Phylogenetic Analysis and Public Health Implications of Salmonella Strains in Southwestern States of Nigeria Using InvA Gene Sequences
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
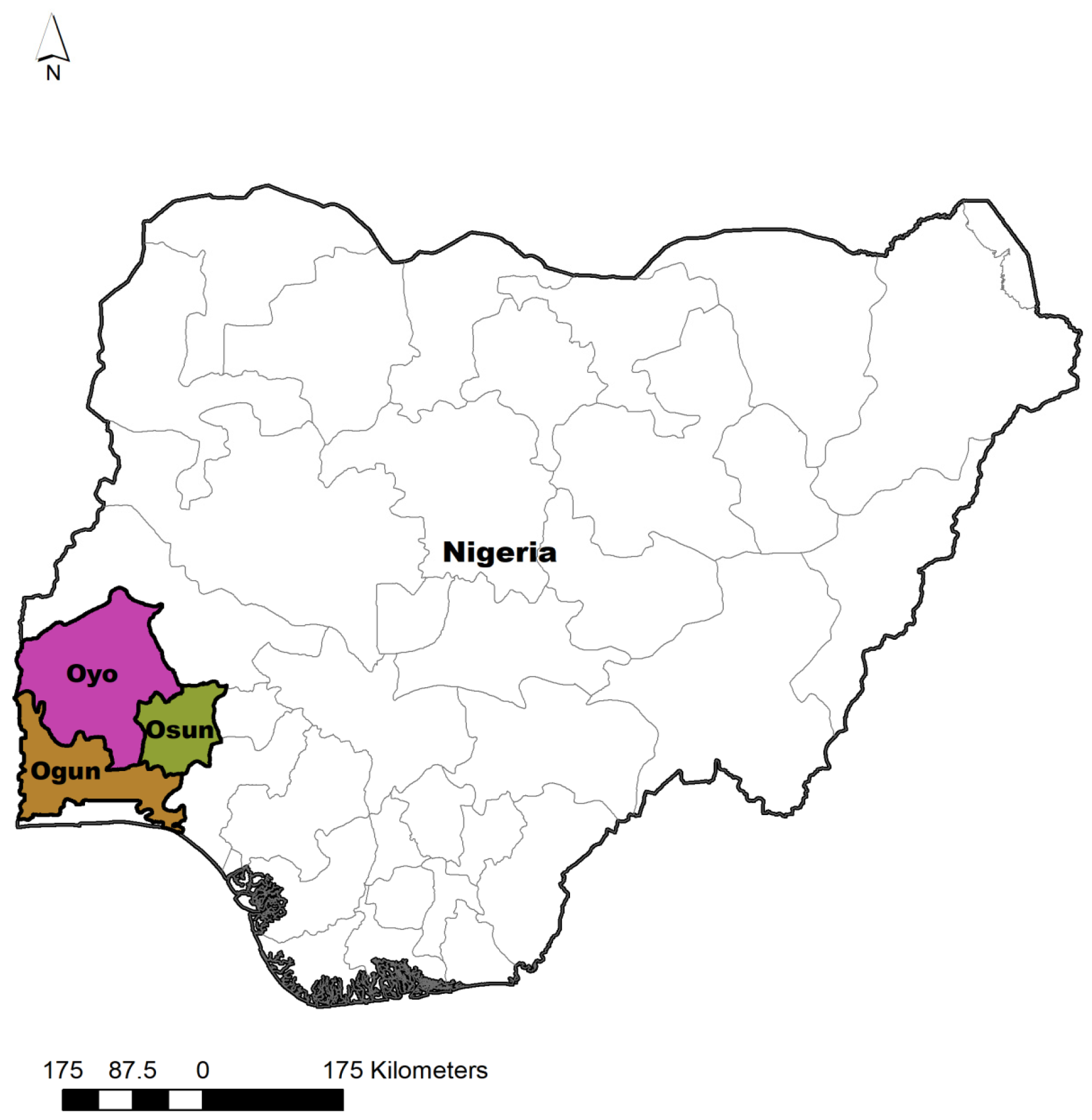
2.1. Study Area
2.2. Sample Size Determination
2.3. Sample Collection
2.4. Isolation of Salmonella
2.5. DNA Extraction from Salmonella Isolates
2.6. Amplification of the invA Gene of Salmonella
2.7. DNA Sequencing of the invA Gene of Salmonella Isolates
2.8. Sequence Alignment and Analysis
2.9. Statistical Analysis
3. Results
3.1. Prevalence of Salmonella in Samples Collected from Poultry Farms and Their Handlers
3.2. Further Characterization of Salmonella Isolates by invA Gene Amplification and Sequencing
4. Discussion
5. Conclusions
- Recommendations from the Research:
- Enhanced Surveillance: Implement systematic monitoring of Salmonella in poultry farms across Southwestern Nigeria. Molecular detection using invA gene PCR should be integrated into routine diagnostics to ensure early and accurate identification.
