Alfalfa and Linseed Oil in Diet for Fattening Rabbits: Performance, Meat Quality, and Nutritional Characteristics
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Chemical Analyses
2.2.1. Proximate Composition of Diet and LTL Meat
2.2.2. Fatty Acid Composition of Diet and LTL Meat
2.2.3. Estimated Lipid Index
2.2.4. Lipid Oxidation of LTL Meat
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cullere, M.; Dalle Zotte, A. Rabbit meat production and consumption: State of knowledge and future perspectives. Meat Sci. 2018, 143, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Crovato, S.; Pinto, A.; Di Martino, G.; Mascarello, G.; Rizzoli, V.; Marcolin, S.; Ravarotto, L. Purchasing habits, sustainability perceptions, and welfare concerns of Italian consumers regarding rabbit meat. Foods 2022, 11, 1205. [Google Scholar] [CrossRef]
- Ismea 2024. Tendenze Settore Cunicolo, n.1/2024. Available online: https://www.ismeamercati.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/13269 (accessed on 13 September 2025).
- Bordignon, F.; Xiccato, G.; Trocino, A.; Zuffellato, A.; Castellini, C.; Mattioli, S.; Berton, M. Environmental impact of rabbit production systems: A farm-based cradle-to-gate analysis. Animal 2025, 19, 101488. [Google Scholar] [CrossRef]
- Cavani, C.; Petracci, M.; Trocino, A.; Xiccato, G. Advances in research on poultry and rabbit meat quality. Ital. J. Anim. Sci. 2009, 8, 741–750. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Castellini, C.; Mancini, S.; Angelucci, E.; Di Federico, F.; Bosa, L.; Mattioli, S. Productive performance and meat nutritional and sensory characteristics of rabbits fed alfalfa-based diet. Meat Sci. 2025, 228, 109897. [Google Scholar] [CrossRef]
- Rodríguez, M.; Rebollar, P.G.; Mattioli, S.; Castellini, C. n-3 PUFA sources (precursor/products): A review of current knowledge on rabbit. Animals 2019, 9, 806. [Google Scholar] [CrossRef]
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Fehri, N.E.; Bejaoui, S.; Brecchia, G.; Jemmali, B. Effect of linseed (Linum usitatissimum) supplementation on the productive and reproductive performance of doe rabbits. Afr. Mediterr. Agric. J.—Al Awamia 2025, 147, 67–74. [Google Scholar]
- Quattrone, A.; Beqiraj, D.; Fehri, N.E.; Belabbas, R.; Vigo, D.; Menchetti, L.; Barbato, O.; Failla, S.; Faustini, M.; Ghoneim, S.S. Impact of Dietary Enrichment with Omega-3 Polyunsaturated Fatty Acids from Extruded Linseed and Padina pavonica Algae Extract on Growth Performance and Metabolic Status in Fattening Rabbits. Animals 2025, 15, 2085. [Google Scholar] [CrossRef]
- Fehri, N.E.; Contò, M.; Castrica, M.; Quattrone, A.; Renzi, G.; Di Giovanni, S.; Agradi, S.; Vigo, D.; Brecchia, G.; Menchetti, L. Effects of diets containing extruded linseed and Padina pavonica algae on meat rabbit: Carcass performance and meat quality. Foods 2025, 14, 274. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Carmona, J.; Cervera, C.; Blas, E. Prediction of the energy value of rabbit feeds varying widely in fibre content. Anim. Feed Sci. Technol. 1996, 64, 61–75. [Google Scholar] [CrossRef]
- de Blas, C.; Mateos, G.G. Feed formulation. In Nutrition of the Rabbit; CAB International: Wallingford, UK, 2020; pp. 243–253. [Google Scholar]
- Blasco Mateu, A.; Ouhayoun, J.; Masoero, G. Harmonization of criteria and terminology in rabbit meat research. World Rabbit Sci. 1993, 1, 3–10. [Google Scholar]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Christie, W.W. A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J. Lipid Res. 1982, 23, 1072–1075. [Google Scholar] [CrossRef]
- Vahmani, P.; Rolland, D.C.; McAllister, T.A.; Block, H.C.; Proctor, S.D.; Guan, L.L.; Prieto, N.; López-Campos, Ó.; Aalhus, J.L.; Dugan, M.E.R. Effects of feeding steers extruded flaxseed on its own before hay or mixed with hay on animal performance, carcass quality, and meat and hamburger fatty acid composition. Meat Sci. 2017, 131, 9–17. [Google Scholar] [CrossRef]
- Bosco, A.D.; Mancinelli, A.C.; Vaudo, G.; Cavallo, M.; Castellini, C.; Mattioli, S. Indexing of Fatty Acids in Poultry Meat for Its Characterization in Healthy Human Nutrition: A Comprehensive Application of the Scientific Literature and New Proposals. Nutrients 2022, 14, 3110. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Cavallo, M.; Menchetti, L.; Angelucci, E.; Cartoni Mancinelli, A.; Vaudo, G.; Marconi, S.; Camilli, E.; Galli, F.; Castellini, C. The Healthy Fatty Index Allows for Deeper Insights into the Lipid Composition of Foods of Animal Origin When Compared with the Atherogenic and Thrombogenicity Indexes. Foods 2024, 13, 1568. [Google Scholar] [CrossRef]
- Ke, P.J.; Ackman, R.G.; Linke, B.A.; Nash, D.M. Differential lipid oxidation in various parts of frozen mackerel. Int. J. Food Sci. Technol. 1977, 12, 37–47. [Google Scholar] [CrossRef]
- StataCorp Stata Statistical Software, version 14; StataCorp LP: College Station, TX, USA, 2015.
