The Role of Short-Chain Fatty Acids (SCFAs) in Colic and Anti-Inflammatory Pathways in Horses
Simple Summary
Abstract
1. Introduction
2. Short-Chain Fatty Acids: Production and Functions
3. SCFA Deficiency and Its Association with Colic
4. Strategies to Enhance SCFA Production for Colic Prevention
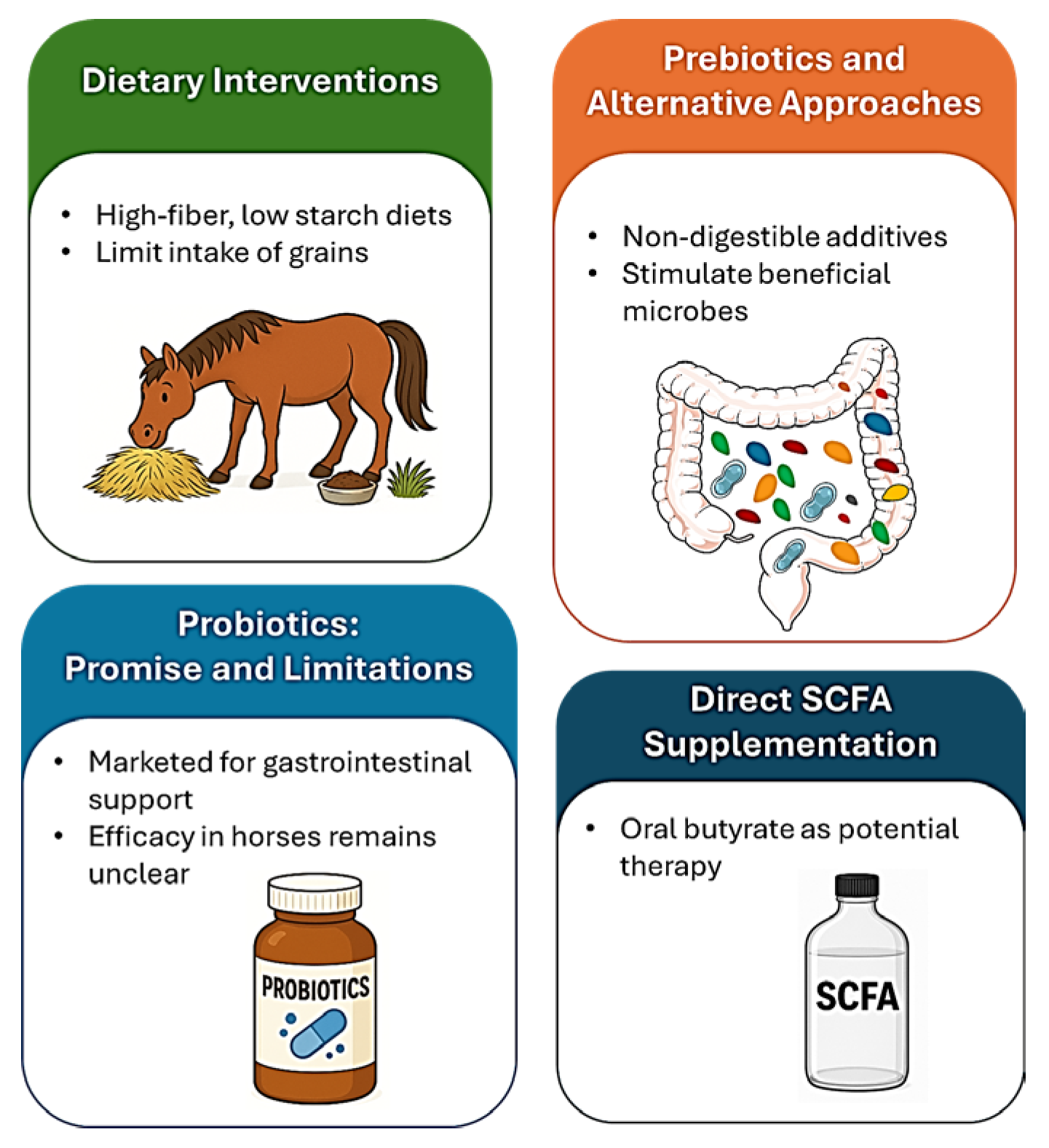
4.1. Dietary Interventions
4.2. Probiotics: Promise and Limitations
4.3. Prebiotics and Alternative Approaches
4.4. Direct SCFA Supplementation
5. Future Research and Therapeutic Potential
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCFA | short-chain fatty acids |
| GPCR | G-protein-coupled receptors |
| GLP-1 | glucagon-like peptide-1 |
| FMT | Fecal microbiota transplantation |
| GABA | γ-aminobutyric acid |
| FOS | fructo-oligosaccharides |
| GOS | galacto-oligosaccharides |
References
- Venable, E.B.; Bland, S.D.; McPherson, J.L.; Francis, J. Role of the gut microbiota in equine health and disease. Anim. Front. 2016, 6, 43–49. [Google Scholar] [CrossRef]
- Salem, S.E.; Maddox, T.W.; Berg, A.; Antczak, P.; Ketley, J.M.; Williams, N.J.; Archer, D.C. Variation in faecal microbiota in a group of horses managed at pasture over a 12-month period. Sci. Rep. 2018, 8, 8510. [Google Scholar] [CrossRef]
- Weese, J.S.; Holcombe, S.J.; Embertson, R.M.; Kurtz, K.A.; Roessner, H.A.; Jalali, M.; Wismer, S.E. Changes in the faecal microbiota of mares precede the development of post partum colic. Equine Vet. J. 2015, 47, 641–649. [Google Scholar] [CrossRef]
- Arnold, C.E.; Pilla, R. What Is the Microbiota and What Is Its Role in Colic? Vet. Clin. N. Am. Equine Pract. 2023, 39, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Lara, F.; Castro, R.; Thomson, P. Changes in the gut microbiome and colic in horses: Are they causes or consequences? Open Vet. J. 2022, 12, 242–249. [Google Scholar] [CrossRef]
- Reed, S.M.; Bayly, W.M.; Sellon, D.C. Equine Internal Medicine; Saunders: Philadelphia, PA, USA, 2017. [Google Scholar]
