Simple Summary
The current review provides a comprehensive classification of common stress types in animal production and detailed methodologies for establishing corresponding stress models. It thoroughly analyzes changes in nutritional requirements—particularly regarding proteins, amino acids, and energy—under stress conditions, and discusses dietary strategies to maintain animal performance. It integrates recent advances in molecular mechanisms, including neuroendocrine signaling, cytokine-mediated pathways, and oxidative stress responses, providing deeper insights into metabolic regulation under stress.
Abstract
Animal stress is a complex physiological state characterized by a suite of non-specific responses (e.g., lethargy, anorexia, and impaired growth) to various internal or external threats, collectively known as stressors. It is the synthesis of non-specific responses produced by the body to various external or internal stimuli. Animals in livestock production are often stressed by breeding density, inappropriate temperature and humidity, harmful gases, noise and complicated immunization. Consequently, the nutritional requirements and underlying metabolic mechanisms in stressed animals are critical and growing research hotspots. Emerging evidence has shown that nutritional intervention can maintain animal health and performance under stresses. In addition, the regulation of metabolic pathways and targets under different stress states is also a potential way to alleviate the stress response of animals. With the rapid development of intensive farming and the aggravation of environmental changes, animals are facing increasing challenges such as heat stress, transportation stress, and pathogen infection. The resulting metabolic disorders and health problems seriously restrict the production efficiency of animals. This review aims to systematically analyze the variation in animal nutritional requirements under stress, clarify the molecular mechanism of metabolic imbalance, and summarize the regulatory targets and effects of nutritional intervention strategies, providing theoretical basis and technical support for solving stress-related problems in livestock production.
1. Introduction
Stress is an instinctive response of animals to external stimuli, mostly referring to a series of non-specific reactions produced by the body when it is stimulated by endogenous or exogenous stressors [1]. When the body is challenged by various unfavorable stressors from the inside or outside, homeostasis is disrupted and lost and stress occurs. In livestock production, common stressors include: environmental factors (e.g., heat, cold, humidity, concentration of toxic gases, radiation, trauma, etc.), disease factors (e.g., diarrhea, inflammation, bacterial infection, etc.), and feeding management (immunization, stocking density, transportation, etc.) [2,3,4]. However, regardless of the nature of the factors stimulating the organism, the stress response is essentially an adaptive and defensive physiological response displayed by the organism during the course of evolution to maintain normal life activities and ensure the recovery of the organism after injury or dysfunction [1,5].
Studies have shown that moderate stress can enhance the animals’ resistance to the external environment, thereby improving productivity and feed conversion rates [6]. Specifically, moderate stress can activate the stress response system of animals, allowing them to better utilize feed in the face of external challenges, which, in turn, enhances growth performance [7]. In addition, moderate stress may also enhance the growth rate of animals by promoting the secretion of growth hormone [8]. On the other hand, excessive stress can cause adverse reactions in animals such as loss of appetite, listlessness, reduced feed conversion rate, decreased production performance, weakened immunity, and even lead to diseases or death [9,10,11,12]. Current studies indicate that stressors such as environmental changes, nutritional deficiencies, and pathogen infections can lead to profound modifications in nutritional requirements and metabolic responses in livestock [13]. With the increasing concern for animal welfare and production efficiency, researchers have gradually recognized the importance of stress on the nutritional requirements of animals. Under stress conditions, the metabolic rate, energy expenditure and nutrient requirements of animals will change significantly. For example, heat stress can lead to decreased appetite, weakened digestion and absorption ability in animals, thereby affecting their nutritional status and growth performance [14]. Previous studies have also shown that transportation stress can lead to the release of large amounts of stress hormones in animals, such as cortisol, which can suppress appetite and decrease feed intake [15]. In addition, excessive cortisol not only directly affects the function of the digestive system but may also lead to metabolic disorders, thereby influencing the growth performance of animals [16]. Based on these findings, the physiological and biochemical mechanisms underlying these changes include alterations in hormonal signaling, immune response modulation, and metabolic shifts that promote energy homeostasis. These studies suggest the necessity for tailored nutritional strategies in livestock management, particularly under stress conditions. These strategies should focus on optimizing nutrient profiles to mitigate the negative effects of stressors on animal physiology and productivity. However, the research on the underlying mechanisms of nutrition metabolism in stressed animals is still not systematic and in depth, which makes the research on the nutritional requirements of stressed animals significantly limited. This review aims to synthesize the recent literature related to the construction of different stress animal models, the requirements and mitigation measures of different nutrients under stress, and the potential mechanisms of nutrient metabolism under different stress conditions, in order to establish a reasonable stress model and provide theoretical basis and potential improvement targets for the nutritional requirements of animals under stress.
2. The Types of Common Stress and the Construction of Stress Models in Animals
2.1. Immune Stress and Model Establishment
Immune stress refers to a non-specific immune activation state in which the immune system is repeatedly activated when the body is exposed to continuous antigenic stimulation (including both specific and non-specific), leading to characteristic clinical manifestations such as fever, anorexia, and immunosuppression [17,18]. In nutritional immunology, immune stress is regarded as one of the key factors that restrict animals from achieving optimal production performance and feed utilization efficiency. Evidence indicates that the inflammatory response can be regarded as a typical marker of immune stress, with its main features being elevated levels of cytokines such as interleukin-1 (IL-1), IL-2, IL-6, and tumor necrosis factor-α (TNF-α) [17,19,20]. At the same time, the production performance and immune function of animals will decline, and the contents of insulin-like growth factor (IGF-1) and growth hormone (GH) will also decrease accordingly [21,22]. To explore the direct impact of immune stress on animals and its potential mechanism of action, it is crucial to establish a successful immune stress model. The establishment of immune stress models includes methods such as lipopolysaccharide (LPS) induction, virus induction, and chemical/physical induction. Among them, LPS, as a commonly used stress inducer, is mostly administered by intraperitoneal injection in practical operations. Although the injection time and dose of LPS vary greatly in different studies, it can successfully induce an immune stress model. In pig production, porcine circovirus (PCV) infection poses a significant threat to piglets. Therefore, directly challenging livestock with pathogens (such as Salmonella typhimurium, Actinobacillus pleuropneumoniae, PCV-2, etc.) may be more relevant to production. In addition, restraint stress can also trigger an immune stress response in animals. Studies have shown that continuous restraint stress treatment of Bama miniature pigs for 18 days leads to an increase in oxidative stress levels in the intestine, resulting in intestinal mucosal damage, including impaired intestinal morphology and a reduction in the number of goblet cells and proliferating cell nuclear antigen-positive cells [23]. Table 1 describes the common methods for inducing immune stress and the applicable animals.

Table 1.
Induction methods for immune stress in animals.
2.2. Oxidative Stress and Model Establishment
When livestock and poultry are exposed to stress stimuli, the imbalance of the redox system leads to excessive generation of highly reactive free radicals, which exceed the threshold of endogenous clearance capacity and induce peroxidation damage. Sohal et al. first proposed the concept of oxidative stress, which refers to the disorder of the redox system and the accumulation of free radical damage when the production rate of reactive oxygen species (ROS, including superoxide anion, hydrogen peroxide, and hydroxyl radicals, etc.) exceeds the clearance capacity of the organism, regardless of physiological or pathological conditions [34]. Under normal physiological conditions, ROS act as redox signaling molecules involved in metabolic regulation, and their levels are strictly regulated by the antioxidant defense system. However, excessive ROS under stress conditions can cause: (1) damage to biological membrane structure and cell function; (2) reduction in the thermal stability of nucleic acids and enzyme activity; (3) oxidation and denaturation of biological macromolecules such as lipids, proteins, and DNA, ultimately leading to metabolic disorders and increased susceptibility to diseases [35]. The clinical manifestations of this pathological process include behavioral abnormalities such as restlessness, vomiting, and anorexia, accompanied by increased levels of oxidative damage products such as malondialdehyde (MDA), protein carbonyl (PCO), and 8-hydroxydeoxyguanosine (8-OHdG) [36], reduced growth performance and meat quality [37], and damage to the intestinal barrier structure [38]. Common stressors used to establish oxidative stress models include hormones (hydrocortisone, corticosterone, dexamethasone, etc.), fatty acid substances (fish oil, soybean oil, coconut oil), diquat, dextran sulfate sodium (DSS), LPS, etc. [36,39,40,41]. Table 2 describes the common methods for constructing oxidative stress in animals, the applicable animals, and the core indicators.

Table 2.
Construction methods for oxidative stress in animals.