- Bosa, L.; Molin, M.; Carnevali, A.; Petricciuolo, M.; Federici, E.; Xiccato, G.; Trocino, A.; Dal Bosco, A.; Mattioli, S.; Castellini, C. Feeding growing rabbits with alfalfa-based diet: Implications for performance, product quality and gut health. Ital. J. Anim. Sci. 2025, 24, 1751–1761. [Google Scholar] [CrossRef]
- Mattioli, S.; Castellini, C.; Mancini, S.; Roscini, V.; Mancinelli, A.C.; Cotozzolo, E.; Pauselli, M.; Dal Bosco, A. Effect of trub and/or linseed dietary supplementation on in vivo oxidative status and some quality traits of rabbit meat. Meat Sci. 2020, 163, 108061. [Google Scholar] [CrossRef]
- Combes, S.; Gidenne, T.; Cauquil, L.L.; Balmisse, E.B.; Aymard, P.; Bonnemere, J.M.; Bannelier, C.; Gabinaud, B.; Segura, M.; Tartie, V. Ingestion of feces in the nest enhances coprophagous behaviour of pups, affects implantation process of caecal microbiota and improves health. In Proceedings of the 15ème Journées de la Recherche Cunicole, Le Mans, France, 20–21 November 2013. [Google Scholar]
- Mattioli, S.; Dal Bosco, A.; Combes, S.; Moscati, L.; Crotti, S.; Cartoni Mancinelli, A.; Cotozzolo, E.; Castellini, C. Dehydrated alfalfa and fresh grass supply in young rabbits: Effect on performance and caecal microbiota biodiversity. Animals 2019, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Dal Bosco, A.; Mugnai, C.; Roscini, V.; Mattioli, S.; Ruggeri, S.; Castellini, C. Effect of dietary alfalfa on the fatty acid composition and indexes of lipid metabolism of rabbit meat. Meat Sci. 2014, 96, 606–609. [Google Scholar] [CrossRef]
- Capra, G.; Grompone, M.A.; Urruzola, N.; Pardo, M.J.; Martínez, R.; Fradiletti, F.; Cozzano, S.; Repiso, L.; Márquez, R. Effect of fresh alfalfa in the diet of growing rabbits on growth performance, carcass characteristics and fat composition. In Proceedings of the IV Congreso Cunicultura de las Américas, Córdoba, Argentina, 22–24 September 2010. [Google Scholar]
