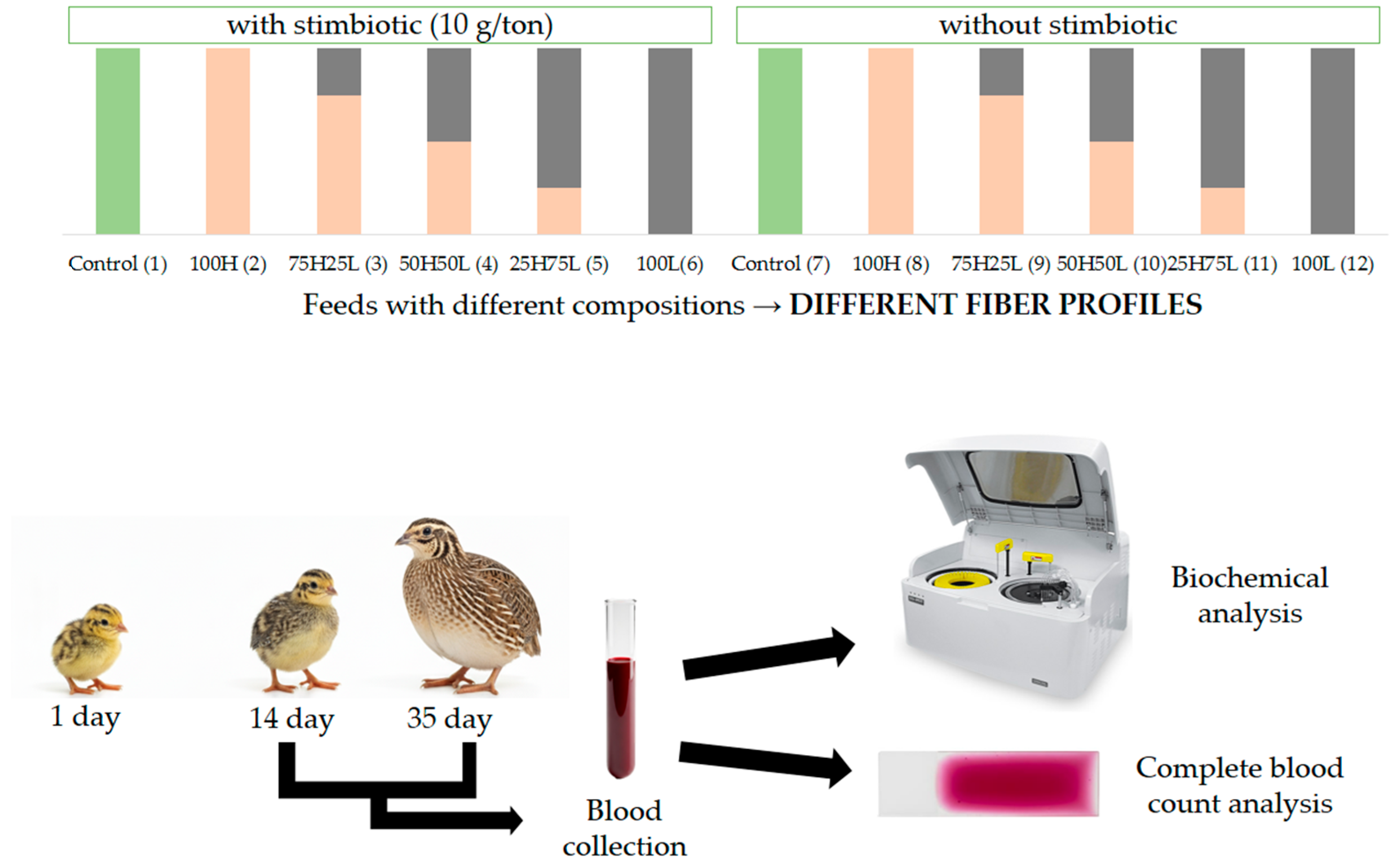
1. Introduction
Quail represent a poultry species of significant economic importance due to the superior quality of their meat, the high nutritional value of their eggs, and distinctive biological characteristics—such as early sexual maturity, elevated laying rates, and rapid growth—which render their farming a fast-return investment requiring minimal space and feed [
1]. The performance and health of these birds are contingent upon balanced diets, particularly during the initial (1–14 days) and growing (15–35 days) phases, when the gastrointestinal tract is still undergoing development. During these stages, nutritional requirements diverge from those of other poultry, characterized by a heightened demand for protein, a reduced requirement for calcium, and an increased need for energy [
2]. These specific nutritional needs, in conjunction with genetic factors (breed and strain), sex, production purpose (meat or eggs), dietary energy density, nutrient bioavailability, and environmental and sanitary conditions, underscore the necessity for tailored nutritional recommendations for quail [
3,
4].