2.3. Environmental Stress and Model Establishment
Environmental stress is particularly common in livestock production. It occurs when the environment itself or the tolerance of livestock to adverse conditions changes. The factors that affect the environment in which livestock live include: excessively high or low temperatures, excessively high or low humidity, transportation, noise, inappropriate lighting, excessive stocking density, and excessive levels of harmful gases [52,53,54]. In actual production, temperature stress is the main type of environmental stress, including cold and heat stress. Studies on heat stress are relatively more numerous, and its occurrence is due to a negative balance between the heat released by the organism into the environment and the heat produced by the organism (producing more than releasing). Heat stress reduces the feed intake of livestock, increases the consumption of nutritional metabolism in the body, and lowers the immune function, resulting in a decrease in the production and reproductive performance of livestock [55]. Low temperature is also one of the stressors. Different animals have different ranges and degrees of cold stress tolerance. Currently, there is no very precise definition for cold stress. It has been reported that low temperature increases environmental energy consumption and energy consumption within the animal’s body [56]. When the requirements for heat generation cannot be met, it also affects production performance. At the same time, cold stress can increase the levels of serum corticosterone, cortisol, and catecholamine hormones in the blood, significantly enhancing the activity of serum creatine kinase, and the effects on different animals still need further study [57,58]. Usually, the establishment of cold and heat stress models is achieved by controlling the environmental temperature of the livestock pens. Livestock are warm-blooded animals, and the external environment temperature at which chickens can maintain a normal body temperature is 16–32 °C [59]; newborn piglets are 27–29 °C, weaned piglets are 21–24 °C, growing and fattening pigs are 15–25 °C, sows giving birth and lactating are 16–18 °C [60], dairy cows are 10–15 °C [61]; sheep are 20–28 °C, and goats are 21–25 °C [62]. When the temperature maintained in the livestock housing environment is higher than the maximum suitable temperature for the organism, livestock will produce heat stress responses, establishing a heat stress model; when the temperature of the livestock breeding environment is lower than the minimum suitable temperature for the organism, or there is a sudden drop in temperature, or the environment is continuously below 4 °C, it will cause cold stress responses in livestock, establishing a cold stress model. Table 3 describes the common methods for constructing animal environmental stress models, the applicable animals, and the core indicators.

Table 3.
Construction methods for transportation stress in animals.
Overall, immune stress is primarily triggered by antigens and modeled via direct immunostimulants (e.g., LPS, viruses) or indirect stressors, leading to inflammation and performance decline. Oxidative stress, induced by chemicals, hormones, or diets, results from ROS overproduction and is marked by biomarkers like MDA and 8-OHdG. Environmental stress, mainly temperature-related, is modeled by controlling ambient conditions and manifests as physiological disruptions.
3. The Impact of Stress on Animal Nutritional Requirements
Stress is essentially a physiological response. In contemporary stress theory, the primary objective of the stress response is to activate all defense mechanisms and capabilities within the body. This mobilization aims to counteract the adverse effects of stressors and preserve the body’s homeostasis in extreme conditions. Additionally, it has been proposed that the concept of using nutrients to regulate the immune system function of animals, which is called immunonutrition (IMN) [75]. This concept applies to any situation where the inflammatory or immune response is regulated by changing nutrient supply. The activation of the immune system leads to a decrease in feed intake, weight gain, and protein deposition in livestock, thereby affecting production performance, changing nutritional requirements and patterns, especially for poultry that are sensitive to stress [76]. Under stress conditions, the catabolic metabolism of the body is significantly higher than the anabolic metabolism, manifested as negative nitrogen balance. This metabolic change is the result of neuroendocrine reactions. After stress occurs, the strengthening of catabolism inevitably leads to imbalance in the internal environment of the animal body, and at the same time, due to the large consumption of energy by tissues, the demand for vitamins and amino acids by the body increases sharply, and the deficiency of various nutrients will cause the body to quickly enter a state of exhaustion [77]. Studies have showed that the recovery from stress requires the body to have good physiological vitality, to quickly adjust the disordered physiological functions, and to accelerate the repair of damaged tissues [78,79,80]. Most stress regulation of nutritional metabolism mainly occurs through direct or indirect actions mediated by cytokines, and is reflected in the metabolic changes of proteins, fats, and carbohydrates.
3.1. Effects of Stress on Protein Nutritional Requirements of Animals
Studies have shown that low protein levels led to poor antioxidant stress resistance, while high protein levels resulted in better antioxidant stress resistance and superior meat quality [81,82,83]. Lee J et al. also observed a similar phenomenon in their study on fattening pigs under heat stress, where pigs fed low-protein diets showed decreased growth performance, nutrient digestibility, intestinal morphology, intestinal integrity, and serum antioxidant markers compared to those fed diets supplemented with lysine [84]. Therefore, when animals are under stress, supplementing them with a high-protein diet can effectively alleviate the impact of stress. However, when the external temperature changes, different physiological responses to heat and cold stress determine different dietary protein adjustments. Cold stress typically promotes increased feed consumption via hypothalamic appetite stimulation, often obviating the need for dietary protein escalation [85]. Conversely, the anorexia characteristic of heat stress compromises protein intake. Although increasing the dietary protein percentage can counter this deficit, caution is warranted due to the higher specific dynamic action (SDA) of protein, which augments metabolic heat production and may aggravate thermal load [86,87]. Therefore, it is recommended to balance the amino acids in the diet and formulate the diet based on the requirements of digestible amino acids to reduce the crude protein content while ensuring adequate amino acid intake, thereby preventing a decline in animal production performance. Morales et al. also demonstrated through their study on nutrient absorption in the intestines of pigs under heat stress that low-protein diets supplemented with amino acids improved intestinal absorption capacity compared to high-protein diets [88]. Furthermore, for weaned piglets, factors other than protein levels, such as palatability and the composition ratios of various feeds, also affect growth performance. However, most studies have indicated that there is no interaction between feed composition and immune stress on growth performance, but they can alleviate the growth retardation caused by stress [89,90,91].
3.2. Effects of Stress on Carbohydrate and Energy Requirements of Animals
Haisan et al. fed dairy cows with different levels of starch as energy sources from 28 days before parturition to 20 days after parturition [92]. The results showed that cows fed with higher levels of starch had higher milk production and lower levels of inflammatory factors in serum, indicating better resistance to immune stress. In addition, it has been reported that heifers were fed with different energy levels of diet for 45 days before parturient, and the high energy diet group showed stronger anti-immune stress and antioxidant capacity [93]. Adebowale et al. also found that weaned piglets require a higher energy feeding level when facing oxidative stress [94]. Moreover, with the deepening of research on nutritional metabolism and the deepening of understanding of the body system, the regulatory role of the immune system in nutritional metabolism has gradually received attention. Under different stress conditions, the digestion and absorption, synthesis and decomposition metabolism of nutrients in animals will change differently, and this process has dynamic characteristics. Therefore, it is difficult to determine the nutritional requirements of animals under different stress conditions through feeding experiments. Therefore, further research and discussion are still needed on the range of changes in nutritional requirements of animals under stress conditions.
4. Research on the Mechanism of Metabolic Regulation in Stressed Animals
4.1. Immune Stress and Metabolic Mechanisms in Animals
The immune system, as a sensing mechanism, can recognize the presence of antigens (such as bacteria, viruses, and exogenous proteins) within the body and transmit relevant information to other parts of the body, thereby triggering a series of changes at the behavioral, cellular, and metabolic levels [95]. The immune system-mediated growth or nutritional regulation mechanisms related to metabolism mainly include the following three aspects: (1) There is a direct connection between immune tissues (thymus, spleen, lymph nodes, etc.) and the central nervous system. Peripheral immune responses can activate the hypothalamic-pituitary-adrenal (HPA) axis in the central nervous system, thereby causing metabolic changes [96,97]. The specific process of HPA axis involvement in immune stress regulation is as follows: When immune stressors stimulate and activate the hypothalamus, the hypothalamus releases corticotropin-releasing factor (CRF); CRF acts on the pituitary gland, causing it to produce adrenocorticotropic hormone (ACTH); ACTH further activates the adrenal cortex, ultimately promoting the secretion of glucocorticoids and thereby causing metabolic changes. (2) There is a regulatory association between immune stress and the endocrine system. For example, the immune system can affect the metabolic process through hormones released by the pituitary gland. Specifically, immune stress reduces the secretion of growth hormone, increases plasma steroid hormone levels, promotes the secretion of catecholamines, and simultaneously lowers insulin-like growth factor levels. These changes alter the metabolic state of animals and their utilization and demand for nutrients [98]. (3) White blood cells release interleukins (including monokines and lymphokines, also known as cytokines). To resist the stimulation of external antigens, various immune active cells in the body secrete a series of cytokines to alter the body’s metabolism. Among them, the three monokines (TNF-α, IL-6, IL-1) produced by macrophages have a profound impact on the behavior, endocrine, and metabolism of animals during the growth process [22,99,100]. Figure 1 illustrates the process of immune stress and subsequent metabolic alterations in animals.