- Chen, J.; Liu, H. Nutritional indices for assessing fatty acids: A mini-review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Fereidoon, S.; Ying, Z. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef]
- Mattioli, S.; Dimauro, C.; Cesarani, A.; Dal Bosco, A.; Bartolini, D.; Galli, F.; Migni, A.; Sebastiani, B.; Signorini, C.; Oger, C.; et al. A Dynamic Model for Estimating the Interaction of ROS–PUFA–Antioxidants in Rabbit. Antioxidants 2022, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, S.; Collodel, G.; Signorini, C.; Cotozzolo, E.; Noto, D.; Cerretani, D.; Micheli, L.; Fiaschi, A.I.; Brecchia, G.; Menchetti, L.; et al. Tissue antioxidant status and lipid peroxidation are related to dietary intake of n-3 polyunsaturated acids: A rabbit model. Antioxidants 2021, 10, 681. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; Lattaioli, P.; Dal Bosco, A.; Beghelli, D. Effect of supranutritional level of dietary α-tocopheryl acetate and selenium on rabbit semen. Theriogenology 2002, 58, 1723–1732. [Google Scholar] [CrossRef]
- Mancini, S.; Mattioli, S.; Dal Bosco, A. Rabbit meat quality with an approach to its processing using spices or specific ingredients as antioxidants. World Rabbit Sci. 2025, 33, 93–102. [Google Scholar] [CrossRef]
- Quattrone, A.; Draghi, S.; Inglesi, A.; Riva, F.; Turmalaj, L.; Filipe, J.; Sulçe, M.; Agradi, S.; Vigo, D.; Muça, G. Effects of Goji Berry Supplementation on Immune-Related and Antioxidant Gene Expression in the Male Rabbit Reproductive Tract. Animals 2025, 15, 1921. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Gerencsér, Z.; Szendro, Z.; Mugnai, C.; Cullere, M.; Kovàcs, M.; Ruggeri, S.; Mattioli, S.; Castellini, C.; Dalle Zotte, A. Effect of dietary supplementation of Spirulina (Arthrospira platensis) and Thyme (Thymus vulgaris) on rabbit meat appearance, oxidative stability and fatty acid profile during retail display. Meat Sci. 2014, 96, 114–119. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Mourvaki, E.; Cardinali, R.; Servili, M.; Sebastiani, B.; Ruggeri, S.; Mattioli, S.; Taticchi, A.; Esposto, S.; Castellini, C. Effect of dietary supplementation with olive pomaces on the performance and meat quality of growing rabbits. Meat Sci. 2012, 92, 783–788. [Google Scholar] [CrossRef]
- Cardinali, R.; Cullere, M.; Dal Bosco, A.; Mugnai, C.; Ruggeri, S.; Mattioli, S.; Castellini, C.; Trabalza Marinucci, M.; Dalle Zotte, A. Oregano, rosemary and vitamin E dietary supplementation in growing rabbits: Effect on growth performance, carcass traits, bone development and meat chemical composition. Livest. Sci. 2015, 175, 83–89. [Google Scholar] [CrossRef]
- Castellini, C.; Mattioli, S.; Moretti, E.; Cotozzolo, E.; Perini, F.; Dal Bosco, A.; Signorini, C.; Noto, D.; Belmonte, G.; Lasagna, E. Expression of genes and localization of enzymes involved in polyunsaturated fatty acid synthesis in rabbit testis and epididymis. Sci. Rep. 2022, 12, 2637. [Google Scholar] [CrossRef]
- Mattioli, S.; Dal Bosco, A.; Maranesi, M.; Petrucci, L.; Rebollar, P.G.; Castellini, C. Dietary fish oil and flaxseed for rabbit does: Fatty acids distribution and Δ6-desaturase enzyme expression of different tissues. Animal 2019, 13, 1943. [Google Scholar] [CrossRef] [PubMed]
- Castellini, C.; Dal Bosco, A.; Mattioli, S.; Davidescu, M.; Corazzi, L.; MacChioni, L.; Rimoldi, S.; Terova, G. Activity, Expression, and Substrate Preference of the δ6-Desaturase in Slow- or Fast-Growing Rabbit Genotypes. J. Agric. Food Chem. 2016, 64, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, T.; Su, C.; Li, Q.; Yu, X. Fortification of Chinese steamed bread with flaxseed flour and evaluation of its physicochemical and sensory properties. Food Chem. X 2022, 13, 100267. [Google Scholar] [CrossRef] [PubMed]