Corn and wheat serve as the primary cereal grains utilized in poultry diets as energy sources, owing to their high starch content. However, these grains exhibit substantial differences in the content and composition of non-starch polysaccharides (NSPs). For example, wheat contains a higher total NSP content, encompassing both soluble and insoluble fractions, compared to corn [
3]. The NSP profile of wheat is predominantly composed of arabinoxylans, whereas corn contains a greater proportion of cellulose and lower levels of soluble arabinoxylans [
5].
Historically, dietary fiber was regarded as a diluting or antinutritional factor due to the presence of NSPs [
6,
7]. Nevertheless, recent years have seen a paradigm shift, with increasing recognition of its beneficial effects on gut health [
8,
9]. Since NSPs are indigestible by poultry enzymes, they undergo fermentation by the gut microbiota, resulting in the production of short-chain fatty acids that promote the proliferation of beneficial bacteria [
9,
10,
11,
12,
13,
14,
15].
In this context, stimbiotic emerges as a promising technological alternative. Comprising xylanase and xylo-oligosaccharides, it acts synergistically to enhance the degradation of non-starch polysaccharides and to stimulate beneficial microbial fermentation in the gut. This process increases the production of short-chain fatty acids, contributing to improved intestinal health, nutrient utilization, and immune function [
16,
17,
18]. Previous studies in broilers and pigs have shown positive effects of stimbiotic inclusion on growth performance, intestinal morphology, and microbiota balance. However, little is known about its physiological and metabolic effects in quails, particularly when combined with diets differing in fiber profile, which represents a critical knowledge gap that this study aims to address.
The analysis of hematological and biochemical parameters is essential for evaluating animal health. These parameters reflect the physiological state of the animal, providing critical insights into organ function, the animal’s capacity to adapt to nutritional and environmental challenges, and are indispensable for identifying metabolic imbalances and various pathologies [
19,
20].
Recent studies have demonstrated that stimbiotic inclusion positively influences gut health, productive performance, and the microbiota of broilers [
9,
14,
15,
21,
22]. However, no research has assessed these effects in meat quail, nor have investigations examined their impacts on hematological and biochemical parameters, particularly during the initial and growing phases. This omission is significant, as inclusion and the definition of diverse fiber profiles directly affect the health, welfare, and physiological responses of the birds.
Consequently, this study aimed to evaluate the effects of different dietary fiber profiles and stimbiotic inclusion on hematological and biochemical parameters of European quails from 1 to 35 days of age, to better understand how these nutritional factors influence metabolism and physiological responses.