- Farm Biosecurity Measures: Poultry farmers should adopt strict biosecurity protocols, including controlled access, regular cleaning and disinfection, safe feed and water handling, and minimizing contact between domestic and wild birds.
- Farmer Education and Training: Conduct regular training to educate poultry farmers on Salmonella risks, prevention strategies, hygiene practices, and vaccination benefits.
- Food Safety Measures: Strengthen regulations for proper handling, processing, and cooking of poultry products to reduce human infection risk. Food safety inspections and public compliance should be emphasized.
- Policy and Regulatory Support: Government and public health authorities should enforce policies for disease surveillance, outbreak response, farm inspections, and zoonotic disease control.
- Future Molecular Studies: Encourage further molecular epidemiological research to:
- Characterize the genetic diversity of Salmonella strains in poultry and humans.
- Detect virulence and antimicrobial resistance genes beyond invA, such as stn, hilA, and spiC.
- Use whole-genome sequencing (WGS) and multilocus sequence typing (MLST) to track transmission pathways, understand evolution, and inform vaccine development.
- Public Health Awareness: Promote campaigns linking poultry health to human health to prevent zoonotic transmission.
- Stakeholder Collaboration: Strengthen cooperation among veterinarians, public health professionals, researchers, and farmers for integrated disease control strategies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fagbamila, I.O.; Barco, L.; Mancini, M.; Kwaga, J.; Ngulukun, S.S.; Zavagnin, P.; Lettini, A.A.; Lorenzetto, M.; Abdu, P.A.; Kabir, J.; et al. Salmonella serovars and their distribution in Nigerian commercial chicken layer farms. PLoS ONE 2017, 12, e0173097. [Google Scholar] [CrossRef] [PubMed]
- Bettridge, J.M.; Lynch, S.E.; Brena, M.C.; Melese, K.; Dessie, T.; Terfa, Z.G. Infection-interactions in Ethiopian village chickens. Prev. Vet. Med. 2014, 117, 358–366. [Google Scholar] [CrossRef]
- Chai, S.; Cole, D.; Nisler, A.; Mahon, B. Poultry: The most common food in outbreaks with known pathogens, United States, 1998–2012. Epidemiol. Infect. 2017, 145, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Majowicz, E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M. The global burden of non-typhoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef]
- Antunes, P.; Mourão, J.; Campos, J.; Peixe, L. Salmonellosis: The role of poultry meat. Clin. Microbiol. Infect. 2016, 22, 110–121. [Google Scholar] [CrossRef]
- Akinyemi, K.O.; Bamiro, B.S.; Coker, A.O. Salmonellosis in Lagos, Nigeria: Incidence of Plasmid-Mediated Resistance. J. Health Popul. Nutr. 2007, 25, 351–358. [Google Scholar]
- Hurley, D.; McCusker, M.P.; Fanning, S.; Martins, M. Salmonella–host interactions–modulation of the host innate immune system. Front. Immunol. 2014, 5, 1–11. [Google Scholar] [CrossRef]
- Asogwa, N.V.; Udeani, T.K.; Obeagu, E.I.; Asogwa, B.E.; Chukwueze, C.M. Prevalence of Salmonella species infection in poultry farming systems in Enugu Metropolis Nigeria. Eur. J. Pharm. Med. Res. 2022, 9, 87–91. [Google Scholar]
