Effects on Rumen Microbial Population and Serum Biochemical Responses to Guanidinoacetic Acid, Ampelopsis grossedentata Flavonoids, and 5,6-Dimethylbenzimidazole Plus Cobalt in Lanping Black-Boned Sheep
Simple Summary
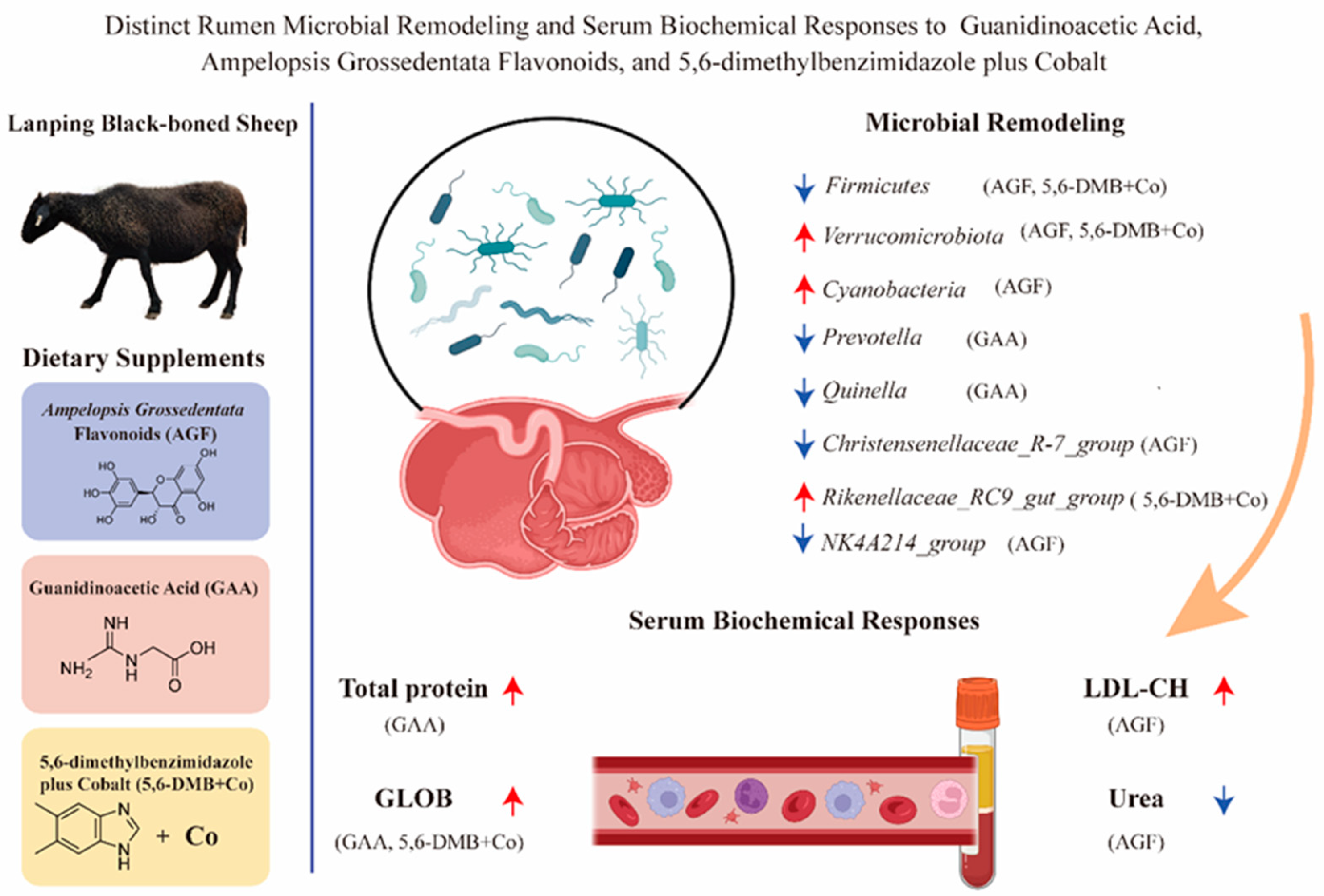
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals, Housing Conditions, and Experimental Design
2.3. Blood Sample Collection and Biochemical Analysis
2.4. Rumen Fluid Collection and Microbial Analysis
2.5. Statistical Analysis
3. Results
3.1. Effects of Dietary Additives on Serum Biochemical Parameters in Lanping Black-Boned Sheep
3.2. Effects of Dietary Additives on Rumen Fluid pH in Lanping Black-Boned Sheep
3.3. Dietary Additives Enhance Rumen Microbial Richness and Diversity in Lanping Black-Boned Sheep
3.4. Differential Remodeling of Rumen Bacterial Composition by Dietary Additives in Lanping Black-Boned Sheep
3.5. Associations Between Rumen Bacterial Genera and Serum Biochemical Parameters in Lanping Black-Boned Sheep
3.6. Differential Bacterial Biomarkers Identified by LEfSe Analysis in Lanping Black-Boned Sheep
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palmonari, A.; Federiconi, A.; Formigoni, A. Animal board invited review: The effect of diet on rumen microbial composition in dairy cows. Anim. Int. J. Anim. Biosci. 2024, 18, 101319. [Google Scholar] [CrossRef] [PubMed]
- Raabis, S.; Li, W.; Cersosimo, L. Effects and immune responses of probiotic treatment in ruminants. Vet. Immunol. Immunopathol. 2019, 208, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Inguglia, E.S.; Song, Z.; Kerry, J.P.; O’Sullivan, M.G.; Hamill, R.M. Addressing clean label trends in commercial meat processing: Strategies, challenges and insights from consumer perspectives. Foods 2023, 12, 2062. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, H.O.; Shikoray, L.; Mohamed, M.I.; Habib, I.; Matsumoto, T. Veterinary drug residues in the food chain as an emerging public health threat: Sources, analytical methods, health impacts, and preventive measures. Foods 2024, 13, 1629. [Google Scholar] [CrossRef]