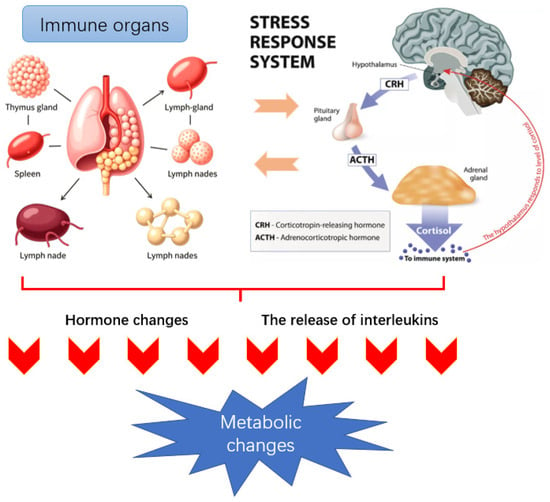
Figure 1.
Immune stress and metabolic mechanisms in animals. Immune challenges (e.g., LPS or pathogens) activate immune cells to release pro-inflammatory cytokines (TNF-α, IL-1, IL-6), which stimulate the HPA axis. This leads to glucocorticoid release and endocrine alterations, suppressing anabolic hormones (e.g., IGF-1, GH) while promoting catabolism. Consequently, nutrient metabolism is reprogrammed away from growth and toward immune support, resulting in reduced performance and metabolic imbalance.
4.2. Oxidative Stress and Metabolic Mechanisms in Animals
When the content of reactive oxygen species (ROS) generated by the body’s metabolism exceeds its clearance rate, the redox balance within the cells is disrupted, thereby triggering oxidative stress responses. During this process, the large amount of free radicals produced cause damage to cells and tissues [101]. Under physiological conditions, redox homeostasis is maintained through intricate feedback mechanisms that regulate the generation and elimination of reactive oxygen species ROS and free radicals. This balance keeps ROS concentrations at specific, non-detrimental levels. Consequently, any basal oxidative damage that occurs is rapidly repaired, preventing its accumulation and ensuring cellular integrity. However, when the content of small molecule reducing substances responsible for eliminating ROS and free radicals decreases, or the activity of antioxidant enzymes is inhibited, the redox balance will be disrupted, thereby triggering oxidative stress responses [102,103]. Oxidative stress can be classified into three types: Firstly, acute oxidative stress, at which the rapidly increased ROS in the body can be fully cleared by the normal antioxidant system and restored to the normal level. The short-term increase in ROS does not immediately trigger oxidative stress but is accompanied by specific biochemical reactions, including the action of enzymes such as superoxide dismutase (SOD) and related antioxidants, to eliminate excess ROS and prevent the occurrence of oxidative stress [104,105]; Secondly, chronic oxidative stress, in this state, the high levels of ROS cannot be restored to normal levels quickly and require a slow reaction process, leading to the disruption of the body’s homeostasis [105,106]; Thirdly, the quasi-static level, in this state, the high levels of ROS in the body are regulated by reduction reactions and cannot return to the initial stable state but establish a new stable state of redox reactions. The excessive accumulation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) not only triggers oxidative stress responses within the body but also causes mitochondrial dysfunction within cells [35,107,108]. Oxidative stress not only causes damage to cell membranes and proteins but also leads to base alterations. The activator protein-1 (AP-1) endonuclease and DNA glycosylase present in mitochondria and the nucleus can remove and repair the altered bases [109]. However, when the cumulative damage to DNA and proteins caused by oxidative stress continues to exceed the body’s own recovery and repair capabilities, it will trigger severe disease responses. To prevent oxidative stress from causing oxidative denaturation of bioactive substances, the body interrupts the synthesis reactions of a large number of substances during oxidative stress, thereby affecting the body’s material basis. The Nrf2/Keap1 pathway is the main approach for the body to respond to mild oxidative stress. This pathway regulates antioxidant-related genes, promotes the production of antioxidant enzymes and the synthesis of other antioxidants, thereby enhancing the body’s antioxidant response [110]. The immune regulatory system centered on AP-1, MAPK and NF-κB is the main regulatory system for the body in a severe oxidative stress state, by upregulating the expression of inflammatory genes and promoting the release of inflammatory factors [101,111]. The process of animal oxidative stress and metabolic mechanisms is shown in Figure 2.
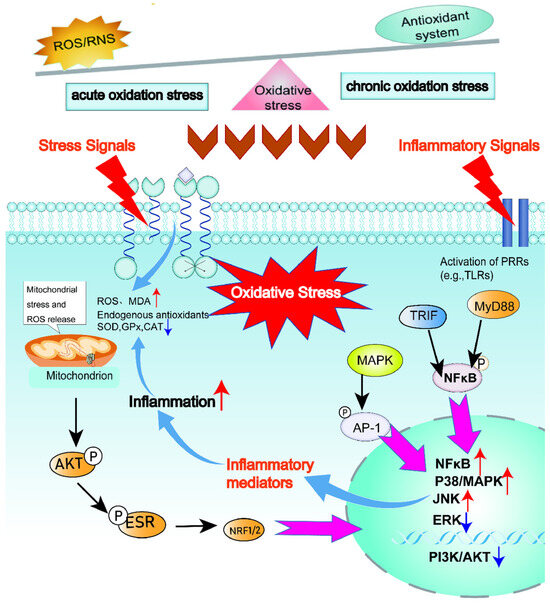
Figure 2.
Oxidative stress and metabolic mechanisms in animals. Oxidative stress is an imbalance between the production of reactive oxygen species (ROS) and the body’s ability to neutralize them. It begins when ROS, primarily generated as metabolic byproducts during mitochondrial energy production, accumulate beyond the capacity of the antioxidant defense system, which includes enzymes like superoxide dismutase and catalase. This imbalance leads to widespread oxidative damage to cellular lipids, proteins, and DNA, disrupting normal function, promoting inflammation, and ultimately contributing to cellular dysfunction, aging, and the pathogenesis of various metabolic diseases.
4.3. Environmental Stress and Metabolic Mechanisms in Animals
Environmental stress refers to the pressure exerted by the external environment on the survival state of animals, which prompts the activation of response mechanisms in internal organs and tissues, thereby causing disruptions in normal physiological functions and leading to stress responses [112,113]. In practical production, animals are prone to varying degrees of physical damage due to the influence of the external environment during transportation. As the degree of stress intensifies, excessive energy consumption can suppress the immune system function. Moreover, due to individual differences, some animals can restore metabolic balance under environmental stress through self-regulation, but weaker groups may experience metabolic imbalance and physiological disorders when exposed to stress beyond their regulatory threshold [114]. Among environmental stresses in livestock production, heat and cold stress are the most studied. Heat stress is generally believed to reduce production and reproductive performance by directly lowering feed intake [115,116]. However, an increasing number of studies have shown that heat stress first reduces feed intake and nutrient absorption, thereby affecting the metabolic level of the body, especially promoting excessive production of free radicals and causing oxidative stress [117,118], ultimately leading to a decline in production and reproductive performance of livestock through cellular and mitochondrial oxidative damage [119,120,121]. Additionally, cold stress, as one of the stressors, can cause varying degrees of cold stress responses in animals exposed to it for a long or short period, which is also known as the “alarm response” [122,123]. Emerging evidence has shown that heat and cold stress affect the production and metabolism of animals, activating the three major nervous systems in the body, namely the sympathetic-adrenal medulla axis (SAM), the hypothalamic-pituitary-adrenal axis (HPA), and the hypothalamic-pituitary-thyroid axis (HPT), promoting the regulation of various hormones such as cortisol, thyroid hormones, and adrenaline to adapt to changes in the external environment as soon as possible [124]. Furthermore, such stressors can dysregulate immune responses, leading to a state of immunosuppression or excessive inflammation. This impaired immune competence not only increases vulnerability to infections but also diverts energy and nutrients away from growth, reproduction, and other production traits, resulting in significant economic losses [125,126]. The core pathway of immune suppression is: Environmental stressor → HPA axis → promoting the secretion of glucocorticoids → inhibiting lymphocyte proliferation → reduction in cellular and humoral immune functions [127]. The process of environmental stress and its mechanism in animals is shown in Figure 3.