- Jibril, A.H.; Okeke, I.N.; Dalsgaard, A.; Kudirkiene, E.; Akinlabi, O.C.; Bello, M.B.; Olsen, J.E. Prevalence and risk factors of Salmonella in commercial poultry farms in Nigeria. PLoS ONE 2020, 15, e0238190. [Google Scholar] [CrossRef]
- Mshelbwala, F.M.; Ibrahim, N.D.G.; Saidu, S.N.; Azeez, A.A.; Akinduti, P.A.; Kwanashie, C.N.; Fakilahyel-Kadiri, A.K.; Muhammed, M.; Fagbamila, I.O.; Luka, P.D. Motile Salmonella serotypes causing high mortality in poultry farms in three South-Western States of Nigeria. Vet. Rec. Open 2017, 4, e000247. [Google Scholar] [CrossRef]
- Orum, T.G.; Ishola, O.O.; Adebowale, O.O. Occurrence and antimicrobial susceptibility patterns of Salmonella species from poultry farms in Ibadan, Nigeria. Afr. J. Lab. Med. 2022, 11, 1606. [Google Scholar] [CrossRef]
- Rahn, K.; De Grandis, S.A.; Clarke, R.C.; McEwen, S.A.; Galán, J.E.; Ginocchio, C.; Curtis, R., III; Gyles, C.L. Amplification of invA gene sequence of Salmonella typhimurium by polymerase chain reaction as a specific method of detection of Salmonella. Mol. Cell. Probes 1992, 6, 271–279. [Google Scholar] [CrossRef]
- Malorny, B.; Bunge, C.; Helmuth, R. Discrimination of d-tartrate-fermenting and non-fermenting Salmonella enterica subsp. enterica isolates by genotypic and phenotypic methods. J. Clin. Microbiol. 2003, 41, 4292–4297. [Google Scholar] [CrossRef]
- Sharma, I.; Das, K. Detection of InvA gene in isolated Salmonella from marketed poultry meat by PCR assay. J. Food Process Technol. 2016, 7, 564. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Shen, J.; Wu, C. Development of a novel hexa-plex PCR method for identification and serotyping of Salmonella species. Foodborne Pathog. Dis. 2013, 11, 75–77. [Google Scholar] [CrossRef]
- Karmi, M. Detection of virulence gene (InvA) in Salmonella isolated from meat and poultry products. Int. J. Genet. 2013, 3, 7–12. [Google Scholar]
- Kadry, M.; Nader, S.M.; Dorgham, S.M.; Kandil, M.M. Molecular diversity of the invA gene obtained from human and egg samples. Vet. World 2019, 12, 1033–1038. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raufu, I.; Bortolaia, V.; Svendsen, C.A.; Ameh, J.A.; Ambali, A.G.; Aarestrup, F.M. The first attempt of an active integrated laboratory-based Salmonella surveillance program in the north-eastern region of Nigeria. J. Appl. Microbiol. 2013, 115, 1059–1067. [Google Scholar] [CrossRef]
- Thrusfield, M. Veterinary Epidemiology, 3rd ed.; Blackwell Science Ltd.: Oxford, UK, 2007. [Google Scholar]
- Barrow, G.I.; Feltham, R.K.A. (Eds.) Cowan and Steel’s Manual for the Identification of Medical Bacteria, 3rd ed.; Cambridge University Press: Cambridge, UK, 2023; ISBN 0-521-32611-7. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Lamichhane, B.; Mawad, A.M.M.; Saleh, M.; Kelley, W.G.; Harrington, P.J.; Lovestad, C.W.; Amezcua, J.; Sarhan, M.M.; El Zowalaty, M.E.; Ramadan, H.; et al. Salmonellosis: An overview of epidemiology, pathogenesis, and innovative approaches to mitigate the antimicrobial resistant infections. Antibiotics 2024, 13, 76. [Google Scholar] [CrossRef]
- Igbinosa, E.O.; Beshiru, A.; Igbinosa, I.H.; Okoh, A.I. Antimicrobial resistance and genetic characterization of Salmonella enterica from retail poultry meats in Benin City, Nigeria. LWT 2022, 169, 114049. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef]