- Arowolo, M.A.; He, J. Use of probiotics and botanical extracts to improve ruminant production in the tropics: A review. Anim. Nutr. 2018, 4, 241–249. [Google Scholar] [CrossRef]
- Fan, Y.; Cheng, Z.; Liu, B.; Hu, X.; Ali, M.; Long, C. An ethnobiological study on traditional knowledge associated with black-boned sheep (Ovis aries) in Northwest Yunnan, China. J. Ethnobiol. Ethnomedicine 2022, 18, 39. [Google Scholar] [CrossRef]
- Xiong, H.; He, X.; Li, J.; Liu, X.; Peng, C.; Xi, D.; Deng, W. Genetic diversity and genetic origin of Lanping black-boned sheep investigated by genome-wide single-nucleotide polymorphisms (SNPs). Arch. Anim. Breed. 2020, 63, 193–201. [Google Scholar] [CrossRef]
- Aminullah, N.; Mostamand, A.; Zahir, A.; Mahaq, O.; Azizi, M.N. Phytogenic feed additives as alternatives to antibiotics in poultry production: A review. Vet. World 2025, 18, 141–154. [Google Scholar] [CrossRef]
- Ibeagha-Awemu, E.M.; Omonijo, F.A.; Piche, L.C.; Vincent, A.T. Alternatives to antibiotics for sustainable livestock production in the context of the One Health approach: Tackling a common foe. Front. Vet. Sci. 2025, 12, 1605215. [Google Scholar] [CrossRef]
- Asiriwardhana, M.; Bertolo, R.F. Guanidinoacetic acid supplementation: A narrative review of its metabolism and effects in swine and poultry. Front. Anim. Sci. 2022, 3, 972868. [Google Scholar] [CrossRef]
- Wang, H.; Li, L.; Yuan, X.; Hong, L.; Pu, L.; Qin, S.; Li, L.; Yang, H.; Zhang, J. Effect of guanidinoacetic acid on the growth performance, serum biochemical indices and metabolites, intestinal morphology, and intestinal flora of Cherry Valley broiler ducks. Poult. Sci. 2025, 104, 105471. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Wang, J.; Ye, B.; Yi, X.; Abudukelimu, A.; Wu, H.; Meng, Q.; Zhou, Z. Guanidinoacetic acid and methionine supplementation improve the growth performance of beef cattle via regulating the antioxidant levels and protein and lipid metabolisms in serum and liver. Antioxidants 2025, 14, 559. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, R.C.V.; Ye, L.; Baek, N.; Teixeira, G.H.A.; O’Keefe, S.F. Vine tea (Ampelopsis grossedentata): A review of chemical composition, functional properties, and potential food applications. J. Funct. Foods 2021, 76, 104317. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, F.; Li, M.; Li, R.; Zhang, Z.; Xu, J.; Wen, L.; Li, R. Supplementation of Ampelopsis grossedentata extract contributes to the improvement of intestinal health in swine. Front. Vet. Sci. 2024, 11, 1417309. [Google Scholar] [CrossRef]
- Wang, H.; Zhan, J.; Zhao, S.; Jiang, H.; Jia, H.; Pan, Y.; Zhong, X.; Huo, J. Microbial-metabolomic exploration of tea polyphenols in the regulation of serum indicators, liver metabolism, rumen microorganisms, and metabolism in Hu sheep. Animals 2024, 14, 2661. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, X.; Lu, Y.; Yue, D.; He, X.; Deng, W.; Zhao, S.; Xi, D. Exploring the impact of Ampelopsis grossedentata flavonoids on growth performance, ruminal microbiota, and plasma physiology and biochemistry of kids. Animals 2023, 13, 2454. [Google Scholar] [CrossRef]
- Tiffany, M.E.; Fellner, V.; Spears, J.W. Influence of cobalt concentration on vitamin B12 production and fermentation of mixed ruminal microorganisms grown in continuous culture flow-through fermentors. J. Anim. Sci. 2006, 84, 635–640. [Google Scholar] [CrossRef]
- Brito, A.; Chiquette, J.; Stabler, S.P.; Allen, R.H.; Girard, C.L. Supplementing lactating dairy cows with a vitamin B12 precursor, 5,6-dimethylbenzimidazole, increases the apparent ruminal synthesis of vitamin B12. Animal 2015, 9, 67–75. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Bergamaschi, M.; Tiezzi, F.; Howard, J.; Huang, Y.J.; Gray, K.A.; Schillebeeckx, C.; McNulty, N.P.; Maltecca, C. Gut microbiome composition differences among breeds impact feed efficiency in swine. Microbiome 2020, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Yan, W.; Mai, C.; Duan, Z.; Zheng, J.; Sun, C.; Yang, N. Joint contributions of the gut microbiota and host genetics to feed efficiency in chickens. Microbiome 2021, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Broom, L.J.; Kogut, M.H. The role of the gut microbiome in shaping the immune system of chickens. Vet. Immunol. Immunopathol. 2018, 204, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Shabat, S.K.; Sasson, G.; Doron-Faigenboim, A.; Durman, T.; Yaacoby, S.; Berg Miller, M.E.; White, B.A.; Shterzer, N.; Mizrahi, I. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME J. 2016, 10, 2958–2972. [Google Scholar] [CrossRef]
- Ortiz-Chura, A.; Corral-Jara, K.F.; Tournayre, J.; Cantalapiedra-Hijar, G.; Popova, M.; Morgavi, D.P. Rumen microbiota associated with feed efficiency in beef cattle are highly influenced by diet composition. Anim. Nutr. 2025, 21, 378–389. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Med. 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Portocarero, N.; Braun, U. The physiological role of guanidinoacetic acid and its relationship with arginine in broiler chickens. Poult. Sci. 2021, 100, 101203. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; Lin, W.; Yang, L.; Hu, X.; Qiu, X. Rumen-protected guanidinoacetic acid improves growth performance in beef cattle under chronic heat stress by reshaping gut microbiota and modulating serum metabolism. Front. Microbiol. 2025, 16, 1529596. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Koli, P.; Bhadoria, B.K.; Agarwal, M.; Lata, S.; Ren, Y.; Du, X. Proanthocyanidins modulate rumen enzyme activities and protein utilization in vitro. Molecules 2022, 27, 5870. [Google Scholar] [CrossRef] [PubMed]
