1. Introduction
Weaning is a critical period in the early life of pigs, typically occurring around 21 days of age when the intestinal tract, immune system, and intestinal microbiota are still immature [
1,
2,
3]. The abrupt transition from maternal milk to solid feed, along with environmental and social stressors, often leads to weaning stress syndrome, which is characterized by intestinal barrier dysfunction, microbial imbalance, diarrhea, and reduced growth performance [
4,
5].
Pharmacological doses of ZnO have traditionally been used to alleviate post-weaning diarrhea due to their antimicrobial and anti-inflammatory effects [
6,
7,
8]. However, environmental concerns about zinc accumulation in soil and water have led to restrictions on its use in many countries [
9,
10]. This shift underscores the need for effective and sustainable nutritional strategies to support gut health during the post-weaning period. Niacinamide, the amide form of niacin, plays an essential role in energy metabolism and cell repair [
11,
12]. Recent evidence suggests that niacinamide can enhance intestinal morphology, improve epithelial barrier integrity, and promote microbial balance [
13,
14,
15], making it a potential nutritional candidate to mitigate weaning-associated intestinal dysfunction.
Concurrently, low-protein diets have been increasingly adopted to reduce nitrogen excretion and control post-weaning diarrhea by limiting undigested protein fermentation in the hindgut of nursery pigs [
16,
17,
18]. However, lowering dietary protein often reduces soybean meal inclusion. Soybean meal serves as the main source of available niacin, with approximately 34 mg/kg of free niacin [
19,
20]. Cereal grains such as maize, commonly used in such diets, contain niacin mainly in a bound, poorly available form [
21,
22]. Negative impacts of a low-protein diet on Zn absorption and retention were also observed [
23,
24]. As a result, nursery pigs on low-protein diets may face an increased risk of suboptimal niacin intake and zinc deficiency.
Previous studies have shown that niacin or niacinamide supplementation can improve intestinal morphology, antioxidant capacity, microbial composition, and immune function in nursery pigs [
14,
25,
26]. However, most of these studies were conducted under standard protein conditions and used limited dosage levels. Thus, the optimal dietary level under more challenging nutritional conditions is not clear. There is a lack of comprehensive dose–response evaluations of niacinamide in low-protein diets, especially under different Zn levels.
Therefore, we hypothesized that the current niacin requirement may be insufficient for nursery pigs fed low-protein diets with no Zn supplementation. Increasing niacinamide level may help compensate for reduced niacin availability and the potential Zn deficiency, further enhancing intestinal health and unleashing growth potential. To test our hypothesis, two experiments were conducted to determine the functional requirements and optimal levels of dietary niacinamide in nursery pigs fed low-protein diets with or without ZnO supplementation. The effects of increasing niacinamide supplementation on intestinal morphology and microbial composition, as well as growth performance, were evaluated. The potential optimized niacinamide level based on these evaluations is suggested for the practical feeding systems.
4. Discussion
This research offers novel perspectives on the functional demand for dietary niacinamide in nursery pigs consuming low-protein diets supplemented with or without pharmacological ZnO [
42]. Although niacin is known to support energy metabolism, redox regulation, and epithelial integrity [
11,
12], its optimal inclusion level under practical feeding strategies remains unclear. Our findings demonstrate that nursery pigs responded to niacinamide in a dose-dependent manner and that the requirement increased in the absence of ZnO. Moreover, the beneficial effects of niacinamide not only improved intestinal morphology, microbial composition, fermentation products, systemic immunity, and host metabolism but also promoted growth performance. These findings support our hypothesis that niacinamide contributes to maintaining intestinal function and physiological homeostasis in nursery pigs, particularly under the current dietary Zn level. The following sections discuss these effects in detail, with a focus on dose-dependent responses across intestinal morphology, microbial composition, fermentation products, metabolic pathways, and systemic biomarkers, as well as growth performance. To compensate for the limited number of replicates, we have verified that consistent results were obtained from the two experiments in growth performance and blood indices, whether the pigs were fed individually or in groups. These results appeared to be supported by the antioxidant markers and intestinal morphology measured in Exp. 2.
