Investigating Sexual Characteristics in Two Frog Species Under Exposure to River Water Polluted with Endocrine Disruptors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Selected Species
2.2. Study Permissions
2.3. Experimental Setup and Rearing of Tadpoles
2.4. Acquiring and Testing Water for Control and Experimental Groups
2.5. Finalizing Exposition
2.6. Measurements
2.7. Digital Measurements
2.8. Anatomical and Histological Analysis
3. Data Analysis
3.1. Water Parameters
3.2. Measurement Precision
3.3. Mortality
3.4. Body Condition
3.5. Allometric Adjustment of Digit Ratios
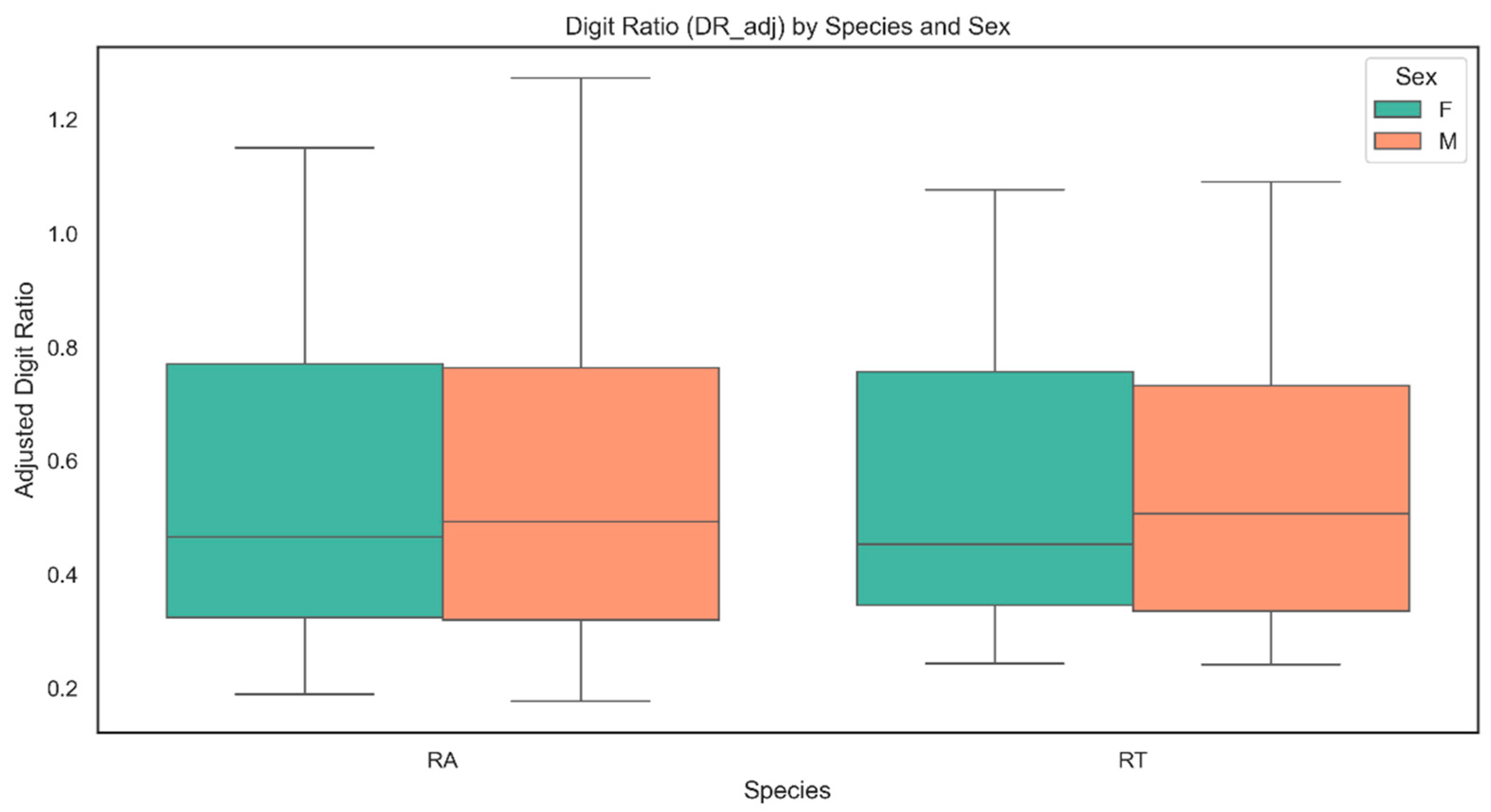
3.6. Statistical Analysis of Digit Ratios
4. Results
4.1. Conditions in Water Tanks
4.2. Concentrations of Endocrine Disruptors in Control and River Water
4.3. Chlorophyl A and Pheophytin A Content
4.4. Mortality
4.5. Body Size and Condition
4.6. Deformities of Rana temporaria Digits
4.7. Digit Ratio: Patterns and Correlations
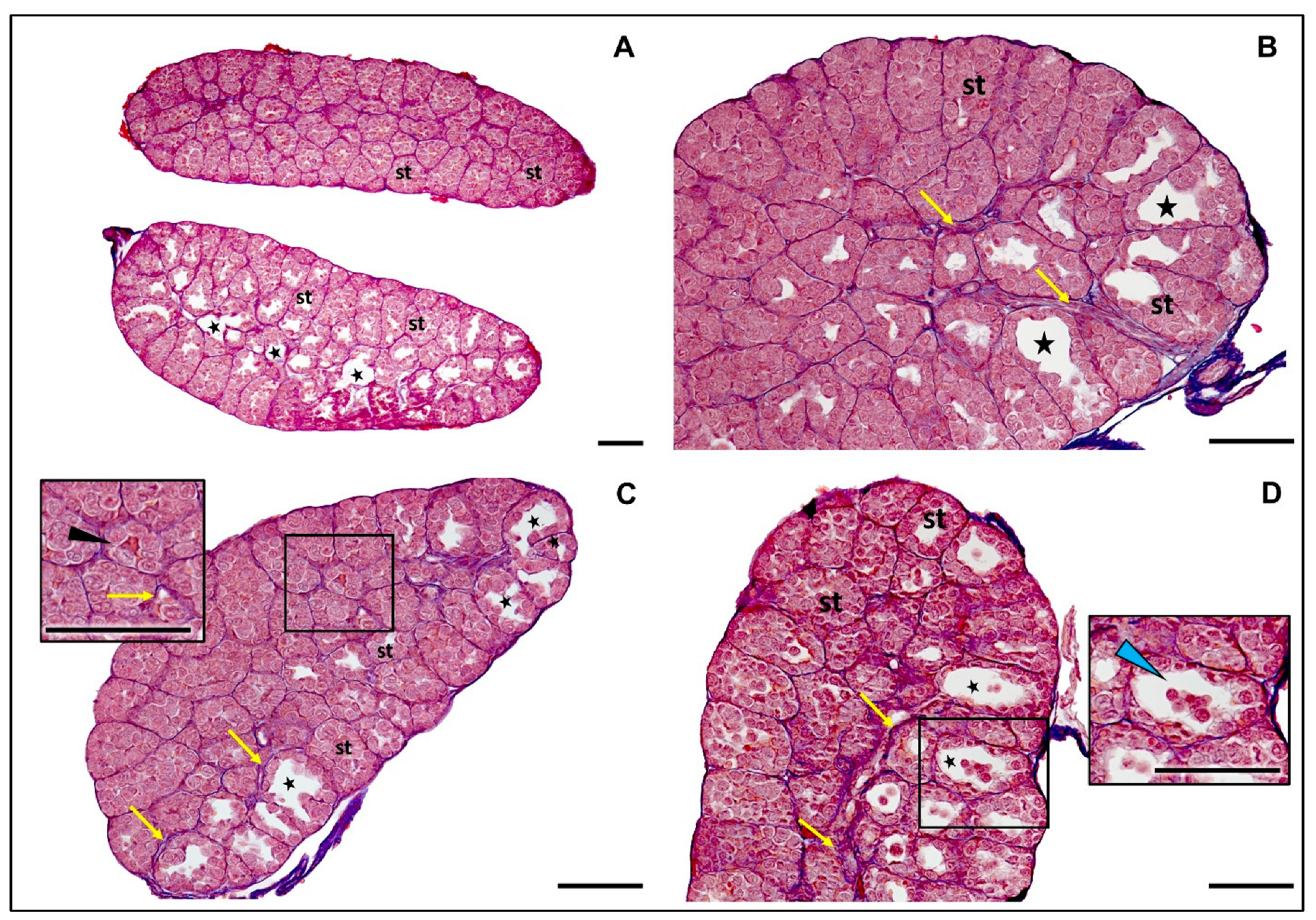
4.8. Sex Identification and Histological Examination of Gonads
4.8.1. Sex Ratio
4.8.2. Histological Examination of Gonads
5. Discussion
5.1. Conditions in Water Tanks
5.2. Mortality and Condition of Individuals
5.3. Syndactyly in Rana temporaria
5.4. Digit Ratio
5.5. Sex Ratio and Gonadal Development
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. European Workshop on the Impact of Endocrine Disrupters on Human Health and Wildlife. In Proceedings of the DG XII Weybridge Environment and Climate Research Programme Report Europe, Weybridge, UK, 2–4 December 1996; p. 17549. [Google Scholar]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the Key Characteristics of Endocrine-Disrupting Chemicals as a Basis for Hazard Identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef]
- Puri, M.; Gandhi, K.; Suresh Kumar, M. A Global Overview of Endocrine Disrupting Chemicals in the Environment: Occurrence, Effects, and Treatment Methods. Int. J. Environ. Sci. Technol. 2023, 20, 12875–12902. [Google Scholar] [CrossRef]
- Jugan, M.L.; Oziol, L.; Bimbot, M.; Huteau, V.; Tamisier-Karolak, S.; Blondeau, J.P.; Lévi, Y. In Vitro Assessment of Thyroid and Estrogenic Endocrine Disruptors in Wastewater Treatment Plants, Rivers and Drinking Water Supplies in the Greater Paris Area (France). Sci. Total Environ. 2009, 407, 3579–3587. [Google Scholar] [CrossRef]
- Esteban, S.; Gorga, M.; Petrovic, M.; González-Alonso, S.; Barceló, D.; Valcárcel, Y. Analysis and Occurrence of Endocrine-Disrupting Compounds and Estrogenic Activity in the Surface Waters of Central Spain. Sci. Total Environ. 2014, 466–467, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Czarczyńska-Goślińska, B.; Zgoła-Grześkowiak, A.; Jeszka-Skowron, M.; Frankowski, R.; Grześkowiak, T. Detection of Bisphenol A, Cumylphenol and Parabens in Surface Waters of Greater Poland Voivodeship. J. Environ. Manag. 2017, 204, 50–60. [Google Scholar] [CrossRef]
- Reichert, G.; Mizukawa, A.; Antonelli, J.; Goulart, F.d.A.B.; Filippe, T.C.; Rodrigues de Azevedo, J.C. Determination of Parabens, Triclosan, and Lipid Regulators in a Subtropical Urban River: Effects of Urban Occupation. Water Air Soil Pollut. 2020, 231, 133. [Google Scholar] [CrossRef]
- Styszko, K.; Proctor, K.; Castrignanò, E.; Kasprzyk-Hordern, B. Occurrence of Pharmaceutical Residues, Personal Care Products, Lifestyle Chemicals, Illicit Drugs and Metabolites in Wastewater and Receiving Surface Waters of Krakow Agglomeration in South Poland. Sci. Total Environ. 2021, 768, 144360. [Google Scholar] [CrossRef]
- Dang, Z.C.; Kienzler, A. Changes in Fish Sex Ratio as a Basis for Regulating Endocrine Disruptors. Environ. Int. 2019, 130, 104928. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, Z.; Luo, Z.; Li, H.; Chen, G. Endocrine Disrupting Chemicals in Wild Freshwater Fishes: Species, Tissues, Sizes and Human Health Risks. Environ. Pollut. 2019, 244, 462–468. [Google Scholar] [CrossRef]
- Wu, N.C.; Seebacher, F. Effect of the Plastic Pollutant Bisphenol A on the Biology of Aquatic Organisms: A Meta-Analysis. Glob. Change Biol. 2020, 26, 3821–3833. [Google Scholar] [CrossRef]
- Kloas, W. Amphibians as a Model for the Study of Endocrine Disruptors. Int. Rev. Cytol. 2002, 216, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Burlibaşa, L. Amphibians as Model Organisms for Study Environmental Genotoxicity. Appl. Ecol. Environ. Res. 2011, 9, 1–15. [Google Scholar] [CrossRef]