| Diets | Control | Alfalfa | Alfalfa+Linseed |
|---|---|---|---|
| Ingredients (g/kg) | |||
| Dehydrated alfalfa meal | 80.00 | 400.00 | 380.00 |
| Alfalfa hay | 170.00 | 300.00 | 300.00 |
| Wheat meal | 150.00 | 140.00 | 140.00 |
| Wheat bran | 120.00 | - | - |
| OGM soybean meal | 140.00 | - | - |
| Sunflower meal | 80.00 | 83.00 | 83.00 |
| Dried beet pulp | 20.00 | 35.50 | 35.00 |
| Barley meal | 210.00 | 25.50 | 25.00 |
| Linseed oil 1 | 20.00 | ||
| Soybean oil | 10.00 | - | - |
| Mineral-vitamin premix 2 | 10.00 | 10.00 | 10.00 |
| Limestone | 5.00 | 5.00 | 5.00 |
| Marine salt | 5.00 | 2.00 | 2.00 |
| Chemical composition (g/100 g D.M.) | |||
| Crude protein (CP) | 16.40 | 15.80 | 15.60 |
| Crude fiber | 15.60 | 22.00 | 21.40 |
| Ether extract (EE) | 3.26 | 1.80 | 2.98 |
| Ash | 7.09 | 7.95 | 7.90 |
| NDF | 30.30 | 40.40 | 38.40 |
| ADF | 18.60 | 25.40 | 23.20 |
| Hemicellulose | 11.70 | 15.00 | 13.20 |
| Cellulose | 14.00 | 23.50 | 18.30 |
| ADL | 1.88 | 4.57 | 4.23 |
| Digestible energy (DE) (kcal/kg) 3 | 2450 | 2180 | 2341 |
| Fatty acids (mg/100 g diet) | |||
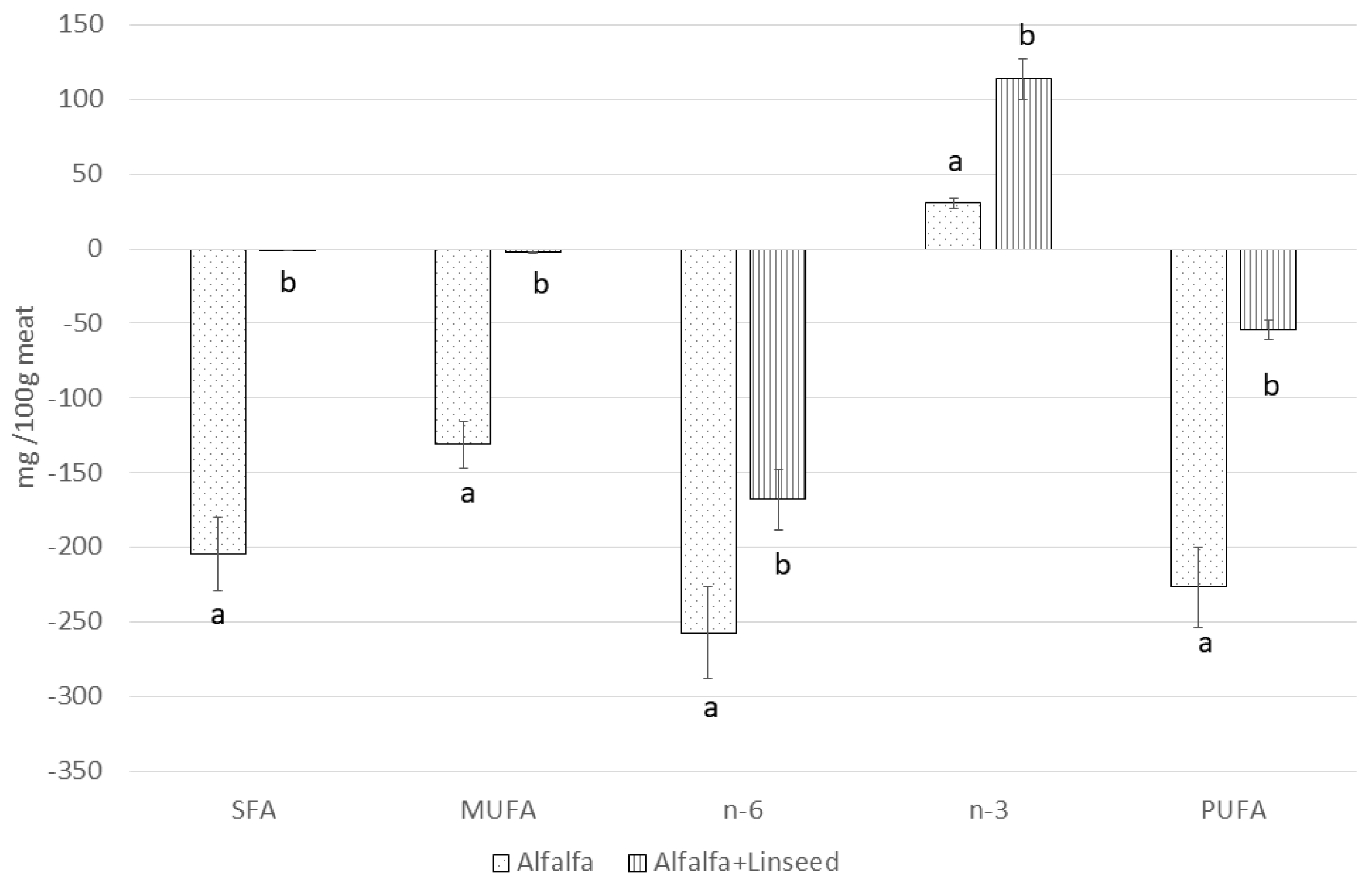
| SFA | 587.56 | 246.62 | 487.25 |
| MUFA | 738.81 | 193.89 | 687.23 |
| PUFA | 1401.30 | 765.96 | 1504.21 |
| C18:2n-6 | 1274.94 | 561.19 | 1054.23 |
| C18:3n-3 | 83.23 | 169.32 | 268.36 |
| Total n-6 | 1318.08 | 596.64 | 1254.02 |
| Total n-3 | 83.23 | 178.15 | 286.22 |
| Control | Alfalfa | Alfalfa+Linseed | RMSE/X2 * | p | |
|---|---|---|---|---|---|
| Weaning weight, 30 d (g) 1 | 618.3 ± 61.0 | 627.2 ± 60.7 | 620.3 ± 59.3 | 10.2 | 0.515 |
| Slaughtering weight, 80 d (g) 2 | 2723.9 B ± 225.4 | 2484.2 A ± 255.1 | 2708.5 B ± 248.1 | 35.2 | 0.001 |
| Feed intake, (g/d) | 137.50 A ± 12.83 | 151.97 B ± 14.17 | 146.17 AB ±14.10 | 9.24 | 0.001 |