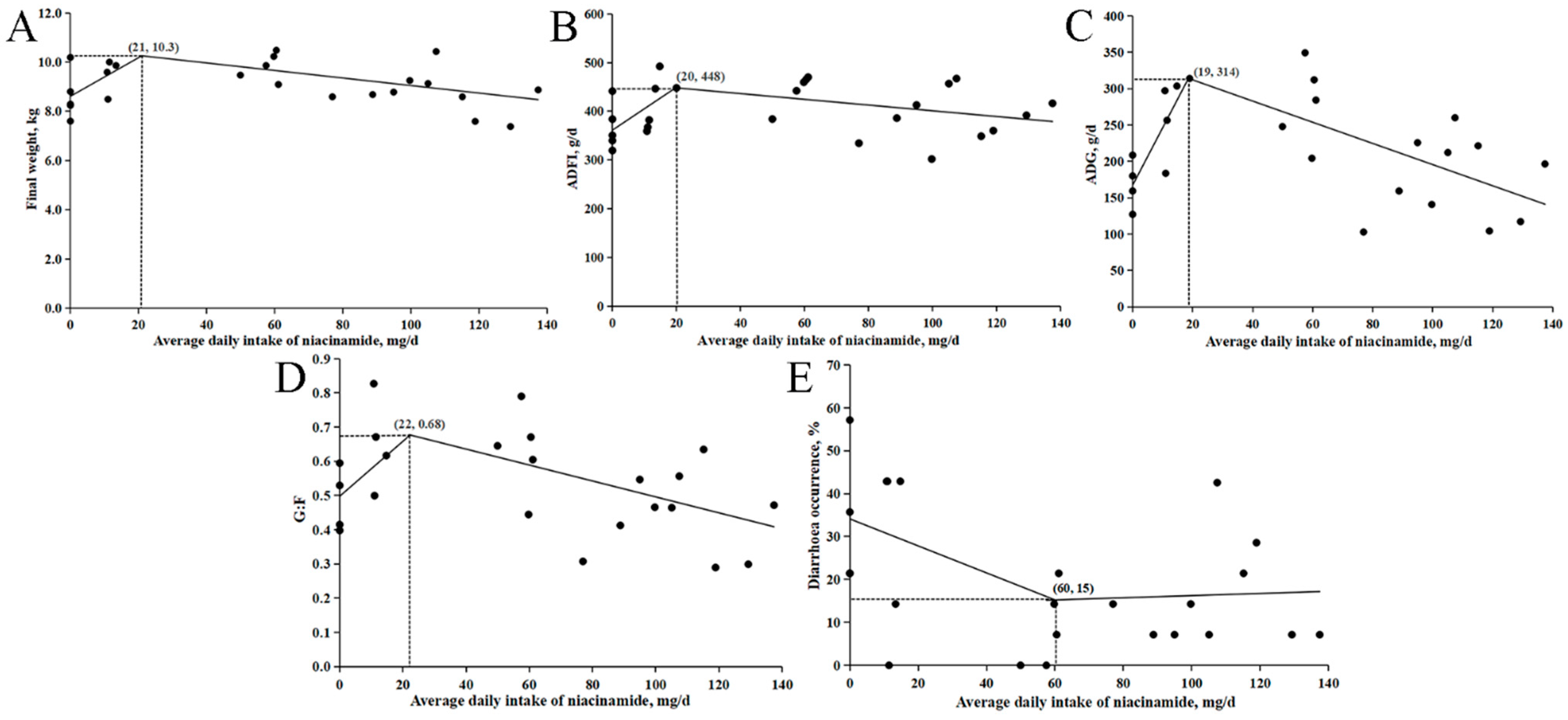
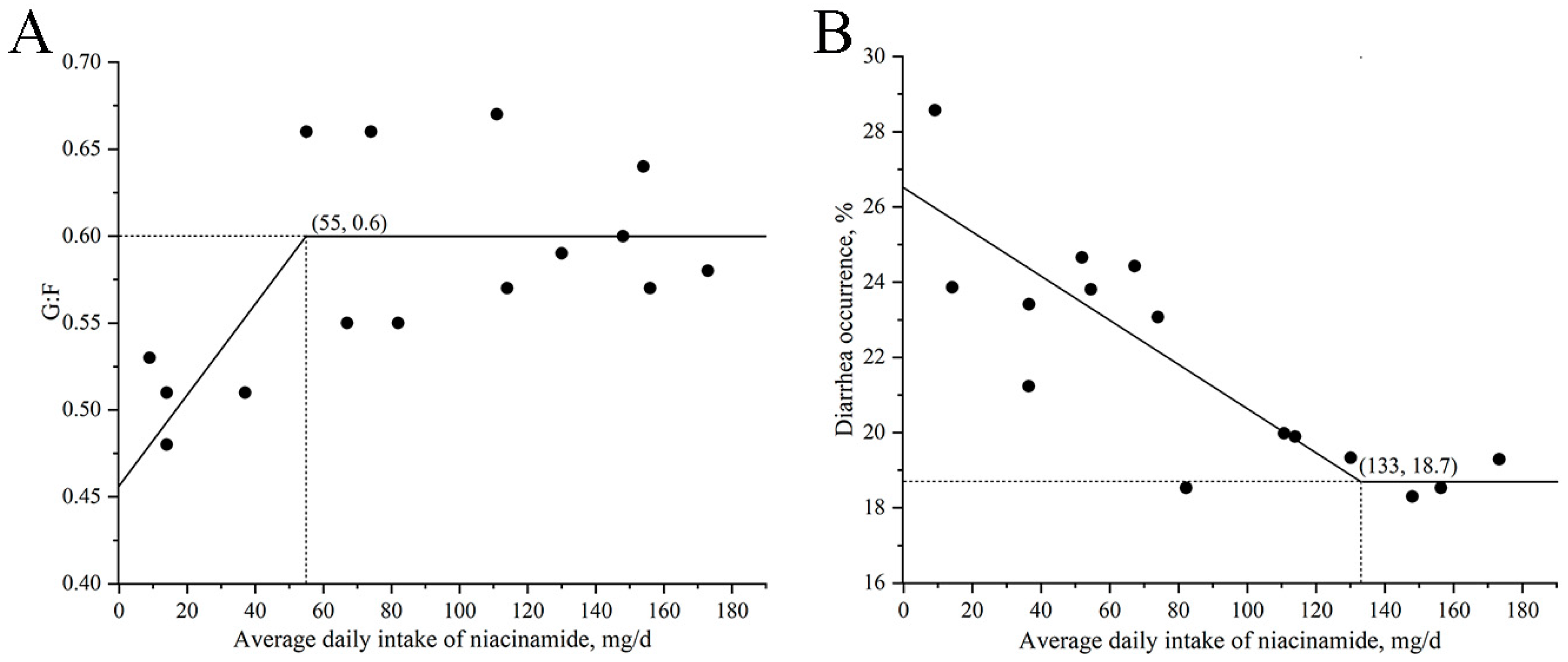
Beneficial responses of weaned pigs in the growth performance to dietary supplementation of niacinamide were observed in both experiments. However, the response patterns to the dose of the supplementation and the estimated optimal levels for niacinamide from pigs with supplemented dietary ZnO were different from those without supplemented ZnO. When ZnO was included in the low-protein diets, BW, ADG, ADFI, and G:F increased quadratically, with peak responses observed at approximately 50 mg/kg of niacinamide. These results suggest that the niacinamide level supplemented to a low-protein diet for optimal growth performance in nursery pigs is associated with the dietary Zn level. This is probably due to the negative impact of a low-protein diet on Zn absorption and efficiency [
23,
24]. Indeed, the G:F was improved linearly with the increase in niacinamide supplementation, with the highest efficiency observed at 130 mg/kg, in Exp. 2 with no ZnO addition. Moreover, the diarrhea occurrence decreased progressively with an increase in niacinamide, reaching a lowest level at approximately 315 mg/kg. These findings indicate that, with no supplementation of ZnO, higher levels of niacinamide are required to sustain feed efficiency and intestinal stability. According to Wu et al., supplementing 360 mg/kg niacinamide in low-protein diets reduced fecal, urinary, and total nitrogen excretion without impairing nitrogen retention or ADG in growing pigs (BW = 40 kg) [
43]. Similarly, Feng et al. observed that feeding 40 mg/d of niacin for three days effectively attenuated the rate of weight loss and diarrhea in weaned piglets under starvation stress [
44]. Collectively, the dose–response curves from both experiments and results reported in previous studies suggest that the dietary requirement of niacinamide is affected by dietary protein level and the growth stage of the animal, in which the dietary composition, especially the availability of functional compounds such as Zn, may play an important role. These findings highlight the importance of evaluating the effect of Zn availability on current niacin recommendations under low-protein diets.
The observed improvements in growth performance were accompanied by positive changes in indicators of intestinal health, suggesting that niacinamide’s beneficial effects are closely linked to its role in maintaining intestinal function. During the early post-weaning period, pigs often experience impaired intestinal structure, microbial imbalance, and immune disruption, all of which compromise nutrient utilization and growth efficiency [