- Kloas, W.; Urbatzka, R.; Opitz, R.; Würtz, S.; Behrends, T.; Hermelink, B.; Hofmann, F.; Jagnytsch, O.; Kroupova, H.; Lorenz, C.; et al. Endocrine Disruption in Aquatic Vertebrates. Ann. N. Y. Acad. Sci. 2009, 1163, 187–200. [Google Scholar] [CrossRef]
- Carey, C.; Bryant, C.J. Possible Interrelations among Environmental Toxicants, Amphibian Development, and Decline of Amphibian Populations. Environ. Health Perspect. 1995, 103, 13–17. [Google Scholar] [CrossRef]
- Hayes, T.B.; Case, P.; Chui, S.; Chung, D.; Haeffele, C.; Haston, K.; Lee, M.; Mai, V.P.; Marjuoa, Y.; Parker, J.; et al. Pesticide Mixtures, Endocrine Disruption, and Amphibian Declines: Are We Underestimating the Impact? Environ. Health Perspect. 2006, 114, 40–50. [Google Scholar] [CrossRef]
- Brühl, C.A.; Schmidt, T.; Pieper, S.; Alscher, A. Terrestrial Pesticide Exposure of Amphibians: An Underestimated Cause of Global Decline? Sci. Rep. 2013, 3, 1135. [Google Scholar] [CrossRef]
- Ruthsatz, K.; Glosv, J. Effects of Pollutants on the Endocrine System of Tadpoles. In Toxicology of Amphibian Tadpoles; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Sinai, N.; Eterovick, P.C.; Kruger, N.; Oetken, B.; Ruthsatz, K. Living in a Multi-Stressor World: Nitrate Pollution and Thermal Stress Interact to Affect Amphibian Larvae. J. Exp. Biol. 2024, 227, jeb247629. [Google Scholar] [CrossRef]
- Kloas, W.; Lutz, I. Amphibians as Model to Study Endocrine Disrupters. J. Chromatogr. A 2006, 1130, 16–27. [Google Scholar] [CrossRef]
- Frątczak, M.; Kaczmarski, M.; Szkudelska, K.; Tryjanowski, P. Assessing Species Bias in Amphibian Research on Endocrine Disruptors: Beyond Xenopus laevis. Front. Environ. Sci. 2025, 13, 1556788. [Google Scholar] [CrossRef]
- Schiesari, L.; Grillitsch, B.; Grillitsch, H. Biogeographic Biases in Research and Their Consequences for Linking Amphibian Declines to Pollution. Conserv. Biol. J. Soc. Conserv. Biol. 2007, 21, 465–471. [Google Scholar] [CrossRef]
- Kaczmarski, M.; Kubicka, A.M.; Tryjanowski, P.; Hromada, M. Females Have Larger Ratio of Second-to-Fourth Digits Than Males in Four Species of Salamandridae, Caudata. Anat. Rec. 2015, 298, 1424–1430. [Google Scholar] [CrossRef]
- Kazimirski, P.P.; Kaczmarski, M.; Zagalska-Neubauer, M.M.; Żołnierowicz, K.M.; Tobółka, M. Absence of Sex Differences in Digit Ratio in Nestlings of the White Stork Ciconia ciconia, a Monomorphic Bird Species. Bird Study 2019, 66, 503–509. [Google Scholar] [CrossRef]
- Kaczmarski, M.; Ziemblińska, K.; Tryjanowski, P. Sand Lizards Lacerta agilis with Higher Digit Ratios Are More Likely to Autotomy. J. Anat. 2020, 237, 1103–1113. [Google Scholar] [CrossRef]
- Kaczmarski, M.; Kaczmarek, J.M.; Jankowiak, Ł.; Kolenda, K.; Tryjanowski, P. Digit Ratio in the Common Toad Bufo bufo: The Effects of Reduced Fingers and of Age Dependency. Zool. Lett. 2021, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Manning, J.T. Digit Ratio: A Pointer to Fertility, Behavior, and Health; Rutgers Series in Human Evolution; Rutgers University Press: New Brunswick, NJ, USA, 2002. [Google Scholar]
- Manning, J.; Kilduff, L.; Cook, C.; Crewther, B.; Fink, B. Digit Ratio (2D:4D): A Biomarker for Prenatal Sex Steroids and Adult Sex Steroids in Challenge Situations. Front. Endocrinol. 2014, 5, 9. [Google Scholar] [CrossRef]
- Romano, M.; Rubolini, D.; Martinelli, R.; Alquati, A.B.; Saino, N. Experimental Manipulation of Yolk Testosterone Affects Digit Length Ratios in the Ring-Necked Pheasant (Phasianus colchicus). Horm. Behav. 2005, 48, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Auger, J.; Denmat, D.L.; Berges, R.; Doridot, L.; Salmon, B.; Canivenc-Lavier, M.C.; Eustache, F. Environmental Levels of Oestrogenic and Antiandrogenic Compounds Feminize Digit Ratios in Male Rats and Their Unexposed Male Progeny. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131532. [Google Scholar] [CrossRef]
- Lofeu, L.; Brandt, R.; Kohlsdorf, T. Phenotypic Integration Mediated by Hormones: Associations among Digit Ratios, Body Size and Testosterone during Tadpole Development. BMC Evol. Biol. 2017, 17, 175. [Google Scholar] [CrossRef]
- Fuse, M.; Sawada, K. Morphological Development of Baculum and Forelimb Second-to-fourth Digit Ratio in Mice. Congenit. Anom. 2019, 59, 24–25. [Google Scholar] [CrossRef] [PubMed]