- Eighner, K. Colic survival rate: Review of 254 Cases. 19 March 2020. Available online: https://vetmed.illinois.edu/2020/03/19/colic-survival-rate/ (accessed on 10 July 2025).
- Orsini, J.A.; Divers, T.J. Equine Emergencies: Treatment and Procedures; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Singer, E.R.; Smith, M.A. Examination of the horse with colic: Is it medical or surgical? Equine Vet. Educ. 2002, 34, 87–96. [Google Scholar] [CrossRef]
- Proudman, C.J.; Smith, J.E.; Edwards, G.B.; French, N.P. Long-term survival of equine surgical colic cases. Part 1: Patterns of mortality and morbidity. Equine Vet. J. 2002, 34, 432–437. [Google Scholar] [CrossRef]
- Proudman, C.J. A two year, prospective survey of equine colic in general practice. Equine Vet. J. 1992, 24, 90–93. [Google Scholar] [CrossRef]
- Tinker, M.K.; White, N.A.; Lessard, P.; Thatcher, C.D.; Pelzer, K.D.; Davis, B.; Carmel, D.K. Prospective study of equine colic incidence and mortality. Equine Vet. J. 1997, 29, 448–453. [Google Scholar] [CrossRef]
- Abutarbush, S.M.; Carmalt, J.L.; Shoemaker, R.W. Causes of gastrointestinal colic in horses in western Canada: 604 cases (1992 to 2002). Can. Vet. J. 2005, 46, 800–805. [Google Scholar]
- Hillyer, M.H.; Taylor, F.G.; French, N.P. A cross-sectional study of colic in horses on thoroughbred training premises in the British Isles in 1997. Equine Vet. J. 2001, 33, 380–385. [Google Scholar] [CrossRef]
- Traub-Dargatz, J.L.; Kopral, C.A.; Seitzinger, A.H.; Garber, L.P.; Forde, K.; White, N.A. Estimate of the national incidence of and operation-level risk factors for colic among horses in the United States, spring 1998 to spring 1999. J. Am. Vet. Med. Assoc. 2001, 219, 67–71. [Google Scholar] [CrossRef]
- United States Department of Agriculture; National Animal Health Monitoring System. Part I: Baseline Reference of 1998 Equine Health and Management; United States Department of Agriculture: Washington, DC, USA, 1998.
