy cows | In vivo | ↑Milk Fat, ↑Milk Protein, ↑Milk lactose, ↑milk kg/d, ↓acetate, ↑propionate, ↓total VFA, ↓A:P |
| 25 | [14] | Fibrolytic enzyme | Dairy cows | In vivo | ↑Milk Fat, ↑Milk Protein, ↑milk kd/d |
| 26 | [43] | Cellulase and xylanase | Dairy cows | In vivo | ↑Milk Fat, ↑Milk Protein, ↓Milk lactose, ↑milk kg/d |
| 27 | [17] | Non-Starch Polysaccharidedase enzyme | Dairy cows | In vivo | ↑Milk Fat, ↑Milk Protein, ↓Milk lactose, ↓milk kg/d, ↑acetate, ↓propionate, ↑total VFA, ↑A:P |
| 28 | [19] | Fibrolytic enzyme | Dairy cows | In vivo | ↓Milk Fat, ↓Milk Protein, ↓Milk lactose, ↑milk kg/d |
| 29 | [44] | α-amylase | Beef cattle | In vivo | ↓Acetate, ↑propionate, ↓A:P |
| 30 | [20] | Fibrolytic enzyme | Dairy cows | In vivo | ↓Acetate, ↑propionate, A:P |
| 31 | [45] | Fibrolytic enzyme | Dairy cows | In vivo | ↑Acetate, ↑propionate, ↑total VFA, ↓A:P |
| 32 | [46] | Xylanase and cellulase | Dairy cows | In vivo | ↓Acetate, ↑propionate, ↑total VFA, ↓A:P |
| 33 | [47] | Xylanase and cellulase | Sheep | In vivo | ↑ADF, ↑NDF, ↑OMD, ↑DMD |
| 34 | [48] | Enzyme extract from Aspergillus oryzae & Aspergillus niger | Dairy cows | In vivo | ~ADF, ~NDF |
| 35 | [49] | exogenous enzymes (Amylase, Protease, Cellulase, Xylanase, Beta glucanase) | Cow | In vivo | ↑DMD, ↓OMD, ↑NDFD, ↑ADFD |
| 36 | [50] | exogenous fibrolytic enzymes | Sheep | In vivo | ↓DMD, ↓OMD, ↑NDFD |
| 37 | [51] | α-amylase | Cow | In vivo | ~DMD, ~OMD, ↑NDFD, CPD, ↑Milk Production, ↑Lactose, ↓Protein, ↓Fat |
| 38 | [52] | fibrolytic enzyme cocktail | Goat | In vivo | ↑DMD, ↑OMD, ↑NDFD, ↑ADFD, ↓Total VFA, ~Acetate, ↑Propionate, ↓A:P |
| 39 | [53] | amylase | Cow | In vivo | ↑DMD, ↑OMD, ↑NDFD, ↑ADFD, ↓Milk Production, ↑Fat, ↓Protein, ↑Lactose, ↓Total VFA, ↑Acetate, ↓Propionate, ↑A:P |
| 40 | [54] | α-amylase | Cow | In vivo | ↑DMD, ↑NDFD, ↑Milk Production, ~Lactose, ↓Protein, ↓Fat |
| 41 | [55] | coated folic acid & coated riboflavin | Cow | In vivo | ↑DMD, ↑OMD, ↑NDFD, ↑ADFD, ↑Total VFA, ↑Acetate, ↓Propionate, ↑A:P |
| 42 | [56] | enzymatic silage ginger straw | Goat | In vivo | ↑DMD, ↑ADFD, ↑NDFD |
| 43 | [57] | exogenous non-starch polysaccharidases | Sheep | In vivo | ↑DMD, ↑OMD, ↑NDFD, ↑ADFD, ~total VFA, ↑Acetate, ↑Propionate, ↓A:P |
| 44 | [58] | fibrolytic enzyme | Dairy cows | In vivo | ↑DMI, ↑Milk Production, ↑Lactose, ↑Protein, ↓Fat |
| Model Results | Heterogeneity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Response Variable | N | Unit | Mean Control ± SE | Mean Experiment ± SE | Estimate | Lower Bound | Upper Bound | SE | p-Value | τ2 | Q | Het p-Value | I2 |
| DMI | 17 | kg/head/d | 25.4 ± 1.56 | 25.4 ± 1.55 | 0.110 | −0.216 | 0.435 | 0.166 | 0.509 | 0.049 | 17.9 | 0.331 | 10.5 |
| OMI | 6 | kg/head/d | 23.8 ± 0.77 | 24.3 ± 0.800 | 0.229 | −0.421 | 0.880 | 0.332 | 0.489 | 0.121 | 6.12 | 0.294 | 18.3 |
| DMD | 41 | % | 67.3 ± 0.820 | 67.9 ± 0.870 | 0.621 | 0.144 | 1.10 | 0.243 | 0.011 | 1.827 | 232 | <0.001 | 83.2 |
| OMD | 35 | % | 68.7 ± 1.00 | 68.7 ± 1.16 | 0.521 | −0.034 | 1.09 | 0.249 | 0.036 | 1.644 | 186 | <0.001 | 81.7 |
| ADFD | 28 | % | 49.3 ± 1.55 | 51.6 ± 1.87 | 0.804 | 0.176 | 1.43 | 0.320 | 0.012 | 1.963 | 159 | <0.001 | 83.1 |
| NDFD | 38 | % | 53.8 ± 1.18 | 56.4 ± 1.39 | 0.791 | 0.304 | 1.28 | 0.248 | 0.001 | 1.662 | 210 | <0.001 | 82.4 |
| Milk Production | 57 | kg/day | 34.6 ± 1.08 | 35.5 ± 1.11 | 0.325 | 0.159 | 0.492 | 0.085 | <0.001 | 0.162 | 105 | <0.001 | 49.9 |