- Leoni, B.; Rubolini, D.; Romano, M.; Di Giancamillo, M.; Saino, N. Avian Hind-limb Digit Length Ratios Measured from Radiographs Are Sexually Dimorphic. J. Anat. 2008, 213, 425–430. [Google Scholar] [CrossRef]
- Van Damme, R.; Wijnrocx, K.; Boeye, J.; Huyghe, K.; Van Dongen, S. Digit Ratios in Two Lacertid Lizards: Sexual Dimorphism and Morphological and Physiological Correlates. Zoomorphology 2015, 134, 565–575. [Google Scholar] [CrossRef]
- Chang, J.L. Sexual Dimorphism of the Second-to-Fourth Digit Length Ratio (2D: 4D) in the Strawberry Poison Dart Frog (Oophaga pumilio) in Costa Rica. J. Herpetol. 2008, 42, 414–416. [Google Scholar] [CrossRef]
- Germano, J.; Cree, A.; Bishop, P. Ruling out the Boys from the Girls: Can Subtle Morphological Differences Identify Sex of the Apparently Monomorphic Frog, Leiopelma pakeka? N. Z. J. Zool. 2011, 38, 161–171. [Google Scholar] [CrossRef]
- Direnzo, G.V.; Stynoski, J.L. Patterns of Second-to-Fourth Digit Length Ratios (2D:4D) in Two Species of Frogs and Two Species of Lizards at La Selva, Costa Rica. Anat. Rec. 2012, 295, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Beaty, L.E.; Emmering, Q.C.; Bernal, X.E. Mixed Sex Effects on the Second-to-Fourth Digit Ratio of Túngara Frogs (Engystomops pustulosus) and Cane Toads (Rhinella marina). Anat. Rec. 2016, 299, 421–427. [Google Scholar] [CrossRef]
- Rajabi, F.; Javanbakht, H. Sexual Dimorphism in Digit Length Ratios in Marsh Frog, Pelophylax ridibundus (Ranidae) from Iran. J. Appl. Biol. Sci. 2019, 13, 33–36. [Google Scholar]
- Frątczak, M.; Kaczmarski, M.; Jankowiak, Ł.; Klessa, J.; Bielicki, K.; Lyskov, B.; Tryjanowski, P. Digit Ratio in the Common Spadefoot Toad Pelobates fuscus (Anura: Mesobatrachia: Pelobatidae): Patterns and Correlations. Eur. Zool. J. 2025, 92, 203–209. [Google Scholar] [CrossRef]
- Rybacki, M. Distribution, Morphology, Ecology and Status of the Moor Frog (Rana arvalis) in Poland. In Der Moorfrosch/The Moor Frog; Laurenti-Verlag: Bielefeld, Germany, 2008. [Google Scholar]
- AmphibiaWeb. Available online: https://amphibiaweb.org/ (accessed on 18 April 2025).
- Berger, L.; Rybacki, M. Growth and Maturity of Brown Frogs, Rana arvalis and Rana remporaria, in Central Poland. Alytes 1993, 11, 17–24. [Google Scholar]
- Lardner, B.; Loman, J. Does Landscape and Habitat Limit the Frogs Rana arvalis and Rana temporaria in Agricultural Landscapes? A Field Experiment. Appl. Herpetol. 2009, 6, 227–236. [Google Scholar] [CrossRef]
- Tattersall, G.J.; Ultsch, G.R. Physiological Ecology of Aquatic Overwintering in Ranid Frogs. Biol. Rev. 2008, 83, 119–140. [Google Scholar] [CrossRef]
- Berman, D.I.; Bulakhova, N.A.; Meshcheryakova, E.N.; Shekhovtsov, S.V. Overwintering and Cold Tolerance in the Moor Frog (Rana arvalis) across Its Range. Can. J. Zool. 2020, 11, 17–24. [Google Scholar] [CrossRef]
- Deoniziak, K. Effects of Wetland Restoration on the Amphibian Community in the Narew River Valley (Northeast Poland). Salamandra 2017, 53, 50–58. [Google Scholar]
- Wojdan, D.; Żeber-Dzikowska, I.; Gworek, B.; Sadowski, M.; Chmielewski, J. Herpetofauna of the Pieprzowe Mountains Nature Reserve and Adjacent Areas. Environ. Prot. Nat. Resour. 2019, 30, 24–31. [Google Scholar] [CrossRef]
- The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/en (accessed on 5 March 2025).
- Polish Minister of the Environment, R. of the P.M. of the Environment. Dz.U. 2016 poz. 2183. In Rozporządzenie Ministra Środowiska w Sprawie Ochrony Gatunkowej Zwierząt, Poland, 2016. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20160002183 (accessed on 20 October 2025).
- Gosner, K.L. A Simplified Table for Staging Anuran Embryos and Larvae with Notes on Identification. Herpetologica 1960, 16, 183–190. [Google Scholar]
- Ogielska, M. The Undifferentiated Amphibian Gonad. In Reproduction of Amphibians, 1st, ed.; Science Publishers: Enfield, NH, USA, 2009; pp. 1–33. [Google Scholar]
- Kaczmarek, J.M.; Kaczmarski, M.; Mazurkiewicz, J.; Kloskowski, J. A Matter of Proportion? Associational Effects in Larval Anuran Communities under Fish Predation. Oecologia 2018, 187, 745–753. [Google Scholar] [CrossRef]
- Ford, J.; Green, D. Captive Rearing Oligotrophic-Adapted Toad Tadpoles in Mesocosms. Herpetol. Rev. 2021, 54, 777–779. [Google Scholar]