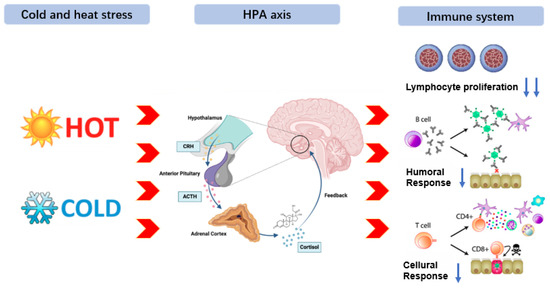
Figure 3.
Environmental stress and metabolic mechanisms in animals. Environmental stressor (heat or cold) activates neuroendocrine axes (SAM, HPA, HPT), inducing release of cortisol, adrenaline, and thyroid hormones to promote adaptation. Concurrent HPA activation and glucocorticoid secretion suppress lymphocyte proliferation, impairing cellular and humoral immunity. This immune dysfunction increases infection susceptibility and diverts nutrients from growth and reproduction, resulting in substantial productivity losses.
5. Conclusions
In conclusion, stress represents a complex physiological response that significantly disrupts homeostasis and alters nutrient metabolism in animals, ultimately impacting health, welfare, and production efficiency. This review has summarized the common types of stress encountered in livestock production, namely immune, oxidative, and environmental stress, as well as methods for establishing representative animal models to study these conditions. A key consensus is that stress invariably increases the animals’ requirements for specific nutrients, including proteins/amino acids and energy-yielding components, while simultaneously impairing intake, digestion, and absorption efficiency. The underlying mechanisms involve intricate interactions between neuroendocrine pathways (e.g., HPA axis activation), immune responses (e.g., cytokine release), and redox signaling, which collectively reprogram metabolic priorities toward maintenance and defense at the expense of growth and productivity.
Despite advances in stress biology, current understanding of the precise alterations in nutritional metabolism under stress remains fragmented. The future research frontier will focus on integrating modern molecular nutrition, genomics, proteomics, and metabolomics, among other multidisciplinary approaches, to systematically analyze the macroscopic to microscopic changes in animal nutritional metabolism and absorption and utilization mechanisms under different stress conditions. Ultimately, integrating insights will be crucial for formulating targeted dietary interventions that enhance resilience, maintain productivity, and improve overall animal welfare in the face of unavoidable stress challenges.
Author Contributions
J.B.: Writing—review & editing; Writing—original draft; Funding acquisition; Formal analysis. X.L. (Xinhang Li): Writing—review & editing; Writing—original draft; Validation; Formal analysis. X.L. (Xinjian Li): Investigation; Project administration. Z.W.: Software, Supervision. K.W.: Validation, Resources. R.Q.: Methodology, Project administration. X.H.: Supervision, Visualization. X.L. (Xiuling Li): Software, Supervision. F.Y.: Validation, Supervision. T.Y.: Software, Validation. T.W.: Software, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Postdoctoral Fellowship Program of China Postdoctoral Science Foundation (Nos. GZB20240203) & Postdoctoral General Funding Project (Nos. 2025M770253) & 14th Five-Year National Key R&D Program (2021YFD1301202) & Pig Industry Technology System Innovation Team Project of Henan Province (HARS-22-12-G4) & Agricultural Breeds Research Project of Henan Province (2022020101) & Key scientific Research Project of Higher Education Institutions in Henan Province (26B230003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No datasets were generated or analysed during the current study.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
ACTH, adrenocorticotropic hormone; AP-1, activator protein-1; CAT, Catalase; CRF, corticotropin-releasing factor; COR, corticosterone; DSS, dextran sulfate sodium; GH, growth hormone; GSH, Glutathione; GST, glutathione S-Transferase; GPX, Glutathione peroxidase; HCT, red blood cell specific volume; HPA, hypothalamic–pituitary–adrenal; HPT, hypothalamic–pituitary–thyroid axis; HSP70, heat shock protein 70; IGF-1, insulin-like growth factor; IL-1, interleukin-1; IL-2, interleukin-2; IL-6, interleukin-6; IMN, immunonutrition; INOS, Inducible Nitric Oxide Synthase; LPS, lipopolysaccharide; MDA, malondialdehyde; MPO, myeloperoxidase; NOX2, NADPH oxidase 2; PCO protein carbonyl; PCV, porcine circovirus; PCV-2, Porcine circovirus type 2; PGF2α, Prostaglandin F2α; RNS, reactive nitrogen species; ROS, reactive oxygen species; SAM, sympathetic-adrenal medulla axis; SDA, specific dynamic action; SOD, superoxide dismutase; T-AOC, total antioxidant capacity; TBARS, thiobarbituric acid reactive substances; TNF-α, tumor necrosis factor-α;UCP1, Uncoupling Protein 1; 8-OHdG, 8-hydroxydeoxyguanosine.
References
- Lu, H.J.; Koju, N.; Sheng, R. Mammalian integrated stress responses in stressed organelles and their functions. Acta Pharmacol. Sin. 2024, 45, 1095–1114. [Google Scholar] [CrossRef]
- Kashiwagi, K.; Yokoyama, T.; Nishimoto, M.; Takahashi, M.; Sakamoto, A.; Yonemochi, M.; Shirouzu, M.; Ito, T. Structural basis for eIF2B inhibition in integrated stress response. Science 2019, 364, 495–499. [Google Scholar] [CrossRef]
- Sawka, M.N.; Leon, L.R.; Montain, S.J.; Sonna, L.A. Integrated physiological mechanisms of exercise performance, adaptation, and maladaptation to heat stress. Compr. Physiol. 2011, 1, 1883–1928. [Google Scholar] [CrossRef]
- Li, Y.; Mao, K.; Zang, Y.; Lu, G.; Qiu, Q.; Ouyang, K.; Zhao, X.; Song, X.; Xu, L.; Liang, H.; et al. Revealing the developmental characterization of rumen microbiome and its host in newly received cattle during receiving period contributes to formulating precise nutritional strategies. Microbiome 2023, 11, 238. [Google Scholar] [CrossRef]
- Tofani, G.S.S.; Leigh, S.J.; Gheorghe, C.E.; Bastiaanssen, T.F.S.; Wilmes, L.; Sen, P.; Clarke, G.; Cryan, J.F. Gut microbiota regulates stress responsivity via the circadian system. Cell Metab. 2025, 37, 138–153.e135. [Google Scholar] [CrossRef]
- Rhoads, M.L. Review: Reproductive consequences of whole-body adaptations of dairy cattle to heat stress. Animal 2023, 17 (Suppl. 1), 100847. [Google Scholar] [CrossRef] [PubMed]
- Oke, O.E.; Akosile, O.A.; Oni, A.I.; Opowoye, I.O.; Ishola, C.A.; Adebiyi, J.O.; Odeyemi, A.J.; Adjei-Mensah, B.; Uyanga, V.A.; Abioja, M.O. Oxidative stress in poultry production. Poult. Sci. 2024, 103, 104003. [Google Scholar] [CrossRef] [PubMed]
- Archer, G.S. Evaluation of an Extract Derived from the Seaweed Ascophyllum nodosum to Reduce the Negative Effects of Heat Stress on Broiler Growth and Stress Parameters. Animals 2023, 13, 259. [Google Scholar] [CrossRef]
- Acharya, R.Y.; Hemsworth, P.H.; Coleman, G.J.; Kinder, J.E. The Animal-Human Interface in Farm Animal Production: Animal Fear, Stress, Reproduction and Welfare. Animals 2022, 12, 487. [Google Scholar] [CrossRef]
- Tang, X.; Xiong, K.; Wassie, T.; Wu, X. Curcumin and Intestinal Oxidative Stress of Pigs With Intrauterine Growth Retardation: A Review. Front. Nutr. 2022, 9, 847673. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Kou, S.; Chen, C.; Raza, S.H.A.; Wang, S.; Ma, X.; Zhang, W.J.; Nie, C. Effects of Clostridium butyricum on growth performance, metabonomics and intestinal microbial differences of weaned piglets. BMC Microbiol. 2021, 21, 85. [Google Scholar] [CrossRef]