| Feed conversion ratio | 3.20 a ± 0.30 | 4.01 b ± 0.38 | 3.43 a ± 0.31 | 0.84 | 0.004 |
| Mortality (%) | 8.34 c | 3.33 b | 0.00 a | 2.41 * | 0.002 |
| Carcass yield (%) 3 | 56.79 ± 3.12 | 57.88 ± 3.87 | 57.67 ± 4.10 | 4.23 | 0.100 |
| Carcass weight (g) 3 | 1574.8 b ± 155.1 | 1429.5 a ± 138.8 | 1562.5 b ± 142.2 | 16.4 | 0.025 |
| Control | Alfalfa | Alfalfa+Linseed | RMSE | p | |
|---|---|---|---|---|---|
| Moisture | 74.12 ± 3.05 | 75.01 ± 2.55 | 74.31 ± 3.10 | 2.28 | 0.357 |
| Protein | 22.51 ± 1.08 | 22.43 ± 1.00 | 22.61 ± 1.85 | 1.02 | 0.107 |
| Lipids | 1.72 B ± 0.08 | 1.05 A ± 0.08 | 1.65 B ± 0.06 | 0.12 | 0.005 |
| Ash | 1.65 ± 0.10 | 1.51 ± 0.09 | 1.43 ± 0.08 | 0.18 | 0.847 |
| Fatty Acids | Control | Alfalfa | Alfalfa+Linseed | RMSE | p |
|---|---|---|---|---|---|
| C14:0 | 2.01 ± 0.46 | 2.04 ± 0.33 | 2.36 ± 0.26 | 0.18 | 0.253 |
| C16:0 | 28.47 ± 1.61 | 28.85 ± 1.88 | 28.05 ± 2.04 | 1.54 | 0.961 |
| C18:0 | 8.12 a ± 0.93 | 9.45 b ± 0.82 | 8.63 a ± 0.79 | 0.58 | 0.015 |
| Others | 1.29 a ± 0.15 | 2.01 b ± 0.18 | 2.45 b ± 0.21 | 0.12 | 0.028 |
| Total SFA | 39.82 ± 3.02 | 42.35 ± 3.24 | 41.49 ± 3.69 | 3.85 | 0.184 |
| C14:1n-6 | 0.10 ± 0.02 | 0.10 ± 0.01 | 0.16 ± 0.03 | 0.01 | 0.662 |
| C16:1n-7 | 1.98 A ± 0.18 | 2.31 A ± 0.22 | 3.22 B ± 0.28 | 0.31 | 0.001 |
| C18:1n-9 | 22.35 ± 1.05 | 22.85 ± 2.41 | 21.47 ± 2.11 | 5.06 | 0.187 |
| Others | 0.43 ± 0.05 | 1.75 ± 0.20 | 0.95 ± 20.09 | 0.49 | 0.247 |
| Total MUFA | 24.76 ± 1.23 | 25.91 ± 2.03 | 25.64 ± 1.98 | 3.11 | 0.113 |
| C18:2n-6 | 28.08 c ± 1.98 | 24.06 b ± 2.08 | 20.02 a ± 2.02 | 3.87 | 0.004 |
| C20:2n-6 | 0.48 ± 0.03 | 0.25 ± 0.02 | 0.28 ± 0.03 | 0.12 | 0.457 |
| C20:4n-6 | 5.48 b ± 0.49 | 2.47 a ± 0.19 | 3.03 a ± 0.29 | 1.16 | 0.008 |
| Total n-6 | 34.04 B ± 2.21 | 26.78 A ± 1.82 | 23.33 A ± 2.01 | 3.02 | <0.001 |
| C18:3n-3 | 1.27 A ± 0.14 | 5.02 B ± 0.50 | 7.36 C ± 0.61 | 0.91 | <0.001 |