45,
46]. Therefore, strategies that promote intestinal development and stabilize the intestinal environment are essential for supporting performance under weaning stress.
Previous studies have shown that niacinamide supplementation contributes to intestinal barrier integrity, epithelial regeneration, and mucosal immunity in pigs [
13,
14,
15,
47]. Consistently, the present study demonstrated that niacinamide reduced diarrhea scores, improved intestinal morphology, enriched beneficial microbial taxa, and modulated microbial fermentation. These coordinated improvements suggest that the intestine is a primary site of niacinamide action, particularly under conditions with no ZnO supplementation, where intestinal health is more vulnerable.
Niacinamide supplementation reduced diarrhea severity in both experiments, showing a clear dose-dependent pattern. In Exp. 1, conducted under ZnO-supplemented conditions, diarrhea occurrence decreased progressively, reaching the lowest levels at approximately 140 mg/kg. In contrast, under ZnO-free conditions in Exp. 2, diarrhea occurrence linearly reduced throughout the whole tested period, with a minimum at around 315 mg/kg. These findings indicate that higher levels of niacinamide are needed to stabilize intestinal function in the absence of ZnO supplementation. The reduction in diarrhea could be attributed to the improved mucosal barrier function, enhanced microbial stability, or modulation of local immune responses, all of which are known targets of niacinamide action [
48,
49]. Although some of these parameters were evaluated in this study, further studies are warranted to elucidate the underlying mechanisms. Similar effects were reported in previous studies, where dietary niacin supplementation linearly decreased fecal scores and intestinal inflammation in weaned pigs [
43,
47] and the incidence of diarrhea under protein-restricted conditions from high-level (330 mg/kg) compared to the low-level niacinamide (30 mg/kg) supplementation [
23]. These findings highlight the protective role of niacinamide in maintaining intestinal stability and suggest that post-weaning diarrhea severity may serve as a sensitive indicator of the functional requirement for this vitamin across different dietary regimens.