4. Discussion
Packed cell volume (PCV), also referred to as hematocrit, denotes the proportion of blood volume occupied by erythrocytes and serves as a critical hematological indicator for evaluating tissue oxygenation and hydration status in avian species. During the growth phase (15 to 35 days) of the present study, a statistically significant increase in this variable was observed in groups supplemented with the stimbiotic. This response may be attributed to enhanced efficiency in the absorption of nutrients vital for erythropoiesis, a process potentially facilitated by the intestinal modulation induced by the additive. According to Veluri et al. [
30], the incorporation of stimbiotics in broiler diets leads to significant improvements in intestinal morphology, characterized by increased villus height, elevated villus-to-crypt ratio, and stimulation of genes associated with epithelial barrier integrity. Such alterations are instrumental in augmenting the absorption of essential minerals, including iron, phosphorus, and magnesium, which are critical for optimal hematopoietic function. Moreover, as emphasized by Martinez et al. [
31], stimbiotics play a role in fostering a more stable and functional intestinal environment, thereby optimizing nutrient digestibility. Consequently, the observed enhancement in PCV may signify a favorable physiological response to inclusion, illustrating the indirect hematological potential of these additives through improved nutritional status.
Concerning hemoglobin levels, a significant increase was observed in both experimental phases among the groups supplemented with stimbiotic, indicating a potential correlation with enhanced iron availability, likely facilitated by improved nutrient absorption attributed to this functional additive. Hemoglobin is a hematological parameter that is directly influenced by iron bioavailability, which is critical for erythrocyte production. According to Thrall et al. [
32], the physiological hemoglobin values in quail range from 4.0 to 5.2 g/L. The data obtained in the current study surpassed this range, indicating levels higher than the normal reference values.
Among the variables that remained constant across both phases were hematimetric indices, including mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), eosinophil counts, basophil counts, monocyte counts, platelet counts, and the heterophil-to-lymphocyte ratio (H/L). During the initial phase, the quail were engaged in hematopoietic and immunological development, which may have curtailed the observable physiological effects of stimbiotic inclusion. Furthermore, Garber and Parra [
33] indicate that stimbiotics exert their influence indirectly by modulating systemic immunity, primarily through the promotion of a favorable intestinal environment that reduces the activation of the immune axis in birds raised under optimal sanitary conditions. Consequently, the lack of significant alterations in certain hematological variables, as noted in the current study, may not indicate the inefficacy of the stimbiotic, but rather reflect the preservation of a physiologically balanced immunological state, negating the necessity for substantial hematopoietic recruitment.
Hematimetric indices, specifically mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC), which evaluate erythrocyte morphological characteristics and mean hemoglobin content, did not exhibit significant variations during either phase of the study. This observation suggests that stimbiotic inclusion, under the conditions examined, did not disrupt erythropoiesis or impair oxygen transport, thereby preserving physiological levels of red blood cell mass.
Total platelet counts, an essential parameter related to hemostatic function, also remained stable with the inclusion of additives during both the initial and growth phases. This finding implies that stimbiotic inclusion did not interfere with primary coagulation mechanisms or physiological platelet activation in European quail (Coturnix coturnix coturnix).
In
Table 3, concerning the initial phase (days 1 to 14), a significant variation in platelet counts was observed in relation to dietary fiber content, with higher fiber levels correlating with lower platelet counts. Platelets play a critical role in maintaining blood hemostasis through the release of thromboplastin and other factors [
34]. The association between a higher-fiber diet and reduced platelet counts may be attributable to enhanced intestinal health, which promotes nutrient absorption in an intestinal environment enriched with beneficial bacteria stimulated by the presence of fiber. Paul et al. [
35] indicated that platelets constitutively express transcripts for both pro- and anti-inflammatory cytokines, suggesting that elevated platelet levels in low-fiber diets may be linked to an inflammatory response elicited by dietary composition.
Regarding leukocyte counts, variations were observed in both experimental phases, with a consistent pattern of increased indices in the groups that were not supplemented with the stimbiotic. This response may be associated with heightened exposure to physiological and environmental stress, as changes in total leukocyte numbers in avian species are commonly linked to factors such as management practices, transportation, subclinical infections, and adverse environmental conditions [
32]. Thus, the data suggest that animals supplemented with the stimbiotic experienced reduced metabolic stress, potentially due to enhanced intestinal integrity and improved nutrient absorption.