- Ehuwa, O.; Jaiswal, A.K.; Jaiswal, S. Salmonella, food safety and food handling practices. Foods 2021, 10, 907. [Google Scholar] [CrossRef]
- Teklemariam, A.D.; Al-Hindi, R.R.; Albiheyri, R.S.; Alharbi, M.G.; Alghamdi, M.A.; Filimban, A.A.R.; Al Mutiri, A.S.; Al-Alyani, A.M.; Alseghayer, M.S.; Almaneea, A.M.; et al. Human salmonellosis: A continuous global threat in the farm-to-fork food safety continuum. Foods 2023, 12, 1756. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of antibiotics and antibiotic resistance, and their impacts on drug development: A narrative review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in 2013. EFSA J. 2015, 13, 3991. [Google Scholar] [CrossRef]
- Cui, M.; Xie, M.; Qu, Z.; Zhao, S.; Wang, J.; Wang, Y.; He, T.; Wang, H.; Zuo, Z.; Wu, C. Prevalence and antimicrobial resistance of Salmonella isolated from an integrated broiler chicken supply chain in Qingdao, China. Food Control. 2016, 62, 270–276. [Google Scholar] [CrossRef]
- Tennant, S.M.; Diallo, S.; Levy, H.; Livio, S.; Sow, S.O.; Tapia, M.; Fields, P.I.; Mikoleit, M.; Tamboura, B.; Kotloff, K.L.; et al. Identification by PCR of non-typhoidal Salmonella enterica serovars associated with invasive infections among febrile patients in Mali. PLoS Negl. Trop. Dis. 2010, 4, e621. [Google Scholar] [CrossRef]
- Mossoro-Kpinde, C.D.; Manirakiza, A.; Mbecko, J.R.; Misatou, P.; Le Faou, A.; Frank, T. Antimicrobial resistance of enteric Salmonella in Bangui, Central African Republic. J. Trop. Med. 2015, 2015, 483974. [Google Scholar] [CrossRef]
- Cibin, V.; Busetti, M.; Longo, A.; Petrin, S.; Knezevich, A.; Ricci, A.; Barco, L.; Losasso, C. Whole genome sequencing of Salmonella Serovar Stanleyville from two Italian outbreaks resulted in unexpected genomic diversity within and between outbreaks. Foodborne Pathog. Dis. 2019, 16, 307–308. [Google Scholar] [CrossRef]
- Andoh, L.A.; Dalsgaard, A.; Obiri-Danso, K.; Newman, M.J.; Barco, L.; Olsen, J.E. Prevalence and antimicrobial resistance of Salmonella serovars isolated from poultry in Ghana. Epidemiol. Infect. 2016, 144, 3288–3299. [Google Scholar] [CrossRef]
- Bertrand, S.; Weill, F.X.; Cloeckaert, A.; Vrints, M.; Mairiaux, E.; Fraud, K.; Dierick, K.; Wildemauve, C.; Godard, C.; Butaye, P.; et al. Clonal emergence of extended-spectrum β-lactamase (CTX-M-2)-producing Salmonella enterica serovar virchow isolates with reduced susceptibilities to ciprofloxacin among poultry and humans in Belgium and France (2000 to 2003). J. Clin. Microbiol. 2006, 44, 2897–2903. [Google Scholar] [CrossRef]
- Parisi, A.; Crump, J.A.; Stafford, R.; Glass, K.; Howden, B.P.; Kirk, M.D. Increasing incidence of invasive nontyphoidal Salmonella infections in Queensland, Australia, 2007–2016. PLoS Negl. Trop. Dis. 2018, 13, 2007–2016. [Google Scholar] [CrossRef]
- Odoch, T.; Wasteson, Y.; L’Abée-Lund, T.; Muwonge, A.; Kankya, C.; Nyakarahuka, L.; Tegule, S.; Skjerve, E. Prevalence, antimicrobial susceptibility and risk factors associated with non-typhoidal Salmonella on Ugandan layer hen farms. BMC Vet. Res. 2017, 13, 1–10. [Google Scholar] [CrossRef]
- Eguale, T. Non-typhoidal Salmonella serovars in poultry farms in central Ethiopia: Prevalence and antimicrobial resistance. BMC Vet. Res. 2018, 14, 1–8. [Google Scholar] [CrossRef]