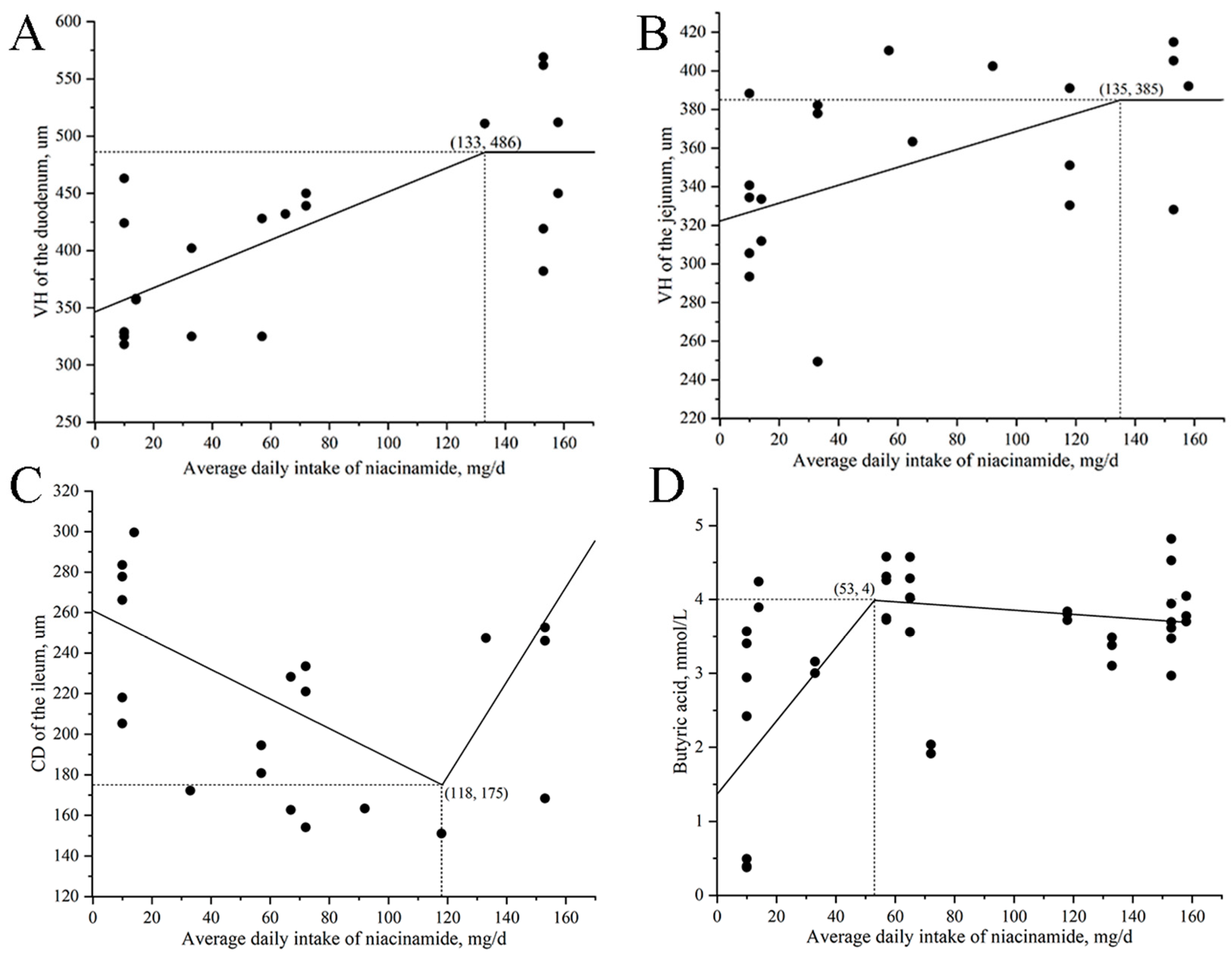
Supplementation with 315 mg/kg of nicotinamide maximized VH in the duodenum and jejunum, while 280 mg/kg minimized CD in the ileum, suggesting that adding an appropriate amount of nicotinamide enhances intestinal absorptive surface area and promotes intestinal maturation. These findings are consistent with the reports in previous studies, demonstrating that niacin or its derivatives support epithelial turnover and preserve mucosal barrier integrity [
40,
50]. For instance, dietary niacin linearly increased jejunal VH, CD, and villus width in weaned pigs at 25 days of age. It promoted the expression of key genes involved in nutrient transport and epithelial integrity, including SLC5A1, SLC15A1, SLC6A19, and tight junction proteins such as occludin and claudin-1 after 14 days of feeding a diet with niacin supplementation [
50]. These effects may be linked to niacinamide’s role in regulating epithelial barrier function, electrolyte transport, and fluid absorption. Taken together, these results suggest that niacinamide supplementation benefits weaned pigs with post-weaning intestinal development and may serve as an effective nutritional strategy to enhance mucosal adaptation, particularly using a low-protein diet with no Zn supplementation.