- Miler, A.T. Variability of the Warta River Water Discharge in the City of Poznań as Influenced by the Jeziorsko Reservoir. Arch. Environ. Prot. 2015, 41, 53–59. [Google Scholar] [CrossRef][Green Version]
- Kołaska, S.; Walkowiak, J.J.; Dymaczewski, Z. Changes of Physicochemical and Microbiologicalparameters of Infiltration Water at Debina Intake in Poznan, Unique Conditions—A Flood. E3S Web Conf. 2018, 30, 01004. [Google Scholar] [CrossRef]
- Goto, Y.; Kitamura, S.; Kashiwagi, K.; Oofusa, K.; Tooi, O.; Yoshizato, K.; Sato, J.; Ohta, S.; Kashiwagi, A. Suppression of Amphibian Metamorphosis by Bisphenol A and Related Chemical Substances. J. Health Sci. 2006, 52, 160–168. [Google Scholar] [CrossRef]
- Heimeier, R.A.; Das, B.; Buchholz, D.R.; Shi, Y.B. The Xenoestrogen Bisphenol A Inhibits Postembryonic Vertebrate Development by Antagonizing Gene Regulation by Thyroid Hormone. Endocrinology 2009, 150, 2964–2973. [Google Scholar] [CrossRef]
- Iwamuro, S.; Sakakibara, M.; Terao, M.; Ozawa, A.; Kurobe, C.; Shigeura, T.; Kato, M.; Kikuyama, S. Teratogenic and Anti-Metamorphic Effects of Bisphenol A on Embryonic and Larval Xenopus laevis. Gen. Comp. Endocrinol. 2003, 133, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Adati, N.; Shinkai, T.; Sakuma, K.; Nishimura, T.; Kurose, K. Bisphenol A Induces Apoptosis in Central Neural Cells during Early Development of Xenopus laevis. Biochem. Biophys. Res. Commun. 2003, 312, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.X.; Xu, Y.; Wen, S. Endocrine-Disrupting Effects of Nonylphenol, Bisphenol A, and p,P′-DDE on Rana nigromaculata Tadpoles. Bull. Environ. Contam. Toxicol. 2005, 75, 1168–1175. [Google Scholar] [CrossRef]
- Wolkowicz, I.H.; Svartz, G.V.; Aronzon, C.M.; Coll, C.P. Developmental Toxicity of Bisphenol A Diglycidyl Ether (Epoxide Resin Badge) during the Early Life Cycle of a Native Amphibian Species. Environ. Toxicol. Chem. 2016, 35, 3031–3038. [Google Scholar] [CrossRef]
- Tamschick, S.; Rozenblut-Kościsty, B.; Ogielska, M.; Kekenj, D.; Gajewski, F.; Krüger, A.; Kloas, W.; Stöck, M. The Plasticizer Bisphenol A Affects Somatic and Sexual Development, but Differently in Pipid, Hylid and Bufonid Anurans. Environ. Pollut. 2016, 216, 282–291. [Google Scholar] [CrossRef]
- Kloas, W.; Lutz, I.; Einspanier, R. Amphibians as a Model to Study Endocrine Disruptors: II. Estrogenic Activity of Environmental Chemicals in Vitro and in Vivo. Sci. Total Environ. 1999, 225, 59–68. [Google Scholar] [CrossRef]
- Mosconi, G.; Carnevali, O.; Franzoni, M.F.; Cottone, E.; Lutz, I.; Kloas, W.; Yamamoto, K.; Kikuyama, S.; Polzonetti-Magni, A.M. Environmental Estrogens and Reproductive Biology in Amphibians. Gen. Comp. Endocrinol. 2002, 126, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.; Lutz, I.; Krüger, A.; Kloas, W. Bisphenol A Induces Feminization in Xenopus laevis Tadpoles. Environ. Res. 2004, 94, 102–111. [Google Scholar] [CrossRef]
- Mackenzie, C.A.; Berrill, M.; Metcalfe, C.; Pauli, B.D. Gonadal Differentiation in Frogs Exposed to Estrogenic and Antiestrogenic Compounds. Environ. Toxicol. Chem. Int. J. 2003, 22, 2466–2475. [Google Scholar] [CrossRef]
- Scaia, M.F.; de Gregorio, L.S.; Franco-Belussi, L.; Succi-Domingues, M.; Oliveira, C. de Gonadal, Body Color, and Genotoxic Alterations in Lithobates catesbeianus Tadpoles Exposed to Nonylphenol. Environ. Sci. Pollut. Res. 2019, 26, 22209–22219. [Google Scholar] [CrossRef]
- Segundo, L.S.; Martini, F.; Pablos, M.V. Gene Expression Responses for Detecting Sublethal Effects of Xenobiotics and Whole Effluents on a Xenopus laevis Embryo Assay. Environ. Toxicol. Chem. 2013, 32, 2018–2025. [Google Scholar] [CrossRef]
- Pohl, J. Thyroid Endocrine Disruption of Propylparaben Assessed Using an Optimized Individual Xenopus tropicalis Metamorphosing Tadpole Exposure System. Master’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2015. [Google Scholar]
- Kang, H.M.; Kim, M.S.; Hwang, U.K.; Jeong, C.B.; Lee, J.S. Effects of Methylparaben, Ethylparaben, and Propylparaben on Life Parameters and Sex Ratio in the Marine Copepod Tigriopus japonicus. Chemosphere 2019, 226, 388–394. [Google Scholar] [CrossRef]
- Richard, J.A.; Leavy, S. Experimental Reversal of Sex in Salamanders by the Injection of Estrone. Proc. Soc. Exp. Biol. Med. 1939, 42, 720–724. [Google Scholar]