- Cohen, N.D.; Peloso, J.G. Risk factors for history of previous colic and for chronic, intermittent colic in a population of horses. J. Am. Vet. Med. Assoc. 1996, 208, 697–703. [Google Scholar] [CrossRef]
- Matyjaszek, S.A.; Morton, A.J.; Freeman, D.E.; Grosche, A.; Polyak, M.M.; Kuck, H. Effects of flunixin meglumine on recovery of colonic mucosa from ischemia in horses. Am. J. Vet. Res. 2009, 70, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.F.; Blikslager, A.T. The effect of nonsteroidal anti-inflammatory drugs on the equine intestine. Equine Vet. J. Suppl. 2011, 43, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Whitfield-Cargile, C.M.; Coleman, M.C.; Cohen, N.D.; Chamoun-Emanuelli, A.M.; DeSolis, C.N.; Tetrault, T.; Sowinski, R.; Bradbery, A.; Much, M. Effects of phenylbutazone alone or in combination with a nutritional therapeutic on gastric ulcers, intestinal permeability, and fecal microbiota in horses. J. Vet. Intern. Med. 2021, 35, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.A.; Kittelmann, S.; Rogers, C.W.; Gee, E.K.; Bolwell, C.F.; Bermingham, E.N.; Thomas, D.G. Faecal microbiota of forage-fed horses in New Zealand and the population dynamics of microbial communities following dietary change. PLoS ONE 2014, 9, e112846. [Google Scholar] [CrossRef]
- Feary, D.J.; Hassel, D.M. Enteritis and colitis in horses. Vet. Clin. N. Am. Equine Pract. 2006, 22, 437–479. [Google Scholar] [CrossRef]
- Dicks, L.; Botha, M.; Dicks, E.; Botes, M. The equine gastro-intestinal tract: An overview of the microbiota, disease and treatment. Livest. Sci. 2014, 160, 69–81. [Google Scholar] [CrossRef]
- Geor, R.J.; Harris, P.A.; Coenen, M. Equine Applied and Clinical Nutrition: Health, Welfare and Performance; Saunders: Philadelphia, PA, USA, 2013. [Google Scholar]
- von Engelhardt, W.; Bartels, J.; Kirschberger, S.; Meyer zu Düttingdorf, H.D.; Busche, R. Role of short-chain fatty acids in the hind gut. Vet. Q. 1998, 20, S52–S59. [Google Scholar] [CrossRef]
- Boucher, L.; Leduc, L.; Leclere, M.; Costa, M.C. Current Understanding of Equine Gut Dysbiosis and Microbiota Manipulation Techniques: Comparison with Current Knowledge in Other Species. Animals 2024, 14, 758. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Julliand, V.; Grimm, P. The Impact of Diet on the Hindgut Microbiome. J. Equine Vet. Sci. 2017, 52, 23–28. [Google Scholar] [CrossRef]
- Garber, A.; Hastie, P.; Murray, J.A. Factors Influencing Equine Gut Microbiota: Current Knowledge. J. Equine Vet. Sci. 2020, 88, 102943. [Google Scholar] [CrossRef] [PubMed]
- Kauter, A.; Epping, L.; Semmler, T.; Antao, E.M.; Kannapin, D.; Stoeckle, S.D.; Gehlen, H.; Lubke-Becker, A.; Gunther, S.; Wieler, L.H.; et al. The gut microbiome of horses: Current research on equine enteral microbiota and future perspectives. Anim. Microbiome 2019, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.P.; McCormick, C.A.; Suen, G. Fibrobacter communities in the gastrointestinal tracts of diverse hindgut-fermenting herbivores are distinct from those of the rumen. Environ. Microbiol. 2017, 19, 3768–3783. [Google Scholar] [CrossRef]