- Barua, H.; Biswas, P.K.; Olsen, K.E.P.; Christensen, J.P. Prevalence and characterization of motile Salmonella in commercial layer poultry farms in Bangladesh. PLoS ONE 2012, 7, e35914. [Google Scholar] [CrossRef]
- Lettini, A.A.; Vo Than, T.; Marafin, E.; Longo, A.; Antonello, K.; Zavagnin, P.; Barco, L.; Mancin, M.; Cibin, V.; Morini, M.; et al. Distribution of Salmonella Serovars and Antimicrobial Susceptibility from Poultry and Swine Farms in Central Vietnam. Zoonoses Public Health 2016, 67, 569–576. [Google Scholar] [CrossRef]
- Fadipe, E.O.; Hölzle, L.E.; Ayinde, J.O. Prevalence of Salmonella outbreak in poultry farms: A comparative study of Osun and Ogun States, Nigeria. Int. J. Agril. Res. Innov. Technol. 2025, 15, 115–126. [Google Scholar] [CrossRef]
- Agbaje, M.; Ayo-Ajayi, P.; Kehinde, O.; Omoshaba, E.; Dipeolu, M.; Fasina, F.O. Salmonella characterization in poultry eggs sold in farms and markets in relation to handling and biosecurity practices in Ogun State, Nigeria. Antibiotics 2021, 10, 773. [Google Scholar] [CrossRef]
- Raji, M.A.; Kazeem, H.M.; Magyigbe, K.A.; Ahmed, A.O.; Lawal, D.N.; Raufu, I.A. Salmonella serovars, antibiotic resistance, and virulence factors isolated from intestinal content of slaughtered chickens and ready-to-eat chicken gizzards in the Ilorin metropolis, Kwara State, Nigeria. Int. J. Food Sci. 2021, 2021, 8872137. [Google Scholar] [CrossRef]
- Fadipe, E.O.; Hölzle, L.E.; Takeet, M.I. Poultry Farmers’ Knowledge and Prevalence of Salmonella Infection in Relation to Handling and Biosecurity Measures in Oyo State, Nigeria. Int. J. Adv. Res. 2025, 13, 438–448. [Google Scholar] [CrossRef]
- Adesiyun, A.; Webb, L.; Musai, L.; Louison, B.; Joseph, G.; Stewart-Johnson, A.; Samlal, S.; Rodrigo, S. Survey of Salmonella contamination in chicken layer farms in three caribbean countries. J. Food Prot. 2014, 77, 1471–1480. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R. Salmonella control in poultry flocks and its public health impact. EFSA J. 2019, 17, e05596. [Google Scholar] [CrossRef]
- Namata, H.; Welby, S.; Aerts, M.; Faes, C.; Abrahantes, J.C.; Imberechts, H.; Vermeersch, K.; Hooyberghs, J.; Méroc, E.; Mintiens, K. Identification of risk factors for the prevalence and persistence of Salmonella in Belgian broiler chicken flocks. Prev. Vet. Med. 2009, 90, 211–222. [Google Scholar] [CrossRef]
- Ibrahim, T.; Ngwai, Y.B.; Ishaleku, D.; Tsaku, P.A.; Abimiku, R.H.; Nkene, I.H. Genetic relatedness of ESBL and non-ESBL Salmonella Typhimurium isolated from poultry birds and poultry handlers in Nasarawa State, Nigeria. FUDMA J. Sci. 2020, 4, 201–206. [Google Scholar] [CrossRef]
- Talukder, H.; Roky, S.A.; Debnath, K.; Sharma, B.; Ahmed, J.; Roy, S. Prevalence and antimicrobial resistance profile of Salmonella isolated from human, animal and environment samples in South Asia: A 10-year meta-analysis. J. Epidemiol. Glob. Health 2023, 13, 637–652. [Google Scholar] [CrossRef]
- Edward, W.; Chisnall, T.; Tano-Debrah, K.; Card, R.M.; Duodu, S. Prevalence and genomic characterization of Salmonella isolates from commercial chicken eggs retailed in traditional markets in Ghana. Front. Microbiol. 2023, 14, 283835. [Google Scholar] [CrossRef]