- Bögi, C.; Schwaiger, J.; Ferling, H.; Mallow, U.; Steineck, C.; Sinowatz, F.; Kalbfus, W.; Negele, R.D.; Lutz, I.; Kloas, W. Endocrine Effects of Environmental Pollution on Xenopus laevis and Rana temporaria. Environ. Res. 2003, 93, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.A.; Gentles, A.; Smith, E.E.; Goleman, W.L.; Urquidi, L.J.; Thuett, K.; Kendall, R.J.; Giesy, J.P.; Gross, T.S.; Solomon, K.R.; et al. Response of Larval Xenopus laevis to Atrazine: Assessment of Growth, Metamorphosis, and Gonadal and Laryngeal Morphology. Environ. Toxicol. Chem. Int. J. 2003, 22, 396–405. [Google Scholar] [CrossRef]
- Hayes, T.B.; Stuart, A.A.; Mendoza, M.; Collins, A.; Noriega, N.; Vonk, A.; Johnston, G.; Liu, R.; Kpodzo, D. Characterization of Atrazine-Induced Gonadal Malformations in African Clawed Frogs (Xenopus laevis) and Comparisons with Effects of an Androgen Antagonist (Cyproterone Acetate) and Exogenous Estrogen (17β-Estradiol): Support for the Demasculinization/Feminization Hypothesis. Environ. Health Perspect. 2006, 114, 134–141. [Google Scholar] [CrossRef]
- Storrs, S.I.; Semlitsch, R.D. Variation in Somatic and Ovarian Development: Predicting Susceptibility of Amphibians to Estrogenic Contaminants. Gen. Comp. Endocrinol. 2008, 156, 524–530. [Google Scholar] [CrossRef]
- Pettersson, I.; Berg, C. Environmentally Relevant Concentrations of Ethynylestradiol Cause Female-Biased Sex Ratios in Xenopus tropicalis and Rana temporaria. Environ. Toxicol. Chem. 2007, 26, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Gyllenhammar, I.; Holm, L.; Eklund, R.; Berg, C. Reproductive Toxicity in Xenopus tropicalis after Developmental Exposure to Environmental Concentrations of Ethynylestradiol. Aquat. Toxicol. 2009, 91, 171–178. [Google Scholar] [CrossRef]
- Hirakawa, I.; Miyagawa, S.; Mitsui, N.; Miyahara, M.; Onishi, Y.; Kagami, Y.; Kusano, T.; Takeuchi, T.; Ohta, Y.; Iguchi, T. Developmental Disorders and Altered Gene Expression in the Tropical Clawed Frog (Silurana tropicalis) Exposed to 17α-Ethinylestradiol. J. Appl. Toxicol. 2013, 33, 1001–1010. [Google Scholar] [CrossRef]
- Tamschick, S.; Rozenblut-Kościsty, B.; Ogielska, M.; Lehmann, A.; Lymberakis, P.; Hoffmann, F.; Lutz, I.; Schneider, R.J.; Kloas, W.; Stöck, M. Impaired Gonadal and Somatic Development Corroborate Vulnerability Differences to the Synthetic Estrogen Ethinylestradiol among Deeply Diverged Anuran Lineages. Aquat. Toxicol. 2016, 177, 503–514. [Google Scholar] [CrossRef]
- Ujhegyi, N.; Bókony, V. Skin Coloration as a Possible Non-Invasive Marker for Skewed Sex Ratios and Gonadal Abnormalities in Immature Common Toads (Bufo Bufo). Ecol. Indic. 2020, 113, 106175. [Google Scholar] [CrossRef]
- Storrs-Méndez, S.I.; Semlitsch, R.D. Intersex Gonads in Frogs: Understanding the Time Course of Natural Development and Role of Endocrine Disruptors. J. Exp. Zool. Part B Mol. Dev. Evol. 2010, 314, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Tompsett, A.R.; Wiseman, S.; Higley, E.; Pryce, S.; Chang, H.; Giesy, J.P.; Hecker, M. Effects of 17α-Ethynylestradiol on Sexual Differentiation and Development of the African Clawed Frog (Xenopus laevis). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 156, 202–210. [Google Scholar] [CrossRef]
- Ślósarczyk, K.; Jakóbczyk-Karpierz, S.; Różkowski, J.; Witkowski, A.J. Occurrence of Pharmaceuticals and Personal Care Products in the Water Environment of Poland: A Review. Water 2021, 13, 2283. [Google Scholar] [CrossRef]
- Lambert, M.R.; Skelly, D.K. Diverse Sources for Endocrine Disruption in the Wild. Endocr. Disruptors 2016, 4, e1148803. [Google Scholar] [CrossRef]
- Säfholm, M.; Norder, A.; Fick, J.; Berg, C. Disrupted Oogenesis in the Frog Xenopus tropicalis after Exposure to Environmental Progestin Concentrations. Biol. Reprod. 2012, 86, 126. [Google Scholar] [CrossRef]
- Ziková, A.; Lorenz, C.; Hoffmann, F.; Kleiner, W.; Lutz, I.; Stöck, M.; Kloas, W. Endocrine Disruption by Environmental Gestagens in Amphibians—A Short Review Supported by New in Vitro Data Using Gonads of Xenopus laevis. Chemosphere 2017, 181, 74–82. [Google Scholar] [CrossRef]
- Kaczmarski, M.; Tryjanowski, P.; Kubicka, A.M. Urban Plums and Toads: Do Fleshy Fruits Affect the Post-Metamorphic Growth of Amphibians? PeerJ 2019, 7, e6337. [Google Scholar] [CrossRef]