- Arnold, C.E.; Pilla, R.; Chaffin, M.K.; Leatherwood, J.L.; Wickersham, T.A.; Callaway, T.R.; Lawhon, S.D.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. The effects of signalment, diet, geographic location, season, and colitis associated with antimicrobial use or Salmonella infection on the fecal microbiome of horses. J. Vet. Intern. Med. 2021, 35, 2437–2448. [Google Scholar] [CrossRef]
- Muhonen, S.; Sadet-Bourgeteau, S.; Julliand, V. Effects of Differences in Fibre Composition and Maturity of Forage-Based Diets on the Microbial Ecosystem and Its Activity in Equine Caecum and Colon Digesta and Faeces. Animals 2021, 11, 2337. [Google Scholar] [CrossRef]
- Lange, O.; Proczko-Stepaniak, M.; Mika, A. Short-Chain Fatty Acids-A Product of the Microbiome and Its Participation in Two-Way Communication on the Microbiome-Host Mammal Line. Curr. Obes. Rep. 2023, 12, 108–126. [Google Scholar] [CrossRef]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef]
- Nilsson, N.E.; Kotarsky, K.; Owman, C.; Olde, B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun. 2003, 303, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Stothart, M.R.; McLoughlin, P.D.; Medill, S.A.; Greuel, R.J.; Wilson, A.J.; Poissant, J. Methanogenic patterns in the gut microbiome are associated with survival in a population of feral horses. Nat. Commun. 2024, 15, 6012. [Google Scholar] [CrossRef]
- Chaucheyras-Durand, F.; Sacy, A.; Karges, K.; Apper, E. Gastro-Intestinal Microbiota in Equines and Its Role in Health and Disease: The Black Box Opens. Microorganisms 2022, 10, 2517. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef]
- Li, M.; van Esch, B.; Wagenaar, G.T.M.; Garssen, J.; Folkerts, G.; Henricks, P.A.J. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 2018, 831, 52–59. [Google Scholar] [CrossRef]
- Correa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Stewart, H.L.; Southwood, L.L.; Indugu, N.; Vecchiarelli, B.; Engiles, J.B.; Pitta, D. Differences in the equine faecal microbiota between horses presenting to a tertiary referral hospital for colic compared with an elective surgical procedure. Equine Vet. J. 2019, 51, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.L.; Singh, B. Neutrophil apoptosis is delayed in an equine model of colitis: Implications for the development of systemic inflammatory response syndrome. Equine Vet. J. 2017, 49, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Tuniyazi, M.; He, J.; Guo, J.; Li, S.; Zhang, N.; Hu, X.; Fu, Y. Changes of microbial and metabolome of the equine hindgut during oligofructose-induced laminitis. BMC Vet. Res. 2021, 17, 11. [Google Scholar] [CrossRef]
- Durham, A.E. The role of nutrition in colic. Vet. Clin. N. Am. Equine Pract. 2009, 25, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Blikslager, A.T. Colic Prevention to Avoid Colic Surgery: A Surgeon’s Perspective. J. Equine Vet. Sci. 2019, 76, 1–5. [Google Scholar] [CrossRef]
- Santos, A.S.; Rodrigues, M.A.; Bessa, R.J.; Ferreira, L.M.; Martin-Rosset, W. Understanding the equine cecum-colon ecosystem: Current knowledge and future perspectives. Animal 2011, 5, 48–56. [Google Scholar] [CrossRef]
- Morrison, P.K.; Newbold, C.J.; Jones, E.; Worgan, H.J.; Grove-White, D.H.; Dugdale, A.H.; Barfoot, C.; Harris, P.A.; Argo, C.M. The equine gastrointestinal microbiome: Impacts of weight-loss. BMC Vet. Res. 2020, 16, 78. [Google Scholar] [CrossRef]