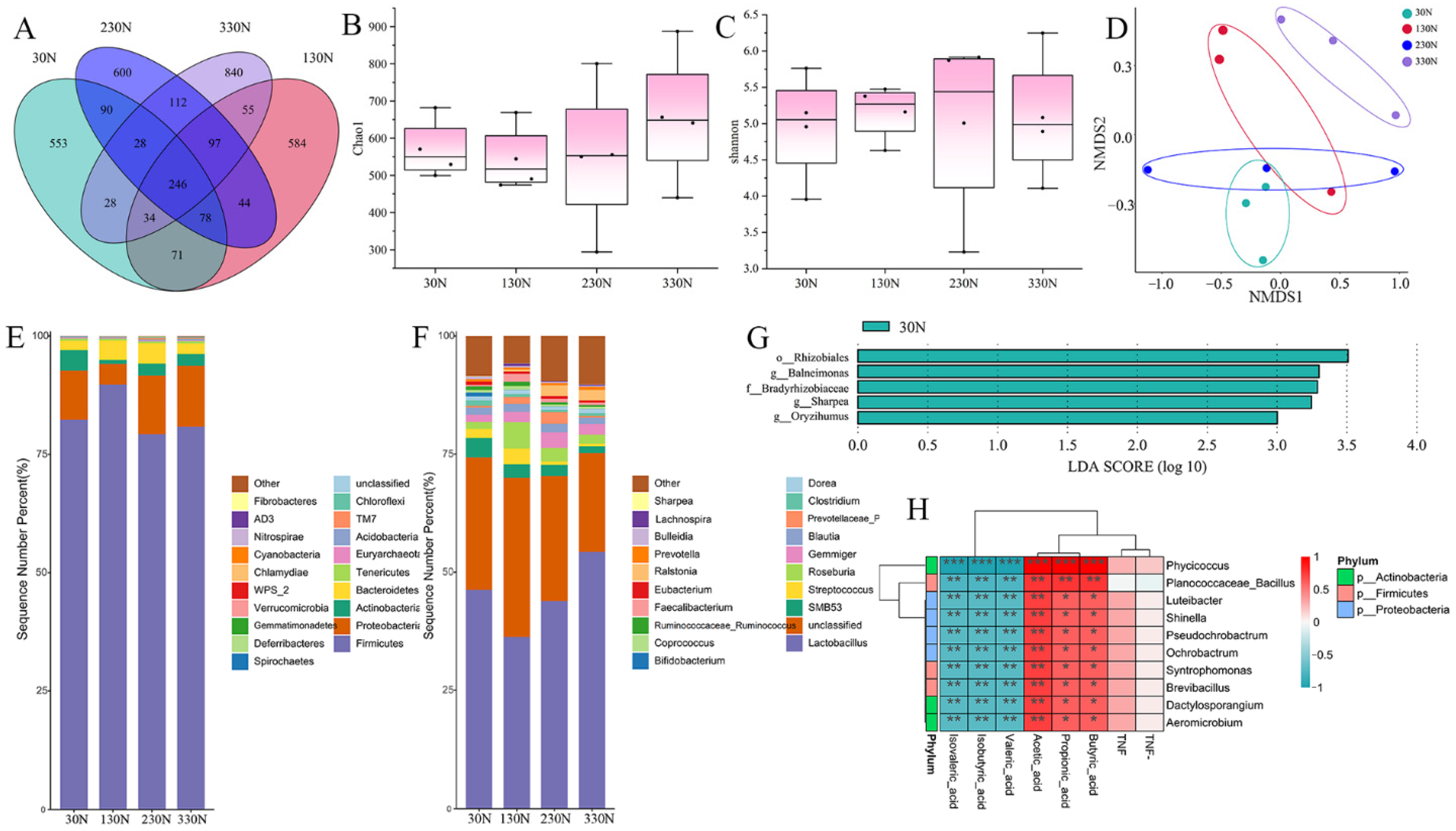
Niacinamide supplementation altered the composition of the intestinal microbiota, characterized by a heightened proportional prevalence of
Lactobacillus and reduced abundance of
Streptococcus in the 330N group. These microbial shifts are considered beneficial for maintaining intestinal homeostasis.
Lactobacillus species are known to enhance epithelial function, produce organic acids, and inhibit pathogenic bacteria [
51,
52]. In contrast, elevated levels of
Streptococcus have been associated with intestinal inflammation and post-weaning diarrhea in pigs [
53,
54]. These microbial shifts might contribute to the observed improvements in villus structure and reduction in diarrhea severity. Consistent with our findings, dietary niacin influences microbial structure and function in the intestine, which has also been reported in previous studies [
47,
55]. For example, Liu et al. [
47] found that niacin supplementation elevated the proportional prevalence of
Lactobacillus and
Dorea, whereas it diminished the proportional prevalence of
Peptococcus in the colon, compared with a multi-probiotic product. Collectively, these results suggest that niacinamide promotes a more favorable microbial community, thereby enhancing intestinal resilience during the critical weaning period.