- Green, S.L. The Laboratory Xenopus S p.; CRC Press: New York, NY, USA, 2009. [Google Scholar]
- Haczkiewicz, K.; Ogielska, M. Gonadal Sex Differentiation in Frogs: How Testes Become Shorter than Ovaries. Zool. Sci. 2013, 30, 125–134. [Google Scholar] [CrossRef]
- Henle, K.; Dubois, A.; Vershinin, V. Studies on Anomalies in Natural Populations of Amphibians. In Commented Glossary, Terminology and Synonymies of Anomalies in Natural Populations of Amphibians. Mertensiella 2017, 25, 9–48. [Google Scholar]
- Ogielska, M.; Chmielewska, M.; Rozenblut-Kościsty, B. Pregametogenesis: The Earliest Stages of Gonad and Germline Differentiation in Anuran Amphibians. Biology 2024, 13, 1017. [Google Scholar] [CrossRef] [PubMed]
- Ogielska, M.; Kotusz, A. Pattern and Rate of Ovary Differentiation with Reference to Somatic Development in Anuran Amphibians. J. Morphol. 2004, 259, 41–54. [Google Scholar] [CrossRef]
- Matesun, J.; Petrik, L.; Musvoto, E.; Ayinde, W.; Ikumi, D. Limitations of Wastewater Treatment Plants in Removing Trace Anthropogenic Biomarkers and Future Directions: A Review. Ecotoxicol. Environ. Saf. 2024, 281, 116610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Q.; Li, G.; Wang, Z.; Yan, C. Levels of Estrogenic Compounds in Xiamen Bay Sediment, China. Mar. Pollut. Bull. 2009, 58, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, L.; Moore, L.J.; Rodgers, J.H.; Bowerman, W.W.; Yarrow, G.K.; Chao, W.Y. Comparative Toxicity of Two Glyphosate Formulations (Original Formulation of Roundup® and Roundup WeatherMAX®) to Six North American Larval Anurans. Environ. Toxicol. Chem. 2011, 30, 2756–2761. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, Y.; Yan, K.; Wu, S.; Han, Z.; Guo, R.; Chen, M.; Yang, Q.; Zhang, S.; Chen, J. Bisphenol Analogues in Surface Water and Sediment from the Shallow Chinese Freshwater Lakes: Occurrence, Distribution, Source Apportionment, and Ecological and Human Health Risk. Chemosphere 2017, 184, 318–328. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, L.; Chang, A.C. Seasonal Variation of Endocrine Disrupting Compounds, Pharmaceuticals and Personal Care Products in Wastewater Treatment Plants. Sci. Total Environ. 2013, 442, 310–316. [Google Scholar] [CrossRef]
- Kot-Wasik, A.; Jakimska, A.; Śliwka-Kaszyńska, M. Occurrence and Seasonal Variations of 25 Pharmaceutical Residues in Wastewater and Drinking Water Treatment Plants. Environ. Monit. Assess. 2016, 188, 661. [Google Scholar] [CrossRef]
- Gmurek, M.; Olak-Kucharczyk, M.; Ledakowicz, S. Photochemical Decomposition of Endocrine Disrupting Compounds—A Review. Chem. Eng. J. 2017, 310, 437–456. [Google Scholar] [CrossRef]
- Nejedly, T.; Klimes, J. A Model of Natural Degradation of 17-α-Ethinylestradiol in Surface Water and Identification of Degradation Products by GC-MS. Environ. Sci. Pollut. Res. 2017, 24, 23196–23206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Wang, C.; Niu, L.; Cai, W. Occurrence of Endocrine Disrupting Compounds in Aqueous Environment and Their Bacterial Degradation: A Review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1–59. [Google Scholar] [CrossRef]
- Im, J.; Löffler, F.E. Fate of Bisphenol A in Terrestrial and Aquatic Environments. Environ. Sci. Technol. 2016, 50, 8403–8416. [Google Scholar] [CrossRef] [PubMed]
- Rusiniak, P.; Kmiecik, E.; Wątor, K.; Duda, R.; Bugno, R. Pharmaceuticals and Personal Care Products in the Urban Groundwater—Preliminary Monitoring (Case Study: Kraków, Southern Poland). Urban Water J. 2021, 18, 364–374. [Google Scholar] [CrossRef]
- McDiarmid, R.W.; Altig, R. Tadpoles: The Biology of Anuran Larvae; University of Chicago Press: Chicago, IL, USA, 2000. [Google Scholar]
- Montaña, C.G.; Silva, S.D.G.T.M.; Hagyari, D.; Wager, J.; Tiegs, L.; Sadeghian, C.; Schriever, T.A.; Schalk, C.M. Revisiting “What Do Tadpoles Really Eat?” A 10-year Perspective. Freshw. Biol. 2019, 64, 2269–2282. [Google Scholar] [CrossRef]
- Kupferberg, S.J.; Marks, J.C.; Power, M.E. Effects of Variation in Natural Algal and Detrital Diets on Larval Anuran (Hyla regilla) Life-History Traits. Copeia 1994, 1994, 446–457. [Google Scholar] [CrossRef]
- Crespi, E.J.; Warne, R.W. Environmental Conditions Experienced During the Tadpole Stage Alter Post-Metamorphic Glucocorticoid Response to Stress in an Amphibian. Integr. Comp. Biol. 2013, 53, 989–1001. [Google Scholar] [CrossRef]