| C20:3n-3 | 0.03 A ± 0.00 | 0.25 B ± 0.02 | 0.43 C ± 0.03 | 0.42 | <0.001 |
| C20:5n-3 | 0.08 A ± 0.00 | 0.35 B ± 0.02 | 0.68 C ± 0.03 | 0.08 | <0.001 |
| C22:6n-3 | 0.07 A ± 0.00 | 0.22 B ± 0.02 | 1.27 C ± 0.12 | 0.04 | <0.001 |
| Total n-3 | 1.42 A ± 0.15 | 5.84 B ± 0.42 | 9.47 C ± 0.78 | 1.81 | <0.001 |
| Total PUFA | 35.46 b ± 2.86 | 32.62 a ± 3.12 | 33.07 a ± 3.09 | 3.21 | 0.004 |
| Fatty Acids | Control | Alfalfa | Alfalfa+Linseed | RMSE | p |
|---|---|---|---|---|---|
| C14:0 | 28.94 b ± 3.25 | 17.75 a ± 2.02 | 32.57 b ± 3.14 | 82.18 | 0.002 |
| C16:0 | 409.97 b ± 28.21 | 251.00 a ± 24.55 | 387.09 b ± 30.01 | 51.18 | 0.009 |
| C18:0 | 115.92 b ± 11.78 | 82.22 a ± 9.46 | 119.09 b ± 10.08 | 38.14 | 0.021 |
| Others | 18.58 ± 2.28 | 17.49 ± 1.52 | 33.81 ± 3.23 | 8.42 | 0.245 |
| Total SFA | 573.41 b ± 49.07 | 368.45 a ± 30.88 | 572.56 b ± 50.27 | 75.14 | 0.004 |
| C14:1n-6 | 1.44 ab ± 0.12 | 0.87 a ± 0.07 | 2.21 b ± 0.23 | 0.48 | 0.002 |
| C16:1n-7 | 28.51 B ± 2.42 | 20.10 A ± 1.82 | 44.44 C ± 3.77 | 8.15 | 0.001 |
| C18:1n-9 | 321.84 B ± 30.91 | 190.10 A ± 20.02 | 296.29 B ± 27.71 | 78.19 | 0.001 |
| Others | 6.19 a ± 0.63 | 15.23 b ± 1.09 | 13.11 b ± 1.10 | 3.75 | 0.004 |
| Total MUFA | 356.54 b ± 30.17 | 225.42 a ± 23.64 | 353.83 b ± 29.51 | 90.36 | 0.008 |
| C18:2n-6 | 404.35 C ± 35.52 | 209.32 A ± 26.24 | 276.28 B ± 30.06 | 85.19 | <0.001 |
| C20:2n-6 | 6.91 B ± 0.41 | 2.18 A ± 0.22 | 3.86 AB ± 0.46 | 1.74 | <0.001 |
| C20:4n-6 | 78.91 b ± 5.08 | 21.49 a ± 2.87 | 41.81 ab ± 3.64 | 9.12 | 0.002 |
| Total n-6 | 490.18 C ± 50.03 | 232.99 A ± 30.56 | 321.95 B ± 30.99 | 97.25 | <0.001 |
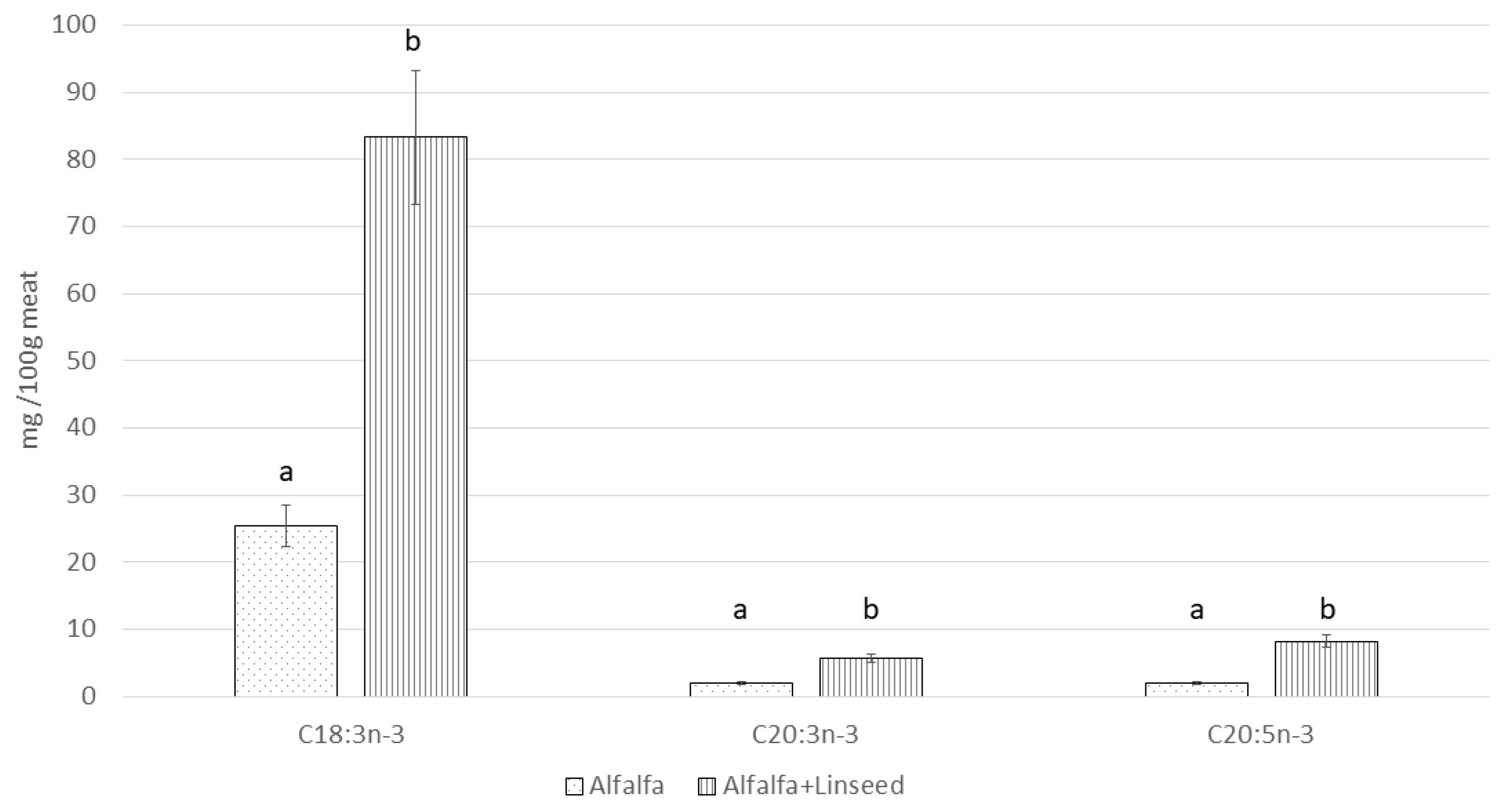
| C18:3n-3 | 18.29 A ± 0.99 | 43.67 B ± 3.11 | 101.57 C ± 8.76 | 15.24 | <0.001 |
| C20:3n-3 | 0.29 A ± 0.02 | 2.18 B ± 0.17 | 5.93 C ± 0.53 | 1.36 | <0.001 |
| C20:5n-3 | 1.15 A ± 0.17 | 3.05 B ± 0.32 | 9.38 C ± 0.88 | 1.94 | <0.001 |