- Castro, F.L.S.; Kim, Y.; Xu, H.; Kim, W.K. The effect of total sulfur amino acid levels on growth performance and bone metabolism in pullets under heat stress. Poult. Sci. 2020, 99, 5783–5791. [Google Scholar] [CrossRef]
- Teixeira, I.; Harter, C.J.; Vargas, J.A.C.; Souza, A.P.; Fernandes, M. Review: Update of nutritional requirements of goats for growth and pregnancy in hot environments. Animal 2024, 18 (Suppl. 2), 101219. [Google Scholar] [CrossRef]
- Liu, L.; Ren, M.; Ren, K.; Jin, Y.; Yan, M. Heat stress impacts on broiler performance: A systematic review and meta-analysis. Poult. Sci. 2020, 99, 6205–6211. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.J.S.; Lee, J.; Kim, D.H.; Beak, S.H.; Hong, S.J.; Jeong, I.H.; Yoo, S.P.; Lee, J.O.; Cho, I.G.; Fassah, D.M.; et al. Effects of stress after road transportation and oral administration of chromium and meloxicam on plasma cortisol concentrations and behavior in dairy calves. Anim. Biosci. 2022, 35, 503–510. [Google Scholar] [CrossRef]
- Choi, W.T.; Ghassemi Nejad, J.; Moon, J.O.; Lee, H.G. Dietary supplementation of acetate-conjugated tryptophan alters feed intake, milk yield and composition, blood profile, physiological variables, and heat shock protein gene expression in heat-stressed dairy cows. J. Therm. Biol. 2021, 98, 102949. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.; Saredy, J.; Yuan, Z.; Yang, X.; Wang, H. Innate-adaptive immunity interplay and redox regulation in immune response. Redox Biol. 2020, 37, 101759. [Google Scholar] [CrossRef]
- Han, Q.; Liu, R.; Wang, H.; Zhang, R.; Liu, H.; Li, J.; Bao, J. Gut Microbiota-Derived 5-Hydroxyindoleacetic Acid Alleviates Diarrhea in Piglets via the Aryl Hydrocarbon Receptor Pathway. J. Agric. Food Chem. 2023, 71, 15132–15144. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.L.; Poller, W.C.; Swirski, F.K.; Russo, S.J. Central regulation of stress-evoked peripheral immune responses. Nat. Rev. Neurosci. 2023, 24, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.J.; Corr, E.M.; van Solingen, C.; Schlamp, F.; Brown, E.J.; Koelwyn, G.J.; Lee, A.H.; Shanley, L.C.; Spruill, T.M.; Bozal, F.; et al. Chronic stress primes innate immune responses in mice and humans. Cell Rep. 2021, 36, 109595. [Google Scholar] [CrossRef]
- Bonetti, A.; Tugnoli, B.; Ghiselli, F.; Markley, G.; Cooper, E.; Piva, A.; Stahl, C.H.; Grilli, E. A microencapsulated blend of botanicals supports weaning piglets during a lipopolysaccharide challenge by modulating liver inflammation and intestinal integrity. J. Anim. Sci. 2024, 102, skae277. [Google Scholar] [CrossRef]
- Rymut, H.E.; Rund, L.A.; Bolt, C.R.; Villamil, M.B.; Southey, B.R.; Johnson, R.W.; Rodriguez-Zas, S.L. The Combined Effect of Weaning Stress and Immune Activation during Pig Gestation on Serum Cytokine and Analyte Concentrations. Animals 2021, 11, 2274. [Google Scholar] [CrossRef]
- Xu, W.; Lu, J.; Chen, Y.; Wang, Z.; Cao, J.; Dong, Y. Impairment of CRH in the intestinal mucosal epithelial barrier of pregnant Bama miniature pig induced by restraint stress. Endocr. J. 2021, 68, 485–502. [Google Scholar] [CrossRef]
- Zhou, J.; Peng, Z.; Wang, J. Trelagliptin Alleviates Lipopolysaccharide (LPS)-Induced Inflammation and Oxidative Stress in Acute Lung Injury Mice. Inflammation 2021, 44, 1507–1517. [Google Scholar] [CrossRef]
- Pi, C.C.; Cheng, Y.C.; Chen, C.C.; Lee, J.W.; Lin, C.N.; Chiou, M.T.; Chen, H.W.; Chiu, C.H. Synergistic fermentation of Cordyceps militaris and herbal substrates boosts grower pig antioxidant and immune function. BMC Vet. Res. 2024, 20, 531. [Google Scholar] [CrossRef] [PubMed]
- Agustinho, B.C.; Mark, A.E.; Laarman, A.H.; Konetchy, D.E.; Rezamand, P. Effect of pH and lipopolysaccharide on tight junction regulators and inflammatory markers in intestinal cells as an experimental model of weaning transition in dairy calves. JDS Commun. 2023, 4, 394–399. [Google Scholar] [CrossRef]
- Rahman, M.A.; Kanda, Y.; Ozawa, M.; Kawamura, T.; Takeuchi, A.; Katakai, T. Transdermal entry of yeast components elicits transient B cell-associated responses in skin-draining lymph nodes. Cell. Immunol. 2020, 355, 104159. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, X.; Zhang, X.; Niu, G.; Yang, L.; Ji, W.; Zhang, L.; Ren, L. PCV2 and PRV Coinfection Induces Endoplasmic Reticulum Stress via PERK-eIF2α-ATF4-CHOP and IRE1-XBP1-EDEM Pathways. Int. J. Mol. Sci. 2022, 23, 4479. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, L.; Wang, J.; Su, D.; Li, D.; Du, Y.; Yang, G.; Zhang, G.; Chu, B. African Swine Fever Virus K205R Induces ER Stress and Consequently Activates Autophagy and the NF-κB Signaling Pathway. Viruses 2022, 14, 394. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, L.; Lucius, R.; Roider, J.; Klettner, A. Interaction of inflammatorily activated retinal pigment epithelium with retinal microglia and neuronal cells. Exp. Eye Res. 2020, 199, 108167. [Google Scholar] [CrossRef]
- Langendijk, P.L.; Soede, N.M. Physiology and management of the peri-parturient sow in the context of changing production conditions. Reprod. Domest. Anim. Zuchthyg. 2023, 58 (Suppl. 2), 84–92. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Peng, Y.; Zhang, Y.; Liu, Y.; Liu, Y.; Yin, Y. Research progress on anti-stress nutrition strategies in swine. Anim. Nutr. (Zhongguo Xu Mu Shou Yi Xue Hui) 2023, 13, 342–360. [Google Scholar] [CrossRef]
- Nemeth, M.; Herrmann, S.M.; Wallner, B.; Millesi, E. Effects of the estrous cycle and sex on stress responses in guinea pigs. Sci. Rep. 2025, 15, 25253. [Google Scholar] [CrossRef]
- Sohal, R.S.; Allen, R.G. Oxidative Stress as a Causal Factor in Differentiation and Aging—A Unifying Hypothesis. Exp. Gerontol. 1990, 25, 499–522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox Rep. Commun. Free Radic. Res. 2022, 27, 45–52. [Google Scholar] [CrossRef]
- Li, D.; Shen, L.; Zhang, D.; Wang, X.; Wang, Q.; Qin, W.; Gao, Y.; Li, X. Ammonia-induced oxidative stress triggered proinflammatory response and apoptosis in pig lungs. J. Environ. Sci. 2023, 126, 683–696. [Google Scholar] [CrossRef]
- Xie, M.; Li, Q.; Qiu, S.; Qi, X.; Shen, H. Effects of Eupolyphaga sinensis Walker polypeptides on growth performance, meat quality, organ indexes and antioxidant capacity of broilers under oxidative stress. Chin. J. Anim. Nutr. 2018, 30, 1726–1735. [Google Scholar]
- Li, X.; Wang, C.; Zhu, J.; Lin, Q.; Yu, M.; Wen, J.; Feng, J.; Hu, C. Sodium Butyrate Ameliorates Oxidative Stress-Induced Intestinal Epithelium Barrier Injury and Mitochondrial Damage through AMPK-Mitophagy Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 3745135. [Google Scholar] [CrossRef]