| Lactose | 53 | % | 4.75 ± 0.019 | 4.78 ± 0.019 | 0.142 | 0.001 | 0.284 | 0.072 | 0.049 | 0.057 | 65.9 | 0.053 | 25.7 |
| Protein | 55 | % | 3.13 ± 0.043 | 3.14 ± 0.041 | −0.060 | −0.189 | 0.069 | 0.066 | 0.362 | 0.024 | 57.6 | 0.245 | 11.4 |
| Fat | 59 | % | 3.78 ± 0.085 | 3.74 ± 0.859 | −0.103 | −0.206 | 0.001 | 0.053 | 0.051 | 0.000 | 48.5 | 0.719 | 0 |
| Total VFA | 29 | mM | 113 ± 3.41 | 115 ± 3.38 | 0.152 | −0.065 | 0.369 | 0.111 | 0.170 | 0.138 | 51.5 | 0.001 | 51.4 |
| Acetate | 32 | mM | 339 ± 49.9 | 329 ± 48.3 | −0.815 | −1.357 | −0.273 | 0.277 | 0.003 | 1.62 | 250.9 | <0.001 | 87.6 |
| Propionate | 32 | mM | 124 ± 17.5 | 134 ± 19.6 | 0.904 | 0.331 | 1.48 | 0.292 | 0.002 | 1.91 | 279.8 | <0.001 | 88.9 |
| A:P | 29 | - | 2.68 ± 0.130 | 2.63 ± 0.140 | 0.232 | −0.254 | 0.717 | 0.248 | 0.350 | 1.39 | 216.7 | <0.001 | 87.1 |
| Model Results | Heterogeneity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Response Variables | N | Unit | Mean Control ± SE | Mean Experiment ± SE | Estimate | Lower Bound | Upper Bound | SE | p-Value | τ2 | Q | Het p-Value | I2 |
| DMD | 39 | % | 58.3 ± 2.76 | 57.0 ± 3.22 | 0.247 | 0.024 | 0.523 | 0.127 | 0.032 | 0.207 | 60.1 | 0.013 | 36.7 |
| OMD | 33 | % | 45.8 ± 3.56 | 32.8 ± 5.96 | 2.08 | 0.318 | 3.85 | 0.901 | 0.021 | 13.3 | 505 | <0.001 | 93.7 |
| CPD | 6 | % | 62.1 ± 1.82 | 70.4 ± 2.27 | 1.76 | −1.73 | 5.25 | 1.78 | 0.322 | 9.26 | 39.4 | <0.001 | 87.3 |
| ADFD | 15 | % | 44.8 ± 3.78 | 49.1 ± 4.20 | 0.936 | 0.078 | 1.79 | 0.438 | 0.032 | 1.54 | 40.1 | <0.001 | 65.1 |
| NDFD | 14 | % | 45.3 ± 3.18 | 44.2 ± 3.89 | 1.410 | 0.489 | 2.33 | 0.470 | 0.003 | 2.30 | 62.6 | <0.001 | 72.8 |
| pH | 25 | - | 6.57 ± 0.066 | 6.51 ± 0.072 | −0.402 | −0.816 | 0.012 | 0.211 | 0.057 | 0.76 | 91.6 | <0.001 | 73.8 |
| GP 12 | 15 | mL/g DM | 51.0 ± 10.5 | 66.7 ± 8.57 | 0.854 | 0.126 | 1.58 | 0.371 | 0.022 | 1.47 | 54.5 | <0.001 | 74.3 |
| GP 24 | 15 | mL/g DM | 77.6 ± 11.5 | 103 ± 9.18 | 0.827 | 0.284 | 1.37 | 0.277 | 0.003 | 0.810 | 50.7 | <0.001 | 72.4 |
| GP 48 | 15 | mL/g DM | 114. ± 11.8 | 150 ± 9.80 | 0.929 | 0.536 | 1.32 | 0.201 | <0.001 | 0.297 | 28.1 | 0.014 | 50.1 |
| GP 72 | 15 | mL/g DM | 138 ± 11.6 | 176 ± 9.55 | 0.823 | 0.427 | 1.22 | 0.202 | <0.001 | 0.308 | 28.9 | 0.011 | 51.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramdani, D.; Rahmatillah, R.S.; Yanza, Y.R.; Jayanegara, A.; Wathoni, N.; Chaudhry, A.S. The Roles of Enzymes as Dietary Additives in Ruminant Diets: A Meta-Analysis. Animals 2025, 15, 3631. https://doi.org/10.3390/ani15243631
Ramdani D, Rahmatillah RS, Yanza YR, Jayanegara A, Wathoni N, Chaudhry AS. The Roles of Enzymes as Dietary Additives in Ruminant Diets: A Meta-Analysis. Animals. 2025; 15(24):3631. https://doi.org/10.3390/ani15243631
Chicago/Turabian StyleRamdani, Diky, Ririn Siti Rahmatillah, Yulianri Rizki Yanza, Anuraga Jayanegara, Nasrul Wathoni, and Abdul Shakoor Chaudhry. 2025. "The Roles of Enzymes as Dietary Additives in Ruminant Diets: A Meta-Analysis" Animals 15, no. 24: 3631. https://doi.org/10.3390/ani15243631
APA StyleRamdani, D., Rahmatillah, R. S., Yanza, Y. R., Jayanegara, A., Wathoni, N., & Chaudhry, A. S. (2025). The Roles of Enzymes as Dietary Additives in Ruminant Diets: A Meta-Analysis. Animals, 15(24), 3631. https://doi.org/10.3390/ani15243631