- Ramtahal, M.A.; Somboro, A.M.; Amoako, D.G.; Abia, A.L.K.; Perrett, K.; Bester, L.A.; Essack, S.Y. Molecular epidemiology of Salmonella enterica in poultry in South Africa using the farm-to-fork approach. Int. J. Microbiol. 2022, 2022, 5121273. [Google Scholar] [CrossRef]
- Waktole, H.; Ayele, Y.; Ayalkibet, Y.; Teshome, T.; Muluneh, T.; Ayane, S.; Borena, B.M.; Abayneh, T.; Deresse, G.; Asefa, Z.; et al. Prevalence, molecular detection, and antimicrobial resistance of Salmonella isolates from poultry farms across central Ethiopia: A cross-sectional study in urban and peri-urban areas. Microorganisms 2024, 12, 767. [Google Scholar] [CrossRef]
- Bouchrif, B.; Paglietti, B.; Murgia, M.; Piana, A.; Cohen, N.; Ennaji, M.M.; Rubino, S.; Timinouni, M. Prevalence and antibiotic-resistance of Salmonella isolated from food in Morocco. J. Infect. Dev. Ctries. 2009, 3, 35–40. [Google Scholar] [CrossRef]
- Lampel, K.A.; Orlandi, P.A.; Kornegay, L. Improved template preparation for PCR-based assay for detection of food-borne bacterial pathogens. Appl. Environ. Microbiol. 2000, 66, 4539–4542. [Google Scholar] [CrossRef]
- Murray, R.A.; Lee, C.A. Invasion genes are not required for Salmonella enterica serovar Typhimurium to breach the intestinal epithelium: Evidence that Salmonella pathogenicity island 1 has alternative functions during infections. Infect. Immun. 2000, 68, 5050–5055. [Google Scholar] [CrossRef]
- Naik, V.K.; Shakya, S.; Patyal, A.; Gade, N.E.; Bhoomika. Isolation and molecular characterization of Salmonella spp. from chevon and chicken meat collected from different districts of Chhattisgarh, India. Vet. World 2015, 8, 702–706. [Google Scholar] [CrossRef]
- Kaushik, P.; Anjay; Kumari, S.; Bharti, S.K.; Dayal, S. Isolation and prevalence of Salmonella from chicken meat and cattle milk collected from local markets of Patna, India. Vet. World 2014, 7, 62–65. [Google Scholar] [CrossRef]
- Abhadionmhen, A.; Anyiam, V.I.; Imarenezor, E.P.K.; Brown, S.T.C. Molecular characterization of Salmonella species isolated from animal sources in southern Taraba State, north-east Nigeria. Int. J. Pathog. Res. 2023, 12, 33–40. [Google Scholar] [CrossRef]
- Yanestria, S.M.; Rahmaniar, R.P.; Wibisono, F.J.; Effendi, M.H. Detection of invA gene of Salmonella from milkfish (Chanos chanos) at Sidoarjo wet fish market, Indonesia, using polymerase chain reaction technique. Vet. World 2019, 12, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Malorny, B.; Hoorfar, J.; Bunge, C.; Helmuth, R. Multicenter validation of the analytical accuracy of Salmonella PCR: Towards an international standard. Appl. Environ. Microbiol. 2003, 69, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Mokgophi, T.M.; Gcebe, N.; Fasina, F.; Adesiyun, A.A. Molecular characterization of virulence and resistance genes in Salmonella strains isolated from chickens sold at the informal chicken market in Gauteng Province, South Africa. J. Food Saf. 2024, 44, e13110. [Google Scholar] [CrossRef]
- Shi, Q. Situation of Salmonella Contamination in Food in Hebei Province of China in 2009–2010. Afr. J. Microbiol. Res. 2012, 6, 371–379. [Google Scholar] [CrossRef]
- Raufu, I.A.; Ahmed, O.A.; Aremu, A.; Ameh, J.A.; Ambali, A. Molecular characterization of Salmonella enterica from poultry farms in Ilorin, north-central Nigeria. Sokoto J. Vet. Sci. 2021, 19, 197–207. [Google Scholar] [CrossRef]