- Veshkini, A.; Gnott, M.; Vogel, L.; Kröger-Koch, C.; Tuchscherer, A.; Tröscher, A.; Bernabucci, U.; Trevisi, E.; Starke, A.; Mielenz, M.; et al. Abomasal infusion of essential fatty acids and conjugated linoleic acid during late pregnancy and early lactation affects immunohematological and oxidative stress markers in dairy cows. J. Dairy Sci. 2023, 106, 5096–5114. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Jiang, J.; Wan, F.; Tan, D.; Zheng, H.; Xue, H.; Hang, Y.; Lu, Y.; Su, Y. Cordyceps militaris Extract and Cordycepin Alleviate Oxidative Stress, Modulate Gut Microbiota and Ameliorate Intestinal Damage in LPS-Induced Piglets. Antioxidants 2024, 13, 441. [Google Scholar] [CrossRef] [PubMed]
- García-Trejo, S.S.; Gómez-Sierra, T.; Eugenio-Pérez, D.; Medina-Campos, O.N.; Pedraza-Chaverri, J. Protective Effect of Curcumin on D-Galactose-Induced Senescence and Oxidative Stress in LLC-PK1 and HK-2 Cells. Antioxidants 2024, 13, 415. [Google Scholar] [CrossRef] [PubMed]
- Ulla, A.; Uchida, T.; Miki, Y.; Sugiura, K.; Higashitani, A.; Kobayashi, T.; Ohno, A.; Nakao, R.; Hirasaka, K.; Sakakibara, I.; et al. Morin attenuates dexamethasone-mediated oxidative stress and atrophy in mouse C2C12 skeletal myotubes. Arch. Biochem. Biophys. 2021, 704, 108873. [Google Scholar] [CrossRef] [PubMed]
- Ambwani, S.; Dolma, R.; Sharma, R.; Kaur, A.; Singh, H.; Ruj, A.; Ambwani, T.K. Modulation of inflammatory and oxidative stress biomarkers due to dexamethasone exposure in chicken splenocytes. Vet. Immunol. Immunopathol. 2023, 262, 110632. [Google Scholar] [CrossRef]
- Yuan, T.; Fu, D.; Xu, R.; Ding, J.; Wu, J.; Han, Y.; Li, W. Corticosterone mediates FKBP51 signaling and inflammation response in the trigeminal ganglion in chronic stress-induced corneal hyperalgesia mice. J. Steroid Biochem. Mol. Biol. 2023, 231, 106312. [Google Scholar] [CrossRef]
- Jafari, Z.; Mehla, J.; Afrashteh, N.; Kolb, B.E.; Mohajerani, M.H. Corticosterone response to gestational stress and postpartum memory function in mice. PLoS ONE 2017, 12, e0180306. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Zhu, J.; Wu, J.; Thompson, C.B.; Jiang, X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc. Natl. Acad. Sci. USA 2020, 117, 31189–31197. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, J.S.; Lee, Y.S.; Han, J.; Lee, D.K.; Kwon, S.W.; Han, D.H.; Lee, Y.H.; Bae, S.H. SQSTM1/p62 activates NFE2L2/NRF2 via ULK1-mediated autophagic KEAP1 degradation and protects mouse liver from lipotoxicity. Autophagy 2020, 16, 1949–1973. [Google Scholar] [CrossRef]
- Park, A.; Koh, H.C. NF-κB/mTOR-mediated autophagy can regulate diquat-induced apoptosis. Arch. Toxicol. 2019, 93, 1239–1253. [Google Scholar] [CrossRef]
- Wang, L.; Tang, L.; Feng, Y.; Zhao, S.; Han, M.; Zhang, C.; Yuan, G.; Zhu, J.; Cao, S.; Wu, Q.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8+ T cells in mice. Gut 2020, 69, 1988–1997. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, X.; Zhong, S.; Yu, W.; Wang, J.; Zhu, W.; Yang, T.; Zhao, G.; Jiang, Y.; Li, Y. Effects of Continuous LPS Induction on Oxidative Stress and Liver Injury in Weaned Piglets. Vet. Sci. 2022, 10, 22. [Google Scholar] [CrossRef]
- Cilenti, F.; Barbiera, G.; Caronni, N.; Iodice, D.; Montaldo, E.; Barresi, S.; Lusito, E.; Cuzzola, V.; Vittoria, F.M.; Mezzanzanica, L.; et al. A PGE2-MEF2A axis enables context-dependent control of inflammatory gene expression. Immunity 2021, 54, 1665–1682.e1614. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, Z.; Bai, Y.; Xu, S. Redox state and metabolic responses to severe heat stress in lenok Brachymystax lenok (Salmonidae). Front. Mol. Biosci. 2023, 10, 1156310. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, M.; Cui, J.; Du, Y.; Teng, X.; Zhang, Z. Heat shock proteins took part in oxidative stress-mediated inflammatory injury via NF-κB pathway in excess manganese-treated chicken livers. Ecotoxicol. Environ. Saf. 2021, 226, 112833. [Google Scholar] [CrossRef]
- Chen, F.; Ling, X.; Zhao, Y.; Fu, S. Hypoxia-induced oxidative stress and apoptosis in gills of scaleless carp (Gymnocypris przewalskii). Fish Physiol. Biochem. 2022, 48, 911–924. [Google Scholar] [CrossRef]
- Bejaoui, B.; Sdiri, C.; Ben Souf, I.; Belhadj Slimen, I.; Ben Larbi, M.; Koumba, S.; Martin, P.; M’Hamdi, N. Physicochemical Properties, Antioxidant Markers, and Meat Quality as Affected by Heat Stress: A Review. Molecules 2023, 28, 3332. [Google Scholar] [CrossRef]
- Takeuchi, K.; Nakano, Y.; Kato, U.; Kaneda, M.; Aizu, M.; Awano, W.; Yonemura, S.; Kiyonaka, S.; Mori, Y.; Yamamoto, D.; et al. Changes in temperature preferences and energy homeostasis in dystroglycan mutants. Science 2009, 323, 1740–1743. [Google Scholar] [CrossRef] [PubMed]
- Saleh, K.M.M.; Tarkhan, A.H.; Al-Zghoul, M.B. Embryonic Thermal Manipulation Affects the Antioxidant Response to Post-Hatch Thermal Exposure in Broiler Chickens. Animals 2020, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Ghassemi Nejad, J.; Lee, H.G. Impact of Cold Stress on Physiological, Endocrinological, Immunological, Metabolic, and Behavioral Changes of Beef Cattle at Different Stages of Growth. Animals 2023, 13, 1073. [Google Scholar] [CrossRef]
- Saeed, M.; Babazadeh, D.; Naveed, M.; Arain, M.A.; Hassan, F.U.; Chao, S. Reconsidering betaine as a natural anti-heat stress agent in poultry industry: A review. Trop. Anim. Health Prod. 2017, 49, 1329–1338. [Google Scholar] [CrossRef]
- Mutua, J.Y.; Marshall, K.; Paul, B.K.; Notenbaert, A.M.O. A methodology for mapping current and future heat stress risk in pigs. Anim. Int. J. Anim. Biosci. 2020, 14, 1952–1960. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yuan, Z.; Dong, G.; Wei, X.; Song, D. Dietary calcium soaps of fatty acids and chromium nicotinate affect lactation performance, physiological and serum biochemical indices of dairy cows under heat stress. Chin. J. Anim. Nutr. 2012, 24, 145–151. [Google Scholar]
- Paranhos Da Costa, M.J.R.; Silva, R.G.D.; Souza, R.C.D. Effect of air temperature and humidity on ingestive behaviour of sheep. Int. J. Biometeorol. 1992, 36, 218–222. [Google Scholar] [CrossRef]
- Silva, P.S.; Hooper, H.B.; Manica, E.; Merighe, G.K.F.; Oliveira, S.A.; Traldi, A.S.; Negrão, J.A. Heat stress affects the expression of key genes in the placenta, placental characteristics, and efficiency of Saanen goats and the survival and growth of their kids. J. Dairy Sci. 2021, 104, 4970–4979. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, L.; Zhu, R.; Zhang, S.; Liu, S.; Wang, Y.; Wu, Y.; Xing, S.; Liao, X.; Mi, J. Porcine gut microbiota in mediating host metabolic adaptation to cold stress. Npj Biofilms Microbiomes 2022, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, M.R.; Neinast, M.D.; Zeng, X.; Chu, Q.; Axsom, J.; Thorsheim, C.; Li, K.; Blair, M.C.; Rabinowitz, J.D.; Arany, Z. Comprehensive quantification of metabolic flux during acute cold stress in mice. Cell Metab. 2023, 35, 2077–2092.e2076. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, X.; Wan, B.; Wang, D.; Li, M.; Cheng, K.; Luo, Q.; Wang, D.; Lu, Y.; Zhu, L. Caveolin-1 accelerates hypoxia-induced endothelial dysfunction in high-altitude cerebral edema. Cell Commun. Signal. CCS 2022, 20, 160. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cui, Y.; Ma, R.; Yu, S.; Zhang, H.; Zhao, P.; He, J. Hypoxia Regulates the Proliferation and Apoptosis of Coronary Artery Smooth Muscle Cells Through HIF-1α Mediated Autophagy in Yak. Biomolecules 2025, 15, 256. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yang, J.; Kim, J.; Jang, Y.; Lee, J.; Han, D.; Kim, H.; Jeong, B.C.; Seong, J.K. Effects of Environmental Noise Stress on Mouse Metabolism. Int. J. Mol. Sci. 2024, 25, 10985. [Google Scholar] [CrossRef]