- Harris, P.A.; Ellis, A.D.; Fradinho, M.J.; Jansson, A.; Julliand, V.; Luthersson, N.; Santos, A.S.; Vervuert, I. Review: Feeding conserved forage to horses: Recent advances and recommendations. Animal 2017, 11, 958–967. [Google Scholar] [CrossRef]
- Cipriano-Salazar, M.; Adegbeye, M.J.; Elghandour, M.M.; Barbabosa-Pilego, A.; Mellado, M.; Hassan, A.; Salem, A.Z.M. The Dietary Components and Feeding Management as Options to Offset Digestive Disturbances in Horses. J. Equine Vet. Sci. 2019, 74, 103–110. [Google Scholar] [CrossRef]
- Schoster, A. Probiotic Use in Equine Gastrointestinal Disease. Vet. Clin. N. Am. Equine Pract. 2018, 34, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S. Microbiologic evaluation of commercial probiotics. J. Am. Vet. Med. Assoc. 2002, 220, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Berreta, A.; Burbick, C.R.; Alexander, T.; Kogan, C.; Kopper, J.J. Microbial Variability of Commercial Equine Probiotics. J. Equine Vet. Sci. 2021, 106, 103728. [Google Scholar] [CrossRef] [PubMed]
- Berreta, A.; Kopper, J. Equine Probiotics-What Are They, Where Are We and Where Do We Need To Go? J. Equine Vet. Sci. 2022, 115, 104037. [Google Scholar] [CrossRef]
- Schoster, A.; Weese, J.S.; Guardabassi, L. Probiotic use in horses—What is the evidence for their clinical efficacy? J. Vet. Intern. Med. 2014, 28, 1640–1652. [Google Scholar] [CrossRef]
- Weinert-Nelson, J.; Williams, C. The Equine Hindgut Microbiome. Rutgers NJAES Fact Sheet. 2023. Available online: https://njaes.rutgers.edu/e375/ (accessed on 15 July 2025).
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Bachmann, M.; Glatter, M.; Bochnia, M.; Greef, J.M.; Breves, G.; Zeyner, A. Degradation of Monosaccharides, Disaccharides, and Fructans in the Stomach of Horses Adapted to a Prebiotic Dose of Fructooligosaccharides and Inulin. J. Equine Vet. Sci. 2021, 105, 103731. [Google Scholar] [CrossRef]
- Bachmann, M.; Glatter, M.; Bochnia, M.; Wensch-Dorendorf, M.; Greef, J.M.; Breves, G.; Zeyner, A. In Vitro Gas Production from Batch Cultures of Stomach and Hindgut Digesta of Horses Adapted to a Prebiotic Dose of Fructooligosaccharides and Inulin. J. Equine Vet. Sci. 2020, 90, 103020. [Google Scholar] [CrossRef]
- Gaggia, F.; Mattarelli, P.; Biavati, B. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 2010, 141 (Suppl. S1), S15–S28. [Google Scholar] [CrossRef]
- Respondek, F.; Goachet, A.G.; Julliand, V. Effects of dietary short-chain fructooligosaccharides on the intestinal microflora of horses subjected to a sudden change in diet. J. Anim. Sci. 2008, 86, 316–323. [Google Scholar] [CrossRef]
- Glatter, M.; Borewicz, K.; van den Bogert, B.; Wensch-Dorendorf, M.; Bochnia, M.; Greef, J.M.; Bachmann, M.; Smidt, H.; Breves, G.; Zeyner, A. Modification of the equine gastrointestinal microbiota by Jerusalem artichoke meal supplementation. PLoS ONE 2019, 14, e0220553. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Y.; Li, J.; Yang, M. Editorial: Unlocking the power of gut microbiota to improving health and welfare in non-ruminant livestock. Front. Vet. Sci. 2025, 12, 1674586. [Google Scholar] [CrossRef]
- Ford, T.; McAdams, Z.L.; Townsend, K.S.; Martin, L.M.; Johnson, P.J.; Ericsson, A.C. Effect of Sugar Beet Pulp on the Composition and Predicted Function of Equine Fecal Microbiota. Biology 2023, 12, 1254. [Google Scholar] [CrossRef] [PubMed]