- Gobert, M.; Martin, B.; Ferlay, A.; Chilliard, Y.; Graulet, B.; Pradel, P.; Bauchart, D.; Durand, D. Plant polyphenols associated with vitamin E can reduce plasma lipoperoxidation in dairy cows given n-3 polyunsaturated fatty acids. J. Dairy. Sci. 2009, 92, 6095–6104. [Google Scholar] [CrossRef]
- Jin, H.; Du, Z.; Fan, X.; Qin, L.; Liu, W.; Zhang, Y.; Ren, J.; Ye, C.; Liu, Q. Effect of guanidinoacetic acid on production performance, serum biochemistry, meat quality and rumen fermentation in Hu sheep. Animals 2024, 14, 2052. [Google Scholar] [CrossRef]
- Degnan, P.H.; Taga, M.E.; Goodman, A.L. Vitamin B12 as a modulator of gut microbial ecology. Cell Metab. 2014, 20, 769–778. [Google Scholar] [CrossRef]
- Zhang, R.; Cheng, Z.; Zang, C.; Cui, C.; Zhang, C.; Jiao, Y.; Li, F.; Li, X.; Yang, K.; Luo, Q. Supplementation of 5,6-dimethylbenzimidazole and cobalt in high-concentrate diet improves the ruminal vitamin B12 synthesis and fermentation of sheep. Fermentation 2023, 9, 956. [Google Scholar] [CrossRef]
- Yu, S.; Li, L.; Zhao, H.; Liu, M.; Jiang, L.; Zhao, Y. Citrus flavonoid extracts alter the profiling of rumen antibiotic resistance genes and virulence factors of dairy cows. Front. Microbiol. 2023, 14, 1201262. [Google Scholar] [CrossRef]
- Zhou, L.; Li, H.; Hou, G.; Wang, J.; Zhou, H.; Wang, D. Effects of Vine tea extract on meat quality, gut microbiota and metabolome of Wenchang broiler. Animals 2022, 12, 1661. [Google Scholar] [CrossRef]
- Qu, X.; Raza, S.H.A.; Zhao, Y.; Deng, J.; Ma, J.; Wang, J.; Alkhorayef, N.; Alkhalil, S.S.; Pant, S.D.; Lei, H.; et al. Effect of Tea saponins on rumen microbiota and rumen function in Qinchuan beef cattle. Microorganisms 2023, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human genetics shape the gut microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zheng, Y.; Tang, W.; Yan, W.; Nie, H.; Fang, J.; Liu, G. Dietary polyphenols in lipid metabolism: A role of gut microbiome. Anim. Nutr. 2020, 6, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Morrow, N.M.; Burke, A.C.; Samsoondar, J.P.; Seigel, K.E.; Wang, A.; Telford, D.E.; Sutherland, B.G.; O’Dwyer, C.; Steinberg, G.R.; Fullerton, M.D.; et al. The citrus flavonoid nobiletin confers protection from metabolic dysregulation in high-fat-fed mice independent of AMPK. J. Lipid Res. 2020, 61, 387–402. [Google Scholar] [CrossRef]
- Yang, Y.; Trevethan, M.; Wang, S.; Zhao, L. Beneficial effects of citrus flavanones naringin and naringenin and their food sources on lipid metabolism: An update on bioavailability, pharmacokinetics, and mechanisms. J. Nutr. Biochem. 2022, 104, 108967. [Google Scholar] [CrossRef]
- Betancur-Murillo, C.L.; Aguilar-Marin, S.B.; Jovel, J. Prevotella: A key player in ruminal metabolism. Microorganisms 2022, 11, 1. [Google Scholar] [CrossRef]
- Kumar, S.; Altermann, E.; Leahy, S.C.; Jauregui, R.; Jonker, A.; Henderson, G.; Kittelmann, S.; Attwood, G.T.; Kamke, J.; Waters, S.M.; et al. Genomic insights into the physiology of Quinella, an iconic uncultured rumen bacterium. Nat. Commun. 2022, 13, 6240. [Google Scholar] [CrossRef]
- Ju, L.; Shao, Q.; Fang, Z.; Trevisi, E.; Chen, M.; Song, Y.; Gao, W.; Lei, L.; Li, X.; Liu, G.; et al. Dietary supplementation with citrus peel extract in transition period improves rumen microbial composition and ameliorates energy metabolism and lactation performance of dairy cows. J. Anim. Sci. Biotechnol. 2024, 15, 152. [Google Scholar] [CrossRef]
- Ricci, S.; Pacifico, C.; Castillo-Lopez, E.; Rivera-Chacon, R.; Schwartz-Zimmermann, H.E.; Reisinger, N.; Berthiller, F.; Zebeli, Q.; Petri, R.M. Progressive microbial adaptation of the bovine rumen and hindgut in response to a step-wise increase in dietary starch and the influence of phytogenic supplementation. Front. Microbiol. 2022, 13, 920427. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Zhang, L.; Wu, J.; Zhao, S.; Jiao, T. Effect of oregano oil and cobalt lactate on sheep in vitro digestibility, fermentation characteristics and rumen microbial community. Animals 2022, 12, 118. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, H.; Zhao, P.; Zhang, C.; Wu, Y.; Li, X.; Huangfu, M.; Chen, Z.; Wang, C.; Liu, B.; et al. Effect of different genetic backgrounds on rumen microbiota and serum metabolic phenotypes in beef cattle. Sci. Rep. 2024, 14, 24005. [Google Scholar] [CrossRef]
- Yu, Y.; Fu, R.; Jin, C.; Han, L.; Gao, H.; Fu, B.; Qi, M.; Li, Q.; Leng, J. Multi-omics insights into rumen microbiota and metabolite interactions regulating milk fat synthesis in Buffaloes. Animals 2025, 15, 248. [Google Scholar] [CrossRef]
- Cantalapiedra-Hijar, G.; Martinez-Fernandez, G.; Forano, E.; Denman, S.E.; Morgavi, D.; McSweeney, C.S. The extent of nitrogen isotopic fractionation in rumen bacteria is associated with changes in rumen nitrogen metabolism. PLoS ONE 2023, 18, e0291243. [Google Scholar] [CrossRef]
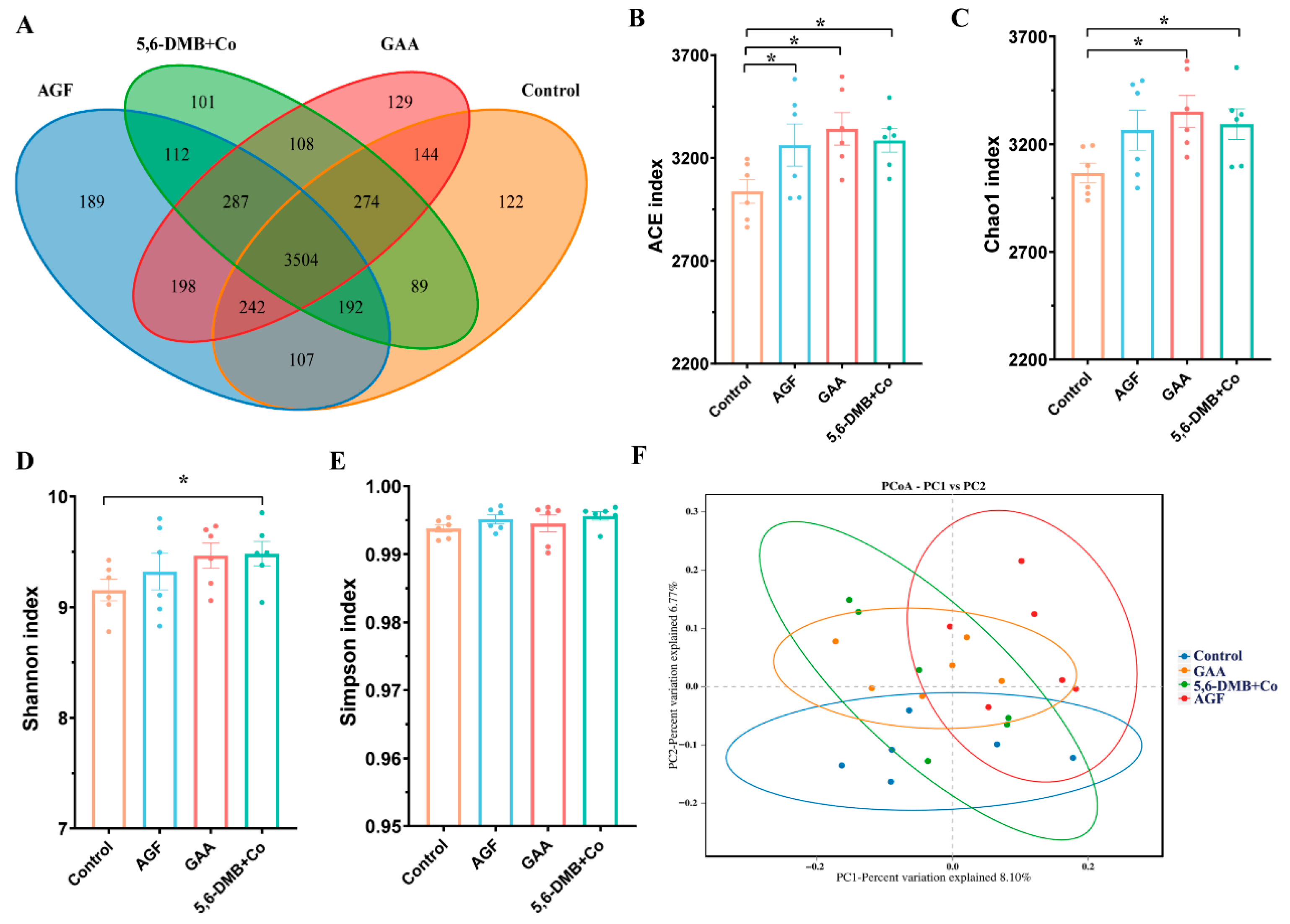
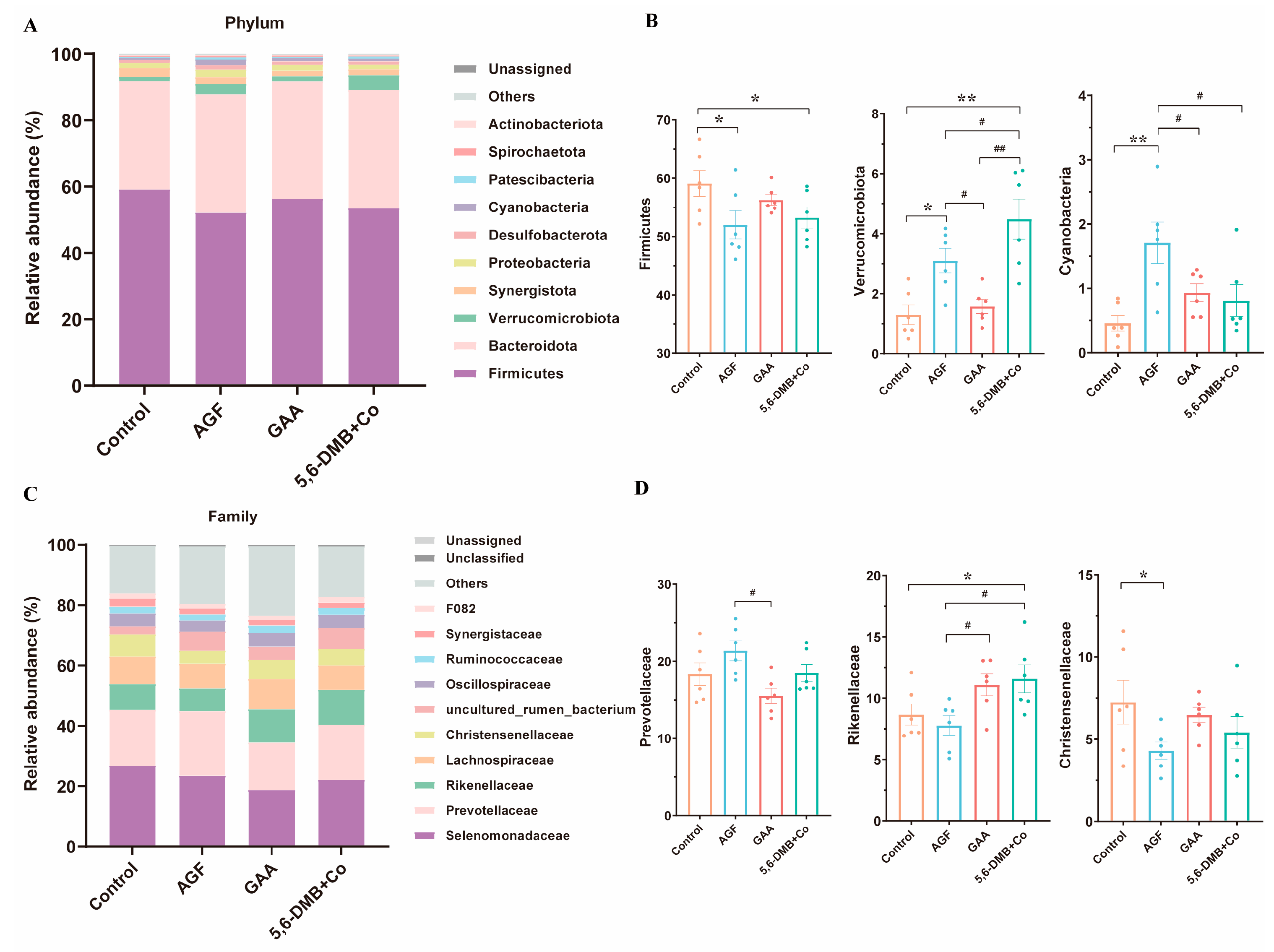
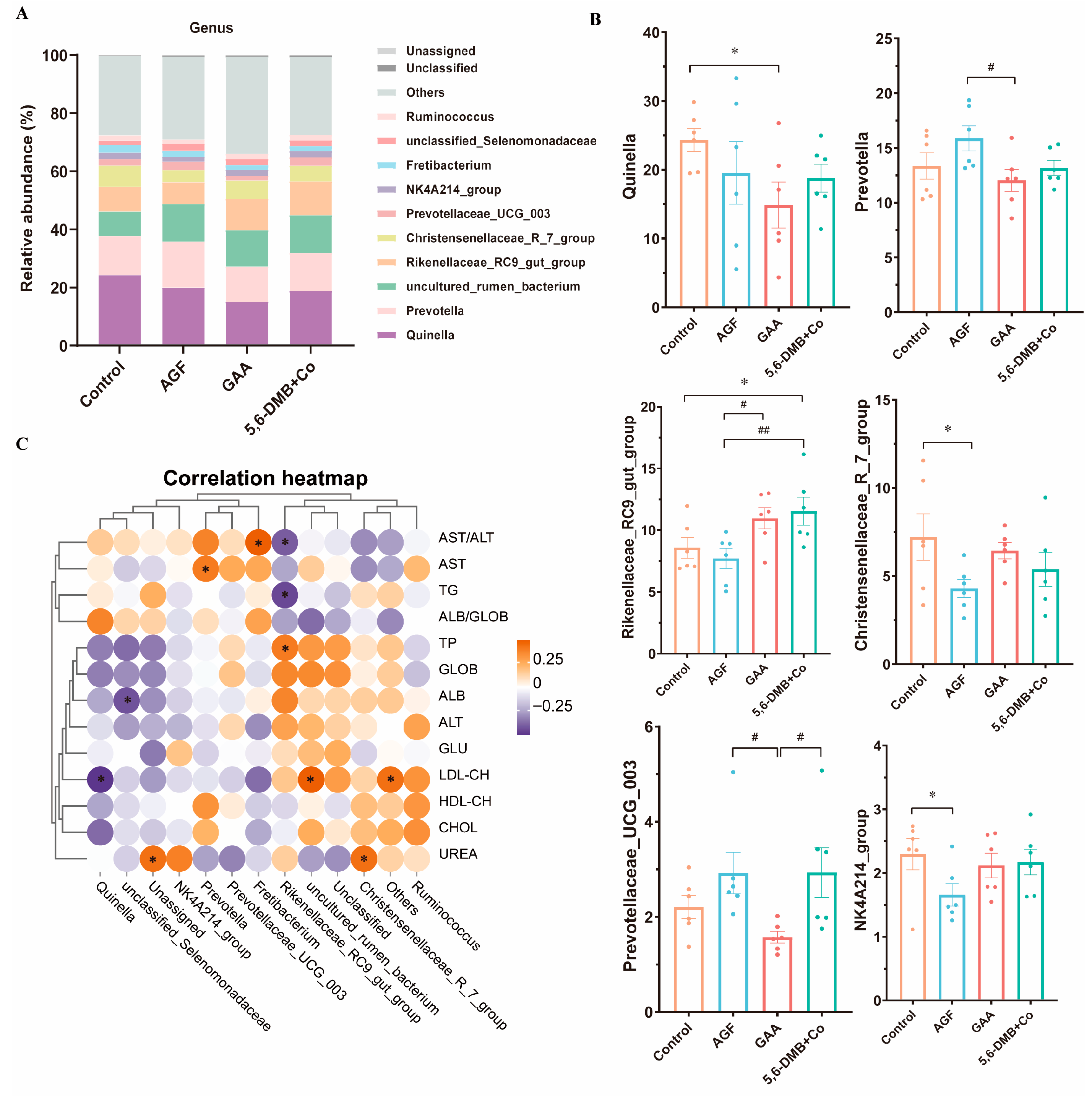
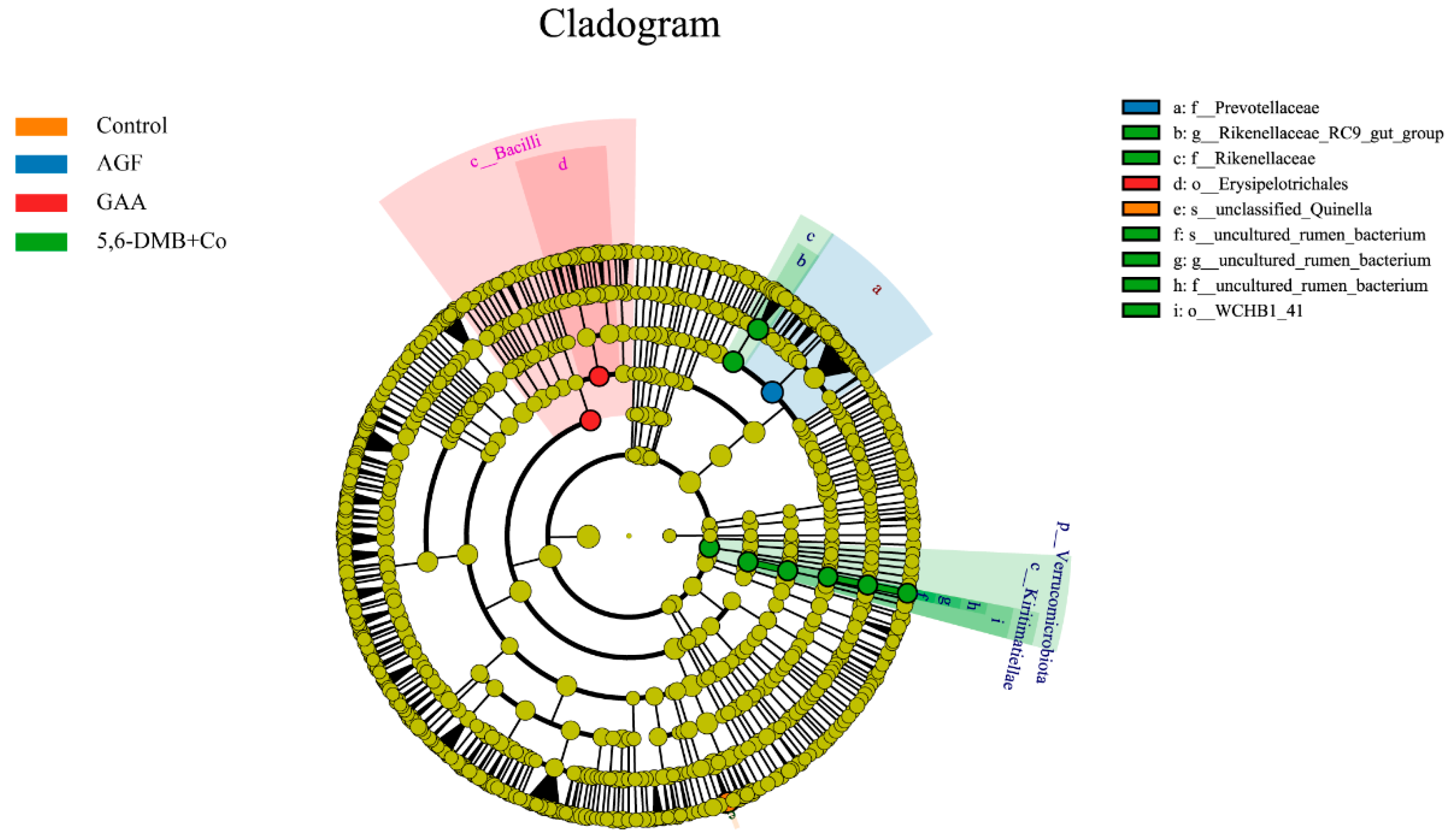
| Group | n | Sex (M/F) | Body Weight (kg, Mean ± SD) | Notes |
|---|---|---|---|---|
| Control | 6 | 3/3 | 50.55 ± 12.78 | Healthy, randomly assigned |
| GAA | 6 | 3/3 | 52.03 ± 8.35 | GAA (1 g/d) |
| AGF | 6 | 3/3 | 52.03 ± 6.61 | AGF (1 g/d) |
| 5,6-DMB + Co | 6 | 3/3 | 51.52 ± 6.05 | 5,6-DMB (100 mg/d) + Co (0.5 mg/d) |
| Item | Control | AGF | GAA | 5,6-DMB + Co |
|---|---|---|---|---|
| GLOB (g/L) | 45.13 ± 5.49 b | 50.56 ± 6.44 ab | 53.90 ± 4.18 a | 52.24 ± 5.55 a |
| ALB (g/L) | 25.83 ± 3.87 | 25.24 ± 2.14 | 27.56 ± 2.24 | 26.29 ± 1.96 |
| ALB/GLOB | 0.57 ± 0.05 | 0.52 ± 0.04 | 0.50 ± 0.00 | 0.52 ± 0.00 |
| TP (g/L) | 70.96 ± 8.64 b | 75.79 ± 8.36 ab | 81.46 ± 6.07 a | 78.52 ± 6.39 ab |
| UREA (mmol/L) | 8.05 ± 1.51 a | 6.54 ± 0.61 b | 7.87 ± 0.79 a | 7.25 ± 0.80 ab |
| AST (U/L) | 150.95 ± 39.60 | 147.08 ± 41.54 | 117.60 ± 12.42 | 159.90 ± 44.25 |
| ALT (U/L) | 22.80 ± 3.73 | 22.25 ± 4.46 | 20.92 ± 5.75 | 23.45 ± 2.51 |