- Ma, T.; Matsuo, R.; Kurogi, K.; Miyamoto, S.; Morita, T.; Shinozuka, M.; Taniguchi, F.; Ikegami, K.; Yasuo, S. Sex-dependent effects of chronic jet lag on circadian rhythm and metabolism in mice. Biol. Sex Differ. 2024, 15, 102. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, W.; Ba, M.; Xuan, S.; Huang, D.; Qi, D.; Pei, X.; Lu, D.; Li, Z. Chronic Jet Lag Disrupts Circadian Rhythms and Induces Hyperproliferation in Murine Lacrimal Glands via ROS Accumulation. Investig. Ophthalmol. Vis. Sci. 2025, 66, 12. [Google Scholar] [CrossRef]
- Shi, Z.; Xi, L.; Wang, Y.; Zhao, X. Chronic Exposure to Environmental Pollutant Ammonia Causes Damage to the Olfactory System and Behavioral Abnormalities in Mice. Environ. Sci. Technol. 2023, 57, 15412–15421. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Chen, S.; Wei, B.; Gao, Y.; Huang, L.; Liu, C.; Huang, T.; Yu, M.; Zhao, S.H.; et al. Ammonia exposure causes lung injuries and disturbs pulmonary circadian clock gene network in a pig study. Ecotoxicol. Environ. Saf. 2020, 205, 111050. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sang, Z.; Zhuo, Y.; Wang, X.; Guo, Z.; He, L.; Zeng, C.; Dai, H. Transport stress induces pig jejunum tissue oxidative damage and results in autophagy/mitophagy activation. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhang, W.; Zhou, L.; Feng, F. Effect of Pre-Slaughter Transport Stress on Protein S-Nitrosylation Levels of Pork during Postmortem Aging. J. Agric. Food Chem. 2023, 71, 11150–11157. [Google Scholar] [CrossRef] [PubMed]
- Grimm, H.; Calder, P.C. Immunonutrition. Br. J. Nutr. 2002, 87, S1. [Google Scholar] [CrossRef]
- Belhadj Slimen, I.; Yerou, H.; Ben Larbi, M.; M’Hamdi, N.; Najar, T. Insects as an alternative protein source for poultry nutrition: A review. Front. Vet. Sci. 2023, 10, 1200031. [Google Scholar] [CrossRef]
- Pasiakos, S.M. Nutritional Requirements for Sustaining Health and Performance During Exposure to Extreme Environments. Annu. Rev. Nutr. 2020, 40, 221–245. [Google Scholar] [CrossRef]
- Racinais, S.; Dablainville, V.; Rousse, Y.; Ihsan, M.; Grant, M.E.; Schobersberger, W.; Budgett, R.; Engebretsen, L. Cryotherapy for treating soft tissue injuries in sport medicine: A critical review. Br. J. Sports Med. 2024, 58, 1215–1223. [Google Scholar] [CrossRef]
- Espina, J.A.; Cordeiro, M.H.; Milivojevic, M.; Pajic-Lijakovic, I.; Barriga, E.H. Response of cells and tissues to shear stress. J. Cell Sci. 2023, 136, jcs260985. [Google Scholar] [CrossRef]
- Cao, X.; Xiao, S.; Shen, C.; Fan, Y. Microdamage in biological hard tissues and its repair mechanisms. Biomed. Eng. Online 2025, 24, 102. [Google Scholar] [CrossRef]
- He, Z.; Cai, Y.; Xiao, Y.; Cao, S.; Zhong, G.; Li, X.; Li, Y.; Luo, J.; Tang, J.; Qu, F.; et al. Intervention of Dietary Protein Levels on Muscle Quality, Antioxidation, and Autophagy in the Muscles of Triploid Crucian Carp (Carassius carassius Triploid). Int. J. Mol. Sci. 2023, 24, 12043. [Google Scholar] [CrossRef]
- Mafra, D.; Brum, I.; Borges, N.A.; Leal, V.O.; Fouque, D. Low-protein diet for chronic kidney disease: Evidence, controversies, and practical guidelines. J. Intern. Med. 2025, 298, 319–335. [Google Scholar] [CrossRef]
- Castro, T.F.; de Matos, N.A.; de Souza, A.B.F.; Costa, G.P.; Perucci, L.O.; Talvani, A.; Cangussu, S.D.; de Menezes, R.C.A.; Bezerra, F.S. Protein restriction during pregnancy affects lung development and promotes oxidative stress and inflammation in C57 BL/6 mice offspring. Nutrition 2022, 101, 111682. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Park, H.; Hong, J.; Kim, Y.; Jeong, Y.; Sa, S.; Choi, Y.; Kim, J. Heat Stress in Growing-Finishing Pigs: Effects of Low Protein with Increased Crystalline Amino Acids on Growth, Gut Health, Antioxidant Status and Microbiome. Animals 2025, 15, 848. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, X.; Feng, Y.; Ding, H.; Sun, H.; Li, Z.; Shi, B. Dietary fat supplementation relieves cold temperature-induced energy stress through AMPK-mediated mitochondrial homeostasis in pigs. J. Anim. Sci. Biotechnol. 2024, 15, 56. [Google Scholar] [CrossRef]
- Chen, X.; Liu, W.; Li, H.; Zhang, J.; Hu, C.; Liu, X. The adverse effect of heat stress and potential nutritional interventions. Food Funct. 2022, 13, 9195–9207. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cui, Z.; Wang, H.; Huang, B.; Ma, H. Dietary supplementation of dimethyl itaconate protects against chronic heat stress-induced growth performance impairment and lipid metabolism disorder in broiler chickens. J. Anim. Sci. 2023, 101, skad120. [Google Scholar] [CrossRef]
- Morales, A.; Gómez, T.; Villalobos, Y.D.; Bernal, H.; Htoo, J.K.; González-Vega, J.C.; Espinoza, S.; Yáñez, J.; Cervantes, M. Dietary protein-bound or free amino acids differently affect intestinal morphology, gene expression of amino acid transporters, and serum amino acids of pigs exposed to heat stress. J. Anim. Sci. 2020, 98, skaa056. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Y.; Zhu, H.; Hong, Y.; Wu, Z.; Hou, Y.; Li, Q.; Ding, B.; Yi, D.; Chen, H. Fish oil attenuates liver injury caused by LPS in weaned pigs associated with inhibition of TLR4 and nucleotide-binding oligomerization domain protein signaling pathways. Innate Immun. 2013, 19, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, D.; Gong, L.; Zhang, W. Effect of immunological stress on immune parameter and endocrine hormones in weaned pigs. Chin. J. Anim. Sci. 2004, 40, 4–6. [Google Scholar]
- Pang, Y.; Jiang, J.; Wang, L.; Zhai, Q.; Song, C. Effects of immunological stress on immune response in different breeds of piglets. Anim. Husb. Feed Sci. 2009, 1, 28–31. [Google Scholar]
- Haisan, J.; Inabu, Y.; Shi, W.; Oba, M. Effects of pre- and postpartum dietary starch content on productivity, plasma energy metabolites, and serum inflammation indicators of dairy cows. J. Dairy Sci. 2021, 104, 4362–4374. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Huasai, S.; Chen, A. Effect of prepartum dietary energy density on beef cow energy metabolites, and birth weight and antioxidative capabilities of neonatal calves. Sci. Rep. 2022, 12, 4828. [Google Scholar] [CrossRef] [PubMed]
- Adebowale, T.; Jiang, Q.; Yao, K. Dietary fat and high energy density diet: Influence on intestinal health, oxidative stress and performance of weaned piglets. J. Anim. Physiol. Anim. Nutr. 2024, 108, 978–986. [Google Scholar] [CrossRef]
- Ma, Y.; Kroemer, G. The cancer-immune dialogue in the context of stress. Nat. Rev. Immunol. 2024, 24, 264–281. [Google Scholar] [CrossRef]