- McGilloway, M.; Manley, S.; Aho, A.; Heeringa, K.N.; Whitacre, L.; Lou, Y.; Squires, E.J.; Pearson, W. Dietary Fermentation Product of Aspergillus Oryzae Prevents Increases in Gastrointestinal Permeability (‘Leaky Gut’) in Horses Undergoing Combined Transport and Exercise. Animals 2023, 13, 951. [Google Scholar] [CrossRef] [PubMed]
- MacNicol, J.L.; Renwick, S.; Ganobis, C.M.; Allen-Vercoe, E.; Weese, J.S.; Pearson, W. The influence of a probiotic/prebiotic supplement on microbial and metabolic parameters of equine cecal fluid or fecal slurry in vitro. J. Anim. Sci. 2023, 101, skad034. [Google Scholar] [CrossRef] [PubMed]
- Adams, V.J.; LeBlanc, N.; Penell, J. Results of a Clinical Trial Showing Changes to the Faecal Microbiome in Racing Thoroughbreds after Feeding a Nutritional Supplement. Vet. Sci. 2022, 10, 27. [Google Scholar] [CrossRef]
- Jacobasch, G.; Schmiedl, D.; Kruschewski, M.; Schmehl, K. Dietary resistant starch and chronic inflammatory bowel diseases. Int. J. Color. Dis. 1999, 14, 201–211. [Google Scholar] [CrossRef]
- Scheppach, W.; Sommer, H.; Kirchner, T.; Paganelli, G.M.; Bartram, P.; Christl, S.; Richter, F.; Dusel, G.; Kasper, H. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology 1992, 103, 51–56. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.M.; Vanhoutvin, S.A.; Troost, F.J.; Rijkers, G.; de Bruine, A.; Bast, A.; Venema, K.; Brummer, R.J. Effect of butyrate enemas on inflammation and antioxidant status in the colonic mucosa of patients with ulcerative colitis in remission. Clin. Nutr. 2010, 29, 738–744. [Google Scholar] [CrossRef]
- Kovanda, L.; Hejna, M.; Du, T.; Liu, Y. Butyrate Derivatives Exhibited Anti-Inflammatory Effects and Enhanced Intestinal Barrier Integrity in Porcine Cell Culture Models. Animals 2025, 15, 1289. [Google Scholar] [CrossRef]
- Niederwerder, M.C. Fecal microbiota transplantation as a tool to treat and reduce susceptibility to disease in animals. Vet. Immunol. Immunopathol. 2018, 206, 65–72. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schank, N.; Cottone, A.; Wulf, M.; Seiter, K.; Thomas, B.; Miller, L.M.J.; Anderson, S.L.; Sahyoun, A.; Abidi, A.H.; Kassan, M.; et al. The Role of Short-Chain Fatty Acids (SCFAs) in Colic and Anti-Inflammatory Pathways in Horses. Animals 2025, 15, 3482. https://doi.org/10.3390/ani15233482
Schank N, Cottone A, Wulf M, Seiter K, Thomas B, Miller LMJ, Anderson SL, Sahyoun A, Abidi AH, Kassan M, et al. The Role of Short-Chain Fatty Acids (SCFAs) in Colic and Anti-Inflammatory Pathways in Horses. Animals. 2025; 15(23):3482. https://doi.org/10.3390/ani15233482
Chicago/Turabian StyleSchank, Nathan, Ashley Cottone, Michelle Wulf, Keely Seiter, Brinley Thomas, Lynda M. J. Miller, Stacy L. Anderson, Amal Sahyoun, Ammaar H. Abidi, Modar Kassan, and et al. 2025. "The Role of Short-Chain Fatty Acids (SCFAs) in Colic and Anti-Inflammatory Pathways in Horses" Animals 15, no. 23: 3482. https://doi.org/10.3390/ani15233482
APA StyleSchank, N., Cottone, A., Wulf, M., Seiter, K., Thomas, B., Miller, L. M. J., Anderson, S. L., Sahyoun, A., Abidi, A. H., Kassan, M., & Verma, A. (2025). The Role of Short-Chain Fatty Acids (SCFAs) in Colic and Anti-Inflammatory Pathways in Horses. Animals, 15(23), 3482. https://doi.org/10.3390/ani15233482