| AST/ALT | 6.74 ± 1.89 | 6.75 ± 2.16 | 6.11 ± 2.30 | 6.97 ± 2.40 |
| TG (mmol/L) | 0.28 ± 0.05 | 0.29 ± 0.05 | 0.27 ± 0.03 | 0.25 ± 0.04 |
| CHOL (mmol/L) | 1.71 ± 0.19 | 1.86 ± 0.19 | 1.66 ± 0.22 | 1.77 ± 0.24 |
| GLU (mmol/L) | 3.28 ± 0.53 | 3.15 ± 0.36 | 3.53 ± 0.88 | 3.39 ± 0.31 |
| HDL-CH (mmol/L) | 1.08 ± 0.15 | 1.09 ± 0.16 | 0.95 ± 0.18 | 1.06 ± 0.14 |
| LDL-CH (mmol/L) | 0.47 ± 0.06 b | 0.58 ± 0.06 a | 0.55 ± 0.06 ab | 0.54 ± 0.09 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.; Lu, Y.; Liu, H.; Huang, D.; Lei, J.; Zhu, J.; Chong, Y.; Deng, W.; Wu, J. Effects on Rumen Microbial Population and Serum Biochemical Responses to Guanidinoacetic Acid, Ampelopsis grossedentata Flavonoids, and 5,6-Dimethylbenzimidazole Plus Cobalt in Lanping Black-Boned Sheep. Animals 2025, 15, 3414. https://doi.org/10.3390/ani15233414
Gao Z, Lu Y, Liu H, Huang D, Lei J, Zhu J, Chong Y, Deng W, Wu J. Effects on Rumen Microbial Population and Serum Biochemical Responses to Guanidinoacetic Acid, Ampelopsis grossedentata Flavonoids, and 5,6-Dimethylbenzimidazole Plus Cobalt in Lanping Black-Boned Sheep. Animals. 2025; 15(23):3414. https://doi.org/10.3390/ani15233414
Chicago/Turabian StyleGao, Zhendong, Ying Lu, Huaijing Liu, Daitao Huang, Jiachen Lei, Junhong Zhu, Yuqing Chong, Weidong Deng, and Jiao Wu. 2025. "Effects on Rumen Microbial Population and Serum Biochemical Responses to Guanidinoacetic Acid, Ampelopsis grossedentata Flavonoids, and 5,6-Dimethylbenzimidazole Plus Cobalt in Lanping Black-Boned Sheep" Animals 15, no. 23: 3414. https://doi.org/10.3390/ani15233414
APA StyleGao, Z., Lu, Y., Liu, H., Huang, D., Lei, J., Zhu, J., Chong, Y., Deng, W., & Wu, J. (2025). Effects on Rumen Microbial Population and Serum Biochemical Responses to Guanidinoacetic Acid, Ampelopsis grossedentata Flavonoids, and 5,6-Dimethylbenzimidazole Plus Cobalt in Lanping Black-Boned Sheep. Animals, 15(23), 3414. https://doi.org/10.3390/ani15233414