- Najafi, P.; Zulkifli, I.; Soleimani, A.F. Inhibition of corticosterone synthesis and its effect on acute phase proteins, heat shock protein 70, and interleukin-6 in broiler chickens subjected to feed restriction. Poult. Sci. 2018, 97, 1441–1447. [Google Scholar] [CrossRef]
- Zhou, Q.; Gao, Y.; Li, Y.; Xie, H.; Liu, X.; Yong, Y.; Li, Y.; Yu, Z.; Ma, X.; Ju, X. Preliminary Proteomic Study of the Porcine Pituitary Gland under Heat Stress. Life 2024, 14, 366. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 587. [Google Scholar] [CrossRef] [PubMed]
- Zinicola, M.; Menta, P.R.; Ribeiro, B.L.; Boisclair, Y.; Bicalho, R.C. Effects of recombinant bovine interleukin-8 (rbIL-8) treatment on health, metabolism, and lactation performance in Holstein cattle III: Administration of rbIL-8 induces insulin resistance in bull calves. J. Dairy Sci. 2019, 102, 10329–10339. [Google Scholar] [CrossRef]
- Lendez, P.A.; Martinez Cuesta, L.; Nieto Farias, M.V.; Vater, A.A.; Ghezzi, M.D.; Mota-Rojas, D.; Dolcini, G.L.; Ceriani, M.C. Alterations in TNF-α and its receptors expression in cows undergoing heat stress. Vet. Immunol. Immunopathol. 2021, 235, 110232. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Muro, P.; Zhang, L.; Li, S.; Zhao, Z.; Jin, T.; Mao, F.; Mao, Z. The emerging role of oxidative stress in inflammatory bowel disease. Front. Endocrinol. 2024, 15, 1390351. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Cai, X.; Wang, C.; Peng, X.; Xu, L.; Gao, Y.; Tian, T.; Zhu, G.; Pan, Y.; Chu, H.; et al. FOXM1 affects oxidative stress, mitochondrial function, and the DNA damage response by regulating p21 in aging oocytes. Theriogenology 2024, 229, 66–74. [Google Scholar] [CrossRef]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2011, 153, 175–190. [Google Scholar] [CrossRef]
- Bal, A.; Panda, F.; Pati, S.G.; Das, K.; Agrawal, P.K.; Paital, B. Modulation of physiological oxidative stress and antioxidant status by abiotic factors especially salinity in aquatic organisms. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2021, 241, 108971. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Storey, K.B. Oxidative stress concept updated: Definitions, classifications, and regulatory pathways implicated. EXCLI J. 2021, 20, 956–967. [Google Scholar] [CrossRef]
- Bonomini, F.; Rodella, L.F.; Rezzani, R. Metabolic syndrome, aging and involvement of oxidative stress. Aging Dis. 2015, 6, 109–120. [Google Scholar] [CrossRef]
- Antonucci, S.; Di Lisa, F.; Kaludercic, N. Mitochondrial reactive oxygen species in physiology and disease. Cell Calcium 2021, 94, 102344. [Google Scholar] [CrossRef] [PubMed]
- Makinde, E.; Ma, L.; Mellick, G.D.; Feng, Y. Mitochondrial Modulators: The Defender. Biomolecules 2023, 13, 226. [Google Scholar] [CrossRef]
- Chen, G.H.; Song, C.C.; Pantopoulos, K.; Wei, X.L.; Zheng, H.; Luo, Z. Mitochondrial oxidative stress mediated Fe-induced ferroptosis via the NRF2-ARE pathway. Free Radic. Biol. Med. 2022, 180, 95–107. [Google Scholar] [CrossRef]
- Bose, A.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in induced pluripotent stem cell models of Parkinson’s disease. Eur. J. Neurosci. 2019, 49, 525–532. [Google Scholar] [CrossRef]
- González-Alvarez, M.E.; Roach, C.M.; Keating, A.F. Scrambled eggs-Negative impacts of heat stress and chemical exposures on ovarian function in swine. Mol. Reprod. Dev. 2023, 90, 503–516. [Google Scholar] [CrossRef]
- Keating, A.F.; Ross, J.W.; Baumgard, L.H. Impact of Real-Life Environmental Exposures on Reproduction: Systemic and ovarian impacts of heat stress in the porcine model. Reproduction 2024, 168, e240217. [Google Scholar] [CrossRef]
- Teng, T.; Song, X.; Sun, G.; Ding, H.; Sun, H.; Bai, G.; Shi, B. Glucose supplementation improves intestinal amino acid transport and muscle amino acid pool in pigs during chronic cold exposure. Anim. Nutr. (Zhongguo Xu Mu Shou Yi Xue Hui) 2023, 12, 360–374. [Google Scholar] [CrossRef]
- Hu, H.; Bai, X.; Shah, A.A.; Wen, A.Y.; Hua, J.L.; Che, C.Y.; He, S.J.; Jiang, J.P.; Cai, Z.H.; Dai, S.F. Dietary supplementation with glutamine and -aminobutyric acid improves growth performance and serum parameters in 22-to 35-day-old broilers exposed to hot environment. J. Anim. Physiol. Anim. Nutr. 2016, 100, 361–370. [Google Scholar] [CrossRef]
- Liu, E.; Sun, M.; He, C.; Mao, K.; Li, Q.; Zhang, J.; Wu, D.; Wang, S.; Zheng, C.; Li, W.; et al. Rumen Microbial Metabolic Responses of Dairy Cows to the Honeycomb Flavonoids Supplement Under Heat-Stress Conditions. Front. Vet. Sci. 2022, 9, 845911. [Google Scholar] [CrossRef] [PubMed]
- Kikusato, M.; Toyomizu, M. Mechanisms underlying the Effects of Heat Stress on Intestinal Integrity, Inflammation, and Microbiota in Chickens. J. Poult. Sci. 2023, 60, 2023021. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Kronenfeld, J.M.; Renquist, B.J. Feed intake-dependent and -independent effects of heat stress on lactation and mammary gland development. J. Dairy Sci. 2020, 103, 12003–12014. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, H.; Yu, D.; Zhao, P.; Liu, Y. Heat stress inhibits the proliferation and differentiation of myoblasts and is associated with damage to mitochondria. Front. Cell Dev. Biol. 2023, 11, 1171506. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, H.; Ouyang, J.; Guo, S.; Zheng, J.; Li, G. Effects of dietary tryptophan supplementation on body temperature, hormone, and cytokine levels in broilers exposed to acute heat stress. Trop. Anim. Health Prod. 2022, 54, 164. [Google Scholar] [CrossRef]
- Fan, H.; Ding, R.; Liu, W.; Zhang, X.; Li, R.; Wei, B.; Su, S.; Jin, F.; Wei, C.; He, X.; et al. Heat shock protein 22 modulates NRF1/TFAM-dependent mitochondrial biogenesis and DRP1-sparked mitochondrial apoptosis through AMPK-PGC1α signaling pathway to alleviate the early brain injury of subarachnoid hemorrhage in rats. Redox Biol. 2021, 40, 101856. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, X.; Fang, H.; Ren, Y.; Li, X.; Ren, W.; Xue, B.; Yang, C. Effect of cold stress on ovarian & uterine microcirculation in rats and the role of endothelin system. Reprod. Biol. Endocrinol. 2020, 18, 29. [Google Scholar] [CrossRef]
- Sun, G.; Song, X.; Zou, Y.; Teng, T.; Jiang, L.; Shi, B. Dietary Glucose Ameliorates Impaired Intestinal Development and Immune Homeostasis Disorders Induced by Chronic Cold Stress in Pig Model. Int. J. Mol. Sci. 2022, 23, 7730. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Li, M.; Li, W.; Guo, Y.; Zhang, J.; Ye, L.; Guo, Z.; Yang, Y.; Liu, W.; Chen, L.; et al. Effects of co-exposure to heat and ozone on lipid metabolism in the liver and adipose tissue of C57BL/6J male mice. J. Hazard. Mater. 2025, 489, 137577. [Google Scholar] [CrossRef] [PubMed]
- Hatch-McChesney, A.; Smith, T.J. Nutrition, Immune Function, and Infectious Disease in Military Personnel: A Narrative Review. Nutrients 2023, 15, 4999. [Google Scholar] [CrossRef] [PubMed]
- Mengal, K.; Kor, G.; Kozak, P.; Niksirat, H. Effects of environmental factors on the cellular and molecular parameters of the immune system in decapods. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2023, 276, 111332. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).