Lymphocyte counts exhibited significant variation across both experimental phases, with a higher proportion of these cells detected in groups that did not receive a diet supplemented with the stimbiotic. Lymphocyte populations in birds can be influenced by a multitude of factors, encompassing physiological, pathological, nutritional, and environmental considerations, as these cells represent the predominant leukocyte population in the peripheral blood of avian species [
36] and play a critical role in the adaptive immune response. A comparative analysis of the results from the two experimental phases revealed that lymphocyte counts were lower in animals receiving the stimbiotic. This reduction may indicate an improved immunological status and a more effective response to pathogenic challenges, thereby underscoring the potential modulatory effect of the stimbiotic on the immune system.
Heterophile counts in birds are subject to variation due to numerous physiological, pathological, and environmental factors and are regarded as significant indicators of the immunological and health status of avian species. Notable changes were observed, with animals receiving the stimbiotic during the initial phase displaying lower heterophile values than those that did not receive the supplement. As noted by Markowiak and Śliżewska [
37], the gastrointestinal tract serves a critical immunological function and constitutes a primary barrier that protects the host from toxins, pathogens, and their inflammatory effects. The presence of a beneficial microbiota, promoted by the stimbiotic, may mitigate pathogen colonization, reduce the necessity for exaggerated inflammatory responses, and ultimately stabilize or lower heterophile counts under healthy conditions.
Regarding leukocytes, the mean values of eosinophils, basophils, and monocytes did not exhibit significant differences between the experimental groups during the two analyzed phases. The heterophil-to-lymphocyte (H/L) ratio, commonly utilized as a biomarker for stress in avian species [
38], also revealed no significant variation between treatments. Given that elevated values of this ratio indicate a heightened level of stress in birds, these findings imply that the stimbiotic, at the dosages and duration administered, did not produce a substantial modulatory effect on the stress response, at least not to a degree sufficient to alter this ratio between treatments.
In the assessment of biochemical parameters, it is essential to acknowledge that these indicators serve as critical reflections of animal physiology, offering insights into organ function, adaptability to nutritional, physiological, and environmental challenges, and aiding in the identification of metabolic imbalances and pathologies [
20]. According to [
32], investigations focusing on the hepatic system in avian species remain relatively limited, with a notable scarcity of data concerning reference values for these parameters as well as the sensitivity and specificity of the associated enzymes.
An analysis of
Table 5 regarding the initial phase indicates that the sole variable exhibiting a significant change was triglycerides. Their concentrations can fluctuate based on sex, diet, and hormonal factors [
20], particularly considering the susceptibility of birds to elevated cortisol levels, especially when subjected to stress from captive rearing systems and social grouping. Rezende et al. [
39] reported triglyceride levels in broiler chickens ranging from 128.9 to 140.2 mg/dL, while Evans et al. [
40] and Silva et al. [
41] documented values between 136 and 166 mg/dL. The values obtained in this study were lower than these referenced parameters, potentially attributable to species and age differences.
In the initial phase (1–14 days), the incorporation of various fiber profiles into the diet significantly affected serum triglyceride concentrations, with the stimbiotic demonstrating a notable impact solely on this variable. High-fiber diets, particularly those derived from whole wheat (100H) or predominantly wheat (75H:25L, 50H:50L), resulted in lower triglyceride levels compared to the control diet and the 100% corn formulation (100L). This reduction can be attributed to the higher proportion of soluble fiber found in wheat, which enhances chyme viscosity, prolongs gastric emptying, and diminishes lipid absorption, as well as potentially interfering with micellization and fatty acid absorption in the small intestine [
42,
43].
The hypolipidemic effect of soluble fiber may also be linked to increased sequestration and excretion of bile acids, compelling the liver to utilize cholesterol for the synthesis of new bile salts, thereby indirectly influencing plasma lipid metabolism [
42].