- Pavon, R.D.N.; Rivera, W.L. Molecular serotyping by phylogenetic analyses of a 1498 bp segment of InvA gene of Salmonella. ASM Sci. J. 2021, 14, 1–9. [Google Scholar]
- Eng, S.K.; Pusparajah, P.; Ab Mutalib, N.S.; Ser, H.L.; Chan, K.G.; Lee, L.H. Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015, 8, 284–293. [Google Scholar] [CrossRef]
- Smith, S.I.; Seriki, A.; Ajayi, A. Typhoidal and non-typhoidal Salmonella infections in Africa. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1913–1922. [Google Scholar] [CrossRef]
- Sivaramalingam, T.; Pearl, D.L.; McEwen, S.A.; Ojkic, D.; Guerin, M.T. A temporal study of Salmonella serovars from fluff samples from poultry breeder hatcheries in Ontario between 1998 and 2008 Can. J. Vet. Res. 2013, 77, 12–23. [Google Scholar]
- EFSA. Salmonella prevalence estimates. EFSA J. 2007, 98, 1–47. [Google Scholar] [CrossRef]

| No | Species | Subspecies | Serovar | Strain | Accession No. |
|---|---|---|---|---|---|
| 1 | S. enterica | enterica | Typhi | R19.2839 | CP046429 |
| 2 | S. enterica | enterica | Gallinarum | RKS2962 | U43273 |
| 3 | S. enterica | enterica | Haifa | EGY 2 | MG001905 |
| 4 | S. enterica | enterica | Typhimurium | EGY 1 | MG001904 |
| 5 | S. enterica | enterica | Typhimurium | SALS 7 | LC111485 |
| 6 | S. enterica | enterica | Typhimurium | CVCC541 | EU348365 |
| 7 | S. enterica | enterica | Typhimurium | RKS4194 | U43237 |
| 8 | S. enterica | enterica | Corvallis | 25B | MG869139 |
| 9 | S. enterica | enterica | Paratyphi | JQ694526 | MH356672 |
| 10 | S. enterica | enterica | Enteritidis | CP018657 | MH356689 |
| 11 | S. enterica | enterica | Enteritidis | CP018655 | MH356670 |
| 12 | S. enterica | enterica | Enteritidis | NCCP16206 | CP041973 |
| No | Sample | No Collected (%) | No Positive (%) | 95% CI |
|---|---|---|---|---|
| 1 | Swabs | 48 | 03 (6.25) | 0.00–13.1 |
| 2 | Feces | 112 | 04 (3.57) | 0.13–7.01 |
| 3 | Water | 51 | 05 (9.80) | 1.64–17.97 |
| 4 | Dust | 52 | 00 (0.00) | 0.00–0.00 |
| 5 | Feed | 51 | 03 (5.88) | 0.00–12.34 |
| 6 | Total | 314 | 15 | 2.42–7.14 |
| Location | No of Farm Sampled | Total No of Sample | No Positive (%) |
|---|---|---|---|
| Abeokuta | 18 | 109 | 03 (2.75%) |
| Oshogbo | 11 | 100 | 06 (6.00%) |
| Ibadan | 20 | 105 | 06 (5.71%) |
| Total | 49 | 314 | 15 (4.78%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadipe, E.O.; Hölzle, L.E. Phylogenetic Analysis and Public Health Implications of Salmonella Strains in Southwestern States of Nigeria Using InvA Gene Sequences. Animals 2025, 15, 3399. https://doi.org/10.3390/ani15233399
Fadipe EO, Hölzle LE. Phylogenetic Analysis and Public Health Implications of Salmonella Strains in Southwestern States of Nigeria Using InvA Gene Sequences. Animals. 2025; 15(23):3399. https://doi.org/10.3390/ani15233399
Chicago/Turabian StyleFadipe, Emmanuel O., and Ludwig E. Hölzle. 2025. "Phylogenetic Analysis and Public Health Implications of Salmonella Strains in Southwestern States of Nigeria Using InvA Gene Sequences" Animals 15, no. 23: 3399. https://doi.org/10.3390/ani15233399
APA StyleFadipe, E. O., & Hölzle, L. E. (2025). Phylogenetic Analysis and Public Health Implications of Salmonella Strains in Southwestern States of Nigeria Using InvA Gene Sequences. Animals, 15(23), 3399. https://doi.org/10.3390/ani15233399