| C22:6n-3 | 0.72 A ± 0.06 | 1.91 B ± 0.24 | 17.53 C ± 1.24 | 3.21 | <0.001 |
| Total n-3 | 20.45 A ± 2.00 | 50.81 B ± 4.26 | 134.41 C ± 10.87 | 18.49 | <0.001 |
| Total PUFA | 510.62 b ± 41.02 | 283.79 a ± 29.03 | 456.37 b ± 30.61 | 99.88 | 0.005 |
| n-6/n-3 | 23.97 C ± 2.65 | 4.59 B ± 0.38 | 2.40 A ± 0.22 | 6.23 | 0.001 |
| Control | Alfalfa | Alfalfa+Linseed | RMSE | ||
|---|---|---|---|---|---|
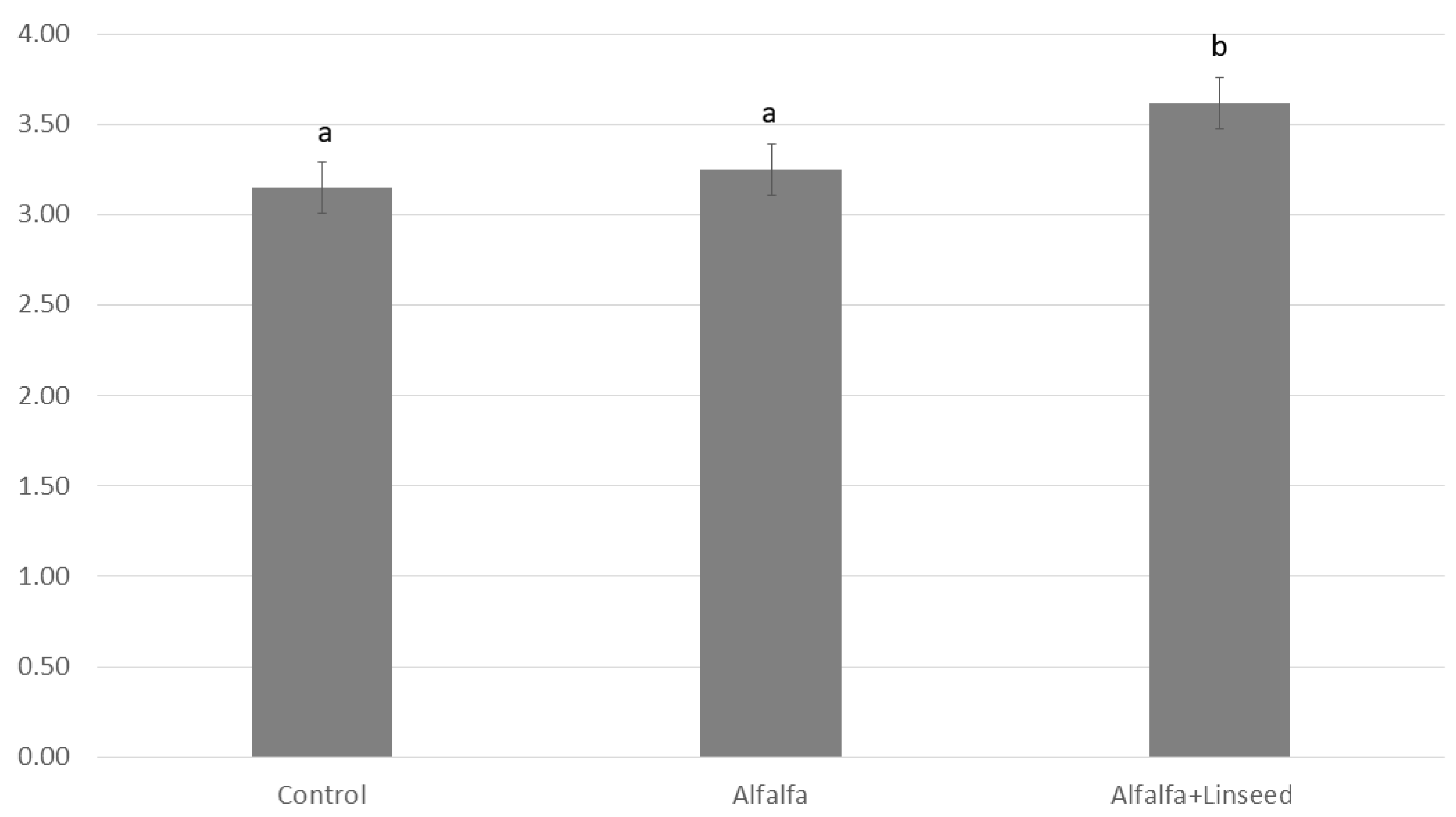
| PUFA n-3 | 11.81 a ± 0.98 | 16.35 b ± 1.43 | 32.33 c ± 2.12 | 1.58 | 0.023 |
| PUFA n-6 | 21.22 c ± 1.41 | 11.32 a ± 0.87 | 16.50 b ± 01.36 | 1.20 | 0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dal Bosco, A.; Castellini, C.; Mancini, S.; Bosa, L.; Di Federico, F.; Nompleggio, L.; Huja, S.; Mattioli, S. Alfalfa and Linseed Oil in Diet for Fattening Rabbits: Performance, Meat Quality, and Nutritional Characteristics. Animals 2025, 15, 3409. https://doi.org/10.3390/ani15233409
Dal Bosco A, Castellini C, Mancini S, Bosa L, Di Federico F, Nompleggio L, Huja S, Mattioli S. Alfalfa and Linseed Oil in Diet for Fattening Rabbits: Performance, Meat Quality, and Nutritional Characteristics. Animals. 2025; 15(23):3409. https://doi.org/10.3390/ani15233409
Chicago/Turabian StyleDal Bosco, Alessandro, Cesare Castellini, Simone Mancini, Luigia Bosa, Francesca Di Federico, Lorenzo Nompleggio, Simona Huja, and Simona Mattioli. 2025. "Alfalfa and Linseed Oil in Diet for Fattening Rabbits: Performance, Meat Quality, and Nutritional Characteristics" Animals 15, no. 23: 3409. https://doi.org/10.3390/ani15233409
APA StyleDal Bosco, A., Castellini, C., Mancini, S., Bosa, L., Di Federico, F., Nompleggio, L., Huja, S., & Mattioli, S. (2025). Alfalfa and Linseed Oil in Diet for Fattening Rabbits: Performance, Meat Quality, and Nutritional Characteristics. Animals, 15(23), 3409. https://doi.org/10.3390/ani15233409