In addition to microbial shifts, niacinamide modulated colonic fermentation activity, as reflected by changes in SCFAs profiles. Acetate concentrations increased linearly with the increased niacinamide levels, while butyrate peaked at 125 mg/kg and did not increase further at higher doses. The rise in acetate may be attributed to the expansion of acetate-producing bacteria such as
Lactobacillus, which were enriched in response to niacinamide [
51,
52]. Acetate plays critical roles in epithelial barrier maintenance, anti-inflammatory signaling, and serves as an energy substrate for peripheral tissues [
56]. The consistent rise in acetate suggests a more active and stable microbial fermentation pattern with higher niacinamide intake. In contrast, the non-linear response of butyrate may be due to the limited responsiveness of key butyrate-producing taxa, primarily obligate anaerobes such as
Ruminococcaceae and
Lachnospiraceae, to niacinamide [
57,
58]. Additionally, enhanced villus development could accelerate butyrate uptake by colonocytes, limiting its accumulation in luminal contents despite continued production. Given the well-established roles of SCFAs in maintaining intestinal integrity and immune regulation, these fermentation changes likely contributed to the suggested improvement in intestinal morphology and reduced diarrhea in this study.
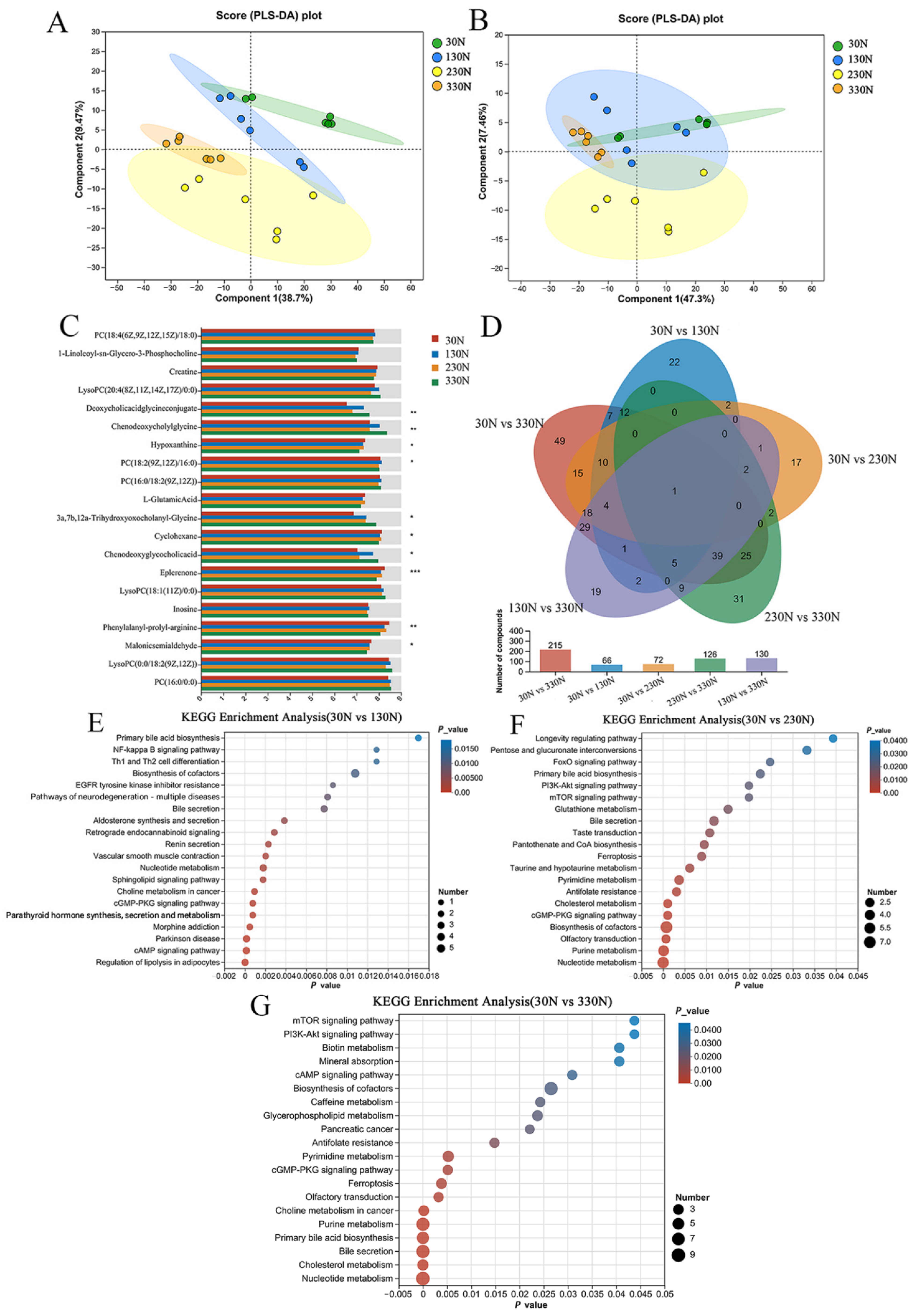
Metabolomic profiling of colonic tissue revealed that niacinamide supplementation influenced several host metabolic pathways, including bile acid metabolism and purine metabolism in the 330N group. These changes indicate that niacinamide not only acts as a dietary precursor of NAD
+ biosynthesis but also modulates host-microbe metabolic crosstalk at the intestinal level [
59]. Specifically, glycocholic acid and taurocholic acid concentrations increased in a dose-dependent manner, while chenodeoxyglycocholic acid was elevated in the 330N compared to the 30N and 230N groups. KEGG pathway enrichment analysis further confirmed the upregulation of primary bile acid biosynthesis in this group. Bile acids are known to exert direct antimicrobial effects and influence microbial composition through signaling pathways such as Takeda G-protein-coupled receptor 5 (TGR5) and farnesoid X receptor (FXR) activation [
60]. Moreover, bile salt hydrolase activity, common in
Bacteroides,
Eubacterium, and
Lactobacillus, promotes deconjugation of bile acids, increasing their hydrophobicity and antimicrobial potency [
61]. These effects may underline the observed enrichment of
Lactobacillus and suppression of
Streptococcus in high-niacinamide groups. Additionally, enhanced purine metabolism may support epithelial renewal, as purine derivatives such as hypoxanthine and guanine are essential for DNA synthesis in rapidly proliferating intestinal cells [
62,
63]. These findings suggest that dietary niacinamide modulates both microbial activity and host epithelial metabolism through multiple metabolic pathways, thereby contributing to improved intestinal health.