- Pabijan, M.; Ogielska, M. Conservation and Declines of Amphibians in Poland. In Amphibian Biology, Volume 11: Status of Conservation and Decline of Amphibians: Eastern Hemisphere, Part 5: Northern Europe; Pelagic Publishing: London, UK, 2019; pp. 26–45. [Google Scholar]
- Agostini, G.; Kacoliris, F.; Demetrio, P.; Natale, G.; Bonetto, C.; Ronco, A. Abnormalities in Amphibian Populations Inhabiting Agroecosystems in Northeastern Buenos Aires Province, Argentina. Dis. Aquat. Organ. 2013, 104, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Srabantika, M.; Sudipta, C.; Nath, B.S. Digital Malformation and Chromosomal Abnormality in Rana tigirina—The Warning Signal of Declining Amphibian Population. Int. J. Res. Biosci. 2014, 3, 48–56. [Google Scholar]
- Carvajal-Castro, J.D.; López-Aguirre, Y.; Ospina-L, A.M.; Santos, J.C.; Rojas, B.; Vargas-Salinas, F. Much More than a Clasp: Evolutionary Patterns of Amplexus Diversity in Anurans. Biol. J. Linn. Soc. 2020, 129, 652–663. [Google Scholar] [CrossRef]
- Duellman, W.E.; Trueb, L. Biology of Amphibians; JHU Press: Baltimore, MD, USA, 1994. [Google Scholar]
- Wells, K.D. The Ecology and Behavior of Amphibians; University of Chicago Press: Chicago, IL, USA, 2010. [Google Scholar]
- Bókony, V.; Üveges, B.; Ujhegyi, N.; Verebélyi, V.; Nemesházi, E.; Csíkvári, O.; Hettyey, A. Endocrine Disruptors in Breeding Ponds and Reproductive Health of Toads in Agricultural, Urban and Natural Landscapes. Sci. Total Environ. 2018, 634, 1335–1345. [Google Scholar] [CrossRef]
- Sowers, A.D.; Mills, M.A.; Klaine, S.J. The Developmental Effects of a Municipal Wastewater Effluent on the Northern Leopard Frog, Rana Pipiens. Aquat. Toxicol. 2009, 94, 145–152. [Google Scholar] [CrossRef]
- Piprek, R.P.; Pecio, A.; Kloc, M.; Kubiak, J.Z.; Szymura, J.M. Evolutionary Trend for Metamery Reduction and Gonad Shortening in Anurans Revealed by Comparison of Gonad Development. Int. J. Dev. Biol. 2015, 58, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Unsicker, K. Fine Structure of the Male Genital Tract and Kidney in the Anura Xenopus laevis Daudin, Rana temporaria L. and Bufo bufo L. under Normal and Experimental Conditions. Cell Tissue Res. 1975, 158, 215–240. [Google Scholar] [CrossRef]
- Brande-Lavridsen, N.; Christensen-Dalsgaard, J.; Korsgaard, B. Effects of Prochloraz and Ethinylestradiol on Sexual Development in Rana temporaria. J. Exp. Zool. Part Ecol. Genet. Physiol. 2008, 309, 389–398. [Google Scholar] [CrossRef]
- Piprek, R.P.; Pecio, A.; Kubiak, J.Z.; Szymura, J.M. Differential Effects of Testosterone and 17β-Estradiol on Gonadal Development in Five Anuran Species. Reproduction 2012, 144, 257–267. [Google Scholar] [CrossRef]
- Ohtani, H.; Miura, I.; Ichikawa, Y. Effects of Dibutyl Phthalate as an Environmental Endocrine Disruptor on Gonadal Sex Differentiation of Genetic Males of the Frog Rana rugosa. Environ. Health Perspect. 2000, 108, 1189–1193. [Google Scholar] [CrossRef]
- Saidapur, S.K.; Gramapurohit, N.P.; Shanbhag, B.A. Effect of Sex Steroids on Gonadal Differentiation and Sex Reversal in the Frog, Rana curtipes. Gen. Comp. Endocrinol. 2001, 124, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Bernabò, I.; Gallo, L.; Sperone, E.; Tripepi, S.; Brunelli, E. Survival, Development, and Gonadal Differentiation in Rana dalmatina Chronically Exposed to Chlorpyrifos. J. Exp. Zool. Part Ecol. Genet. Physiol. 2011, 315, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Meng, T.; Li, Y.; Gao, K.; Qin, Z. Effects of Triclosan on Gonadal Differentiation and Development in the Frog Pelophylax nigromaculatus. J. Environ. Sci. 2018, 64, 157–165. [Google Scholar] [CrossRef]
- Lent, E.M.; Babbitt, K.J.; Sower, S.A. Gonadal Histology and Reproductive Steroidogenesis in Lithobates pipiens Exposed to Atrazine. Toxicol. Environ. Chem. 2019, 100, 583–600. [Google Scholar] [CrossRef]
- Li, X.; Shen, Y.; Lang, B.; Zhao, J.; Wang, H.; Zhang, Y. Influence of Octylphenol on Gene Expression of Gonadotropins and Their Receptors, Testicular Structure and Mating Behavior of Male Rana chensinensis. Environ. Toxicol. Pharmacol. 2021, 87, 103694. [Google Scholar] [CrossRef] [PubMed]






| Compound | Impact on Amphibians | Presence and Relevance |
|---|---|---|