The administration of the stimbiotic resulted in elevated mean serum triglyceride levels compared to diets devoid of the additive, irrespective of the fiber profile. This increase may be associated with the modulatory effects of the stimbiotic on intestinal microbiota, which enhances the fermentation of undigested carbohydrates and elevates the production of short-chain fatty acids, particularly acetate, which serves as a precursor for hepatic lipogenesis in avian species [
44].
Although no significant interaction was noted for triglycerides, the primary effects suggest that both fiber composition and the presence of the stimbiotic independently influenced the modulation of this variable [
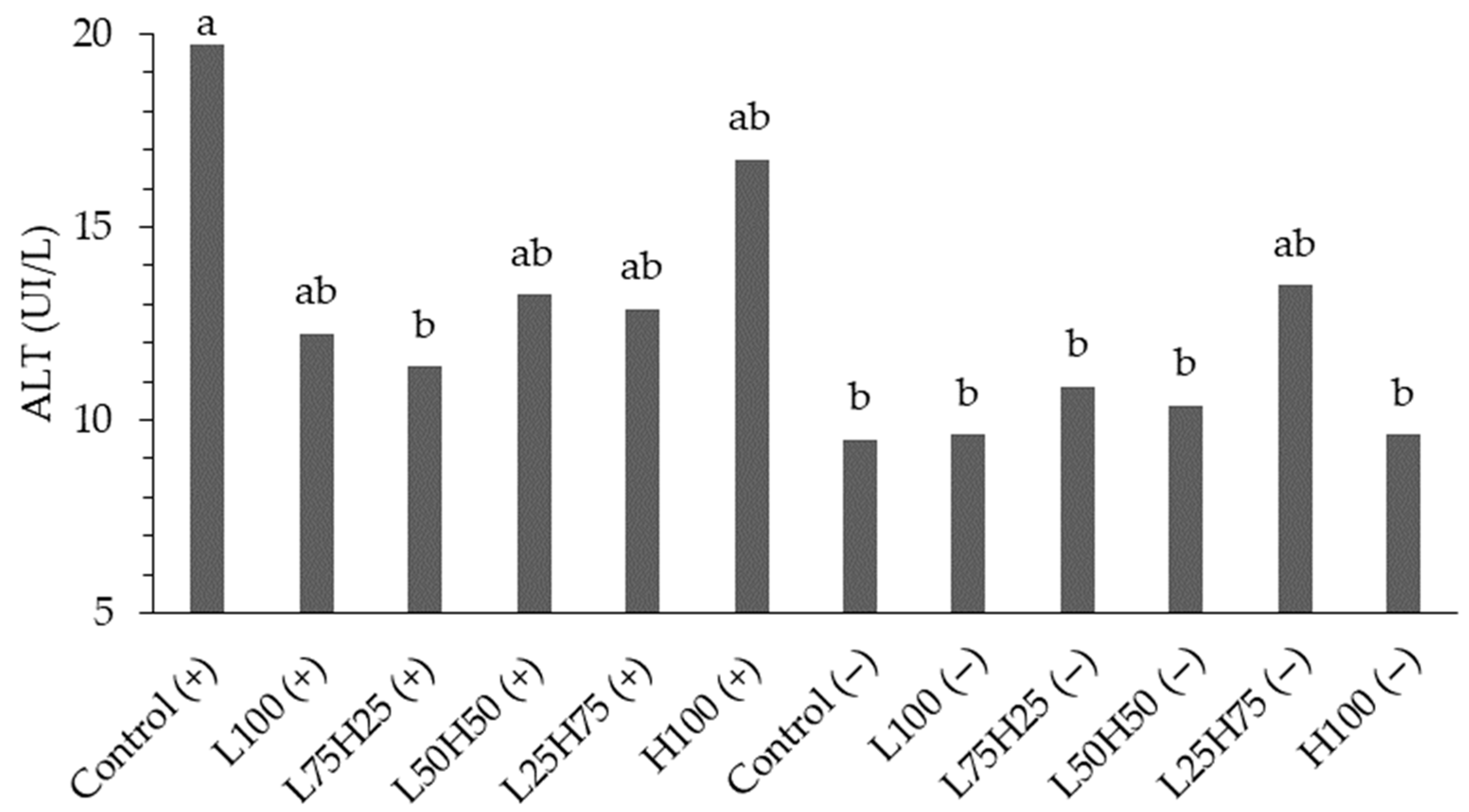
45]. The other biochemical parameters (albumin, AST, GGT, total protein, and cholesterol) did not exhibit statistically significant differences, with the exception of ALT, which displayed a significant interaction, indicating the sensitivity of this enzyme to the specific combination of fiber profile and additive.