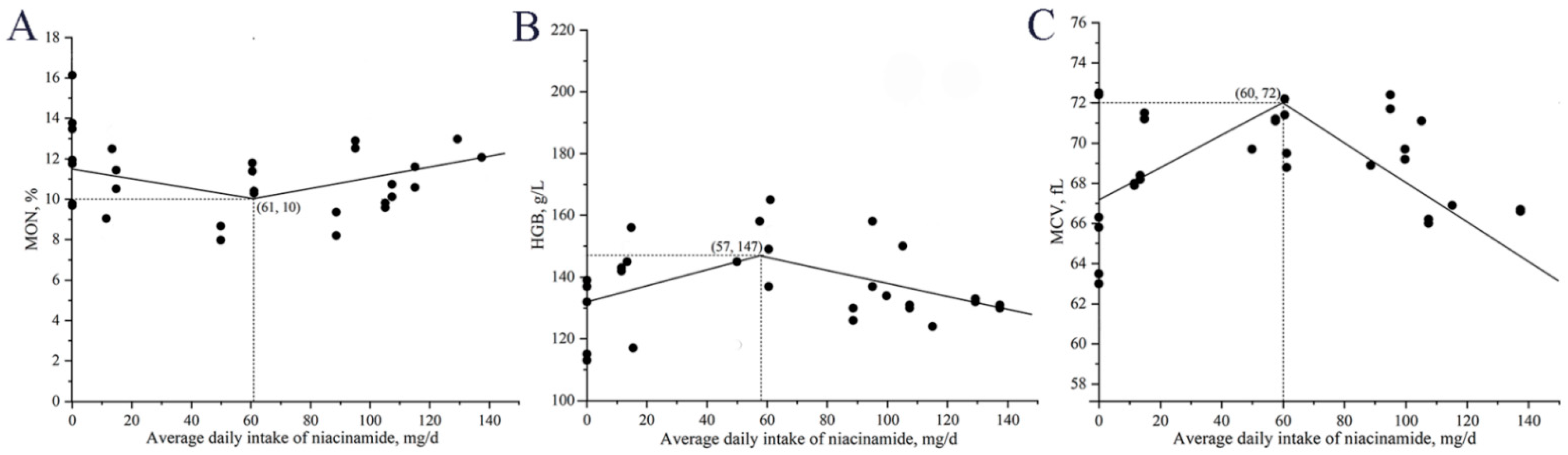
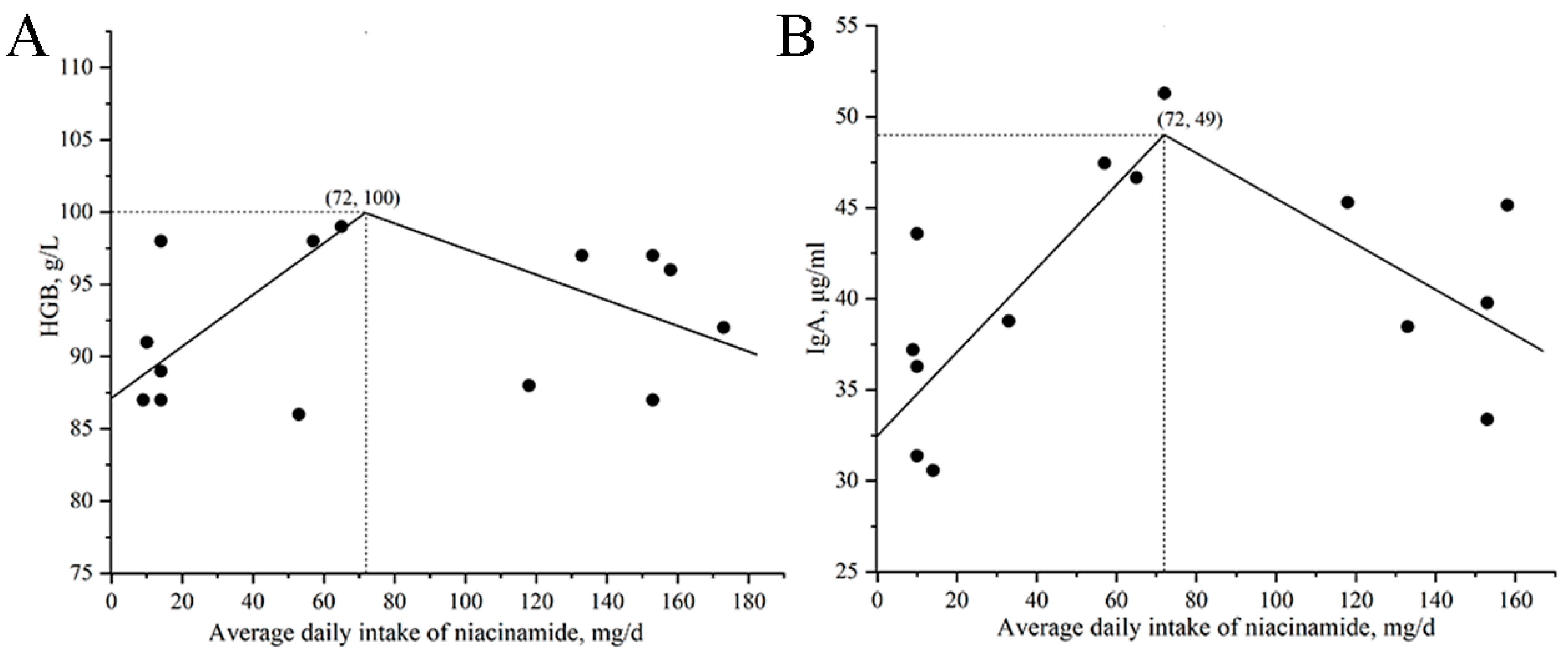
Beyond local intestinal effects, niacinamide supplementation also altered several systemic blood biomarkers associated with immune regulation and nutritional status. In Exp. 1, monocyte counts increased quadratically, peaking at 140 mg/kg, suggesting enhanced innate immune responses. In Exp. 2, serum IgA concentrations increased with niacinamide supplementation, reaching the highest level at 170 mg/kg. As a key component of humoral immunity, IgA binds to antigens and helps modulate immune responses [
64]. The increase in IgA indicates that niacinamide may support B-cell maturation and contribute to immune homeostasis during the post-weaning period [
65]. Hematological indicators related to oxygen transport also responded positively. Hemoglobin concentrations increased quadratically in both experiments, with the peak at 140 mg/kg for the number of monocytes in Exp. 1 and 170 mg/kg for serum IgA in Exp. 2. Mean corpuscular volume also showed a quadratic increase, reaching its maximum at 140 mg/kg in Exp. 1. These improvements suggest more efficient oxygen delivery and energy metabolism, which are critical for sustaining growth in nursery pigs [
66]. The increase in effective dose required for a diet with no ZnO supplementation confirmed that Zn availability is important for the efficiency of niacinamide supplementation in the diet of nursery pigs, especially in the low-protein diet.
In summary, the improvements in growth performance observed in this study can be attributed to coordinated enhancements in intestinal health, optimization in microbial ecology, and homeostasis in systemic physiological function. These beneficiaries of niacinamide supplementation are supported by the reduced diarrhea, which tended to