These findings reinforce the modulatory role of dietary fiber—particularly wheat—on lipid metabolism in juvenile quails, likely associated with the immaturity of the gastrointestinal tract at this developmental stage, rendering the animals more responsive to alterations in dietary composition [
43].
A direct relationship was also observed between a high-fiber diet and increased eosinophil counts in birds during the initial phase. This observation contrasts with low-fiber diets, which exhibited lower eosinophil counts. Research conducted by Cīrule et al. [
46] suggests that this variation may be linked to stress, as eosinophil numbers tend to decline under stressful conditions. The underlying mechanism posits that fibers, when hydrolyzed into xylo-oligosaccharides, encourage microbial fermentation and the production of volatile fatty acids. As detailed by Parra et al. [
16], these acids enhance digestion and nutrient absorption. This improvement in the digestive process may mitigate stress in the animals, thereby elucidating the higher eosinophil levels observed in high-fiber diets.
When assessing the interaction with the ALT variable, it is crucial to recognize that dietary fiber plays a significant role in the metabolic and digestive health of avian species. In the case of the European quails analyzed, fiber sources such as wheat and corn possess attributes that directly influence ALT levels. Interpreting the increase in serum ALT concentration presents challenges, as it may stem from tissue damage [
47] or from physiological variations, such as aging, which can elevate ALT values [
32].
Wheat fiber, primarily composed of soluble and insoluble fractions, exhibits a higher fermentation capacity in the intestine, potentially resulting in the production of volatile fatty acids that are advantageous to the intestinal mucosa and overall metabolism. According to Faria et al. [
20], avian species with significant liver damage may present normal or even reduced ALT values, signifying that the enzymatic activity of ALT in the hepatic tissue of certain species may be diminished. Most avian species demonstrate serum ALT values ranging from 19 to 50 IU/L [
48,
49].
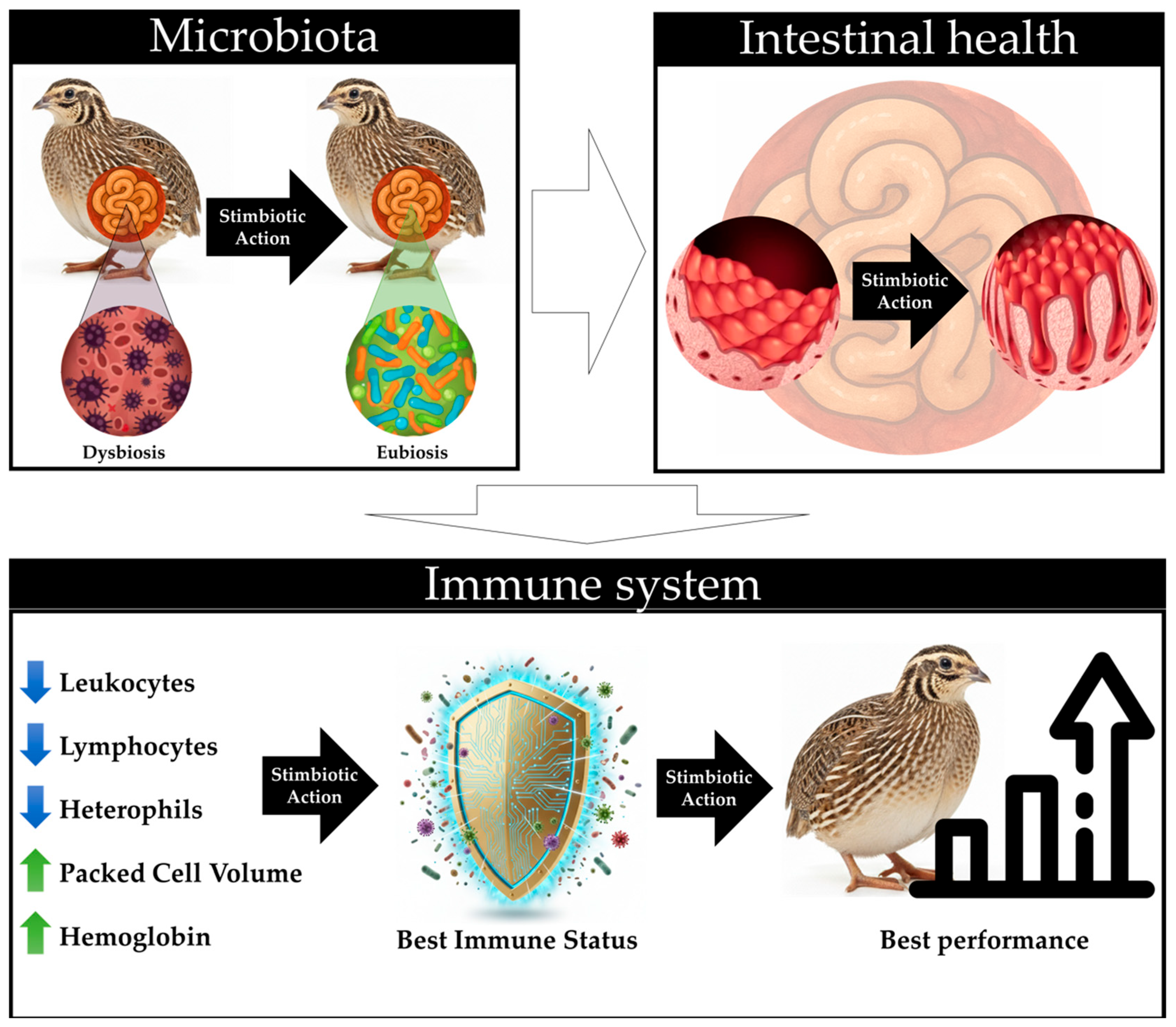
Previous studies have indicated that inclusion with stimbiotics enhances productive performance, feed efficiency, intestinal microbiota composition, and gut health in broiler chickens [
9,
14,
15,
21,
22]. Although the present study did not directly assess these parameters, the hematological and biochemical findings suggest an enhancement in the immunological status of quails, as evidenced by reductions in leukocytes, lymphocytes, and heterophils, alongside increases in packed cell volume and hemoglobin. These results imply that the stimbiotic likely induced effects similar to those reported in prior studies, fostering a healthier intestinal environment, optimizing nutrient absorption, and enhancing the physiological and immunological balance in the birds (
Figure 4).
The findings of the present study indicate that inclusion with a stimbiotic and variations in fiber profiles independently modulated hematological and biochemical parameters in broiler quails. The inclusion of the stimbiotic resulted in increases in packed cell volume and hemoglobin levels, alongside reductions in leukocytes, lymphocytes, and heterophils, which suggest a decrease in physiological stress and an improvement in immunological balance. Conversely, the introduction of different fiber profiles independently affected specific variables, such as platelet counts and triglyceride levels, thereby underscoring their significance in intestinal and lipid metabolism. Although productive performance and gut health were not explicitly assessed, the results imply that the stimbiotic may have elicited effects analogous to those previously documented in broiler chickens, enhancing nutrient absorption, physiological equilibrium, and the maintenance of a healthy immunological status in the subjects.