improve villus morphology, increased beneficial bacteria such as Lactobacillus, and promoted the production of SCFAs, particularly acetate and butyrate, in this study. These changes fully explain that niacinamide supplementation promotes a more stable and functional intestinal environment. In addition, results from the metabolomic analysis of colonic tissue revealed that niacinamide upregulated bile acid and purine metabolic pathways, thereby enhancing host–microbiota interactions and epithelial renewal. Furthermore, increased HGB, MCV, and IgA levels in circulation further reflected the improvements in nutrient delivery and immune competence. Therefore, these outcomes indicate that niacinamide supports nursery pig growth through multiple interrelated mechanisms.
The different peak responses obtained from the two experiments suggest that the supplemental level of niacinamide in low-protein diets for optimal benefits will be affected by dietary Zn level. Because protein is a major source of dietary Zn and the absorption of Zn is associated with dietary protein level and Zn availability [
23,
24], the presence of ZnO in the diet increased Zn availability, improved intestinal stability, and reduced weaning stress, thereby reducing the amount of niacinamide required for the peak responses. The negative impact of a low-protein diet on Zn absorption and utilization was also recently reported in broiler chicks [
67]. Therefore, these findings highlight that dietary supplementation of niacinamide for optimal benefits is affected by other nutrients in the diet. Current nutritional recommendations may underestimate niacin needs for optimizing performance in nursery pigs, particularly the use of a low-protein diet, in which Zn may play an important role in compensatory functions and mechanisms of niacinamide.