| Bisphenol A (BPA) | Affected thyroid function in Xenopus laevis, Xenopus tropicalis, Glandirana rugosa [57,58]. Disrupted somatic development in X. laevis, X. tropicalis, G. rugosa, Rhinella arenarum, Pelophylax nigromaculatus [57,59,60,61,62]. Negatively affected testes development in X. laevis [63]. Feminized sex ratio in X. laevis [64,65,66]. | One of the most widely studied endocrine-disrupting compounds (EDCs). Present in polycarbonate plastics, epoxy resins, and various consumer products. Found in surface waters, affecting wildlife and human health [6]. |
| Nonylphenol (NP) | Had feminizing effect on sex ratio in X. laevis [64] and gonadal development in L. pipiens, L. sylvaticus [67] and Lithobates catesbeianus [68]. Impacted levels of sexual hormones in Pelophylax kl. esculentus [65]. | A persistent environmental contaminant from the degradation of alkylphenols. Used in surfactants and detergents. Commonly found in aquatic environments, affecting reproductive functions [65]. |
| Parabens | Methylparaben impaired gene expression and development in X. laevis embryos at relatively high concentrations [69]. Propylparaben affected the thyroid function in X. tropicalis [70]. | Widely used as preservatives in cosmetics, pharmaceuticals, and personal care products. Frequently detected in surface waters. Emerging EDCs require further research on their effects on amphibians [71]. |
| Estrogens | Estrone (E1) caused sex reversal in Ambystoma tigrinum [72]. 17β-estradiol (E2) had feminizing effect on sex ratio and gonadal development in X. laevis [66,73,74,75]; Anaxyrus (Bufo) americanus, Dryophytes versicolor and Lithobates sphenocephalus [76]. 17-ethinylestradiol (EE2) had a feminizing effect on sex ratio and gonadal development of X. laevis and X. tropicalis [77,78,79]; Hyla arborea, Bufotes viridis [80]; Bufo bufo [81], Rana temporaria [77], L. pipiens [67,82] and L. sylvaticus [83]. | Found in surface waters and wastewater effluents, originating from natural and synthetic hormones, including those used in contraceptive pills and hormone therapies. One of the most potent EDCs affecting amphibians [84,85]. |
| Progesterone | Negatively affected oogenesis and disrupted metamorphosis in X. laevis [86,87]. | Present in the environment due to pharmaceutical use in contraceptives and cancer treatment therapies. Its effects on amphibian populations remain poorly understood, requiring further investigation [87]. |
| Compound | Week | RA C1 [µg/I] | RA C2 [µg/I] | RA E1 [µg/I] | RA E2 [µg/I] | RT C1 [µg/I] | RT C2 [µg/I] | RT E1 [µg/I] | RT E2 [µg/I] |
|---|---|---|---|---|---|---|---|---|---|
| bisphenol A | 1 | <0.095 | <0.095 | <0.095 | <0.095 | <0.095 | <0.095 | <0.095 | <0.095 |
| bisphenol A | 3 | <0.095 | <0.095 | <0.095 | <0.095 | <0.095 | <0.095 | <0.095 | <0.095 |
| bisphenol A | 5 | <0.095 | <0.095 | <0.095 | <0.095 | <0.095 | <0.095 | <0.095 | <0.095 |
| bisphenol A | 7 | <0.095 | <0.095 | <0.095 | <0.095 | <0.095 | <0.095 | <0.095 | <0.095 |
| bisphenol A | 9 | <0.095 | <0.095 | <0.095 | <0.095 | <0.095 | <0.095 | <0.095 | <0.095 |
| nonylphenol | 1 | N.D. | N.D. | N.D. | N.D. | 0.1697 | N.D. | N.D. | N.D. |
| nonylphenol | 3 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| nonylphenol | 5 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| nonylphenol | 7 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| nonylphenol | 9 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| estradiol | 1 | N.D. | N.D. | <0.084 | N.D. | N.D. | N.D. | N.D. | <0.084 |
| estradiol | 3 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| estradiol | 5 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| estradiol | 7 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| estradiol | 9 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| estrone | 1 | N.D. | N.D. | N.D. | <0.071 | 0.1640 | N.D. | 4.5275 | <0.071 |
| estrone | 3 | N.D. | N.D. | N.D. | N.D. | 0.1100 | N.D. | 1.3800 | N.D. |
| estrone | 5 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 1.0400 | N.D. |
| estrone | 7 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| estrone | 9 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| ethinylestradiol | 1 | 2.0284 | 1.2656 | 2.0300 | 3.6314 | 3.4168 | 1.5508 | 5.5474 | 2.7144 |
| ethinylestradiol | 3 | 1.0300 | 0.9700 | 0.5500 | 1.4300 | 2.3600 | 1.4700 | <0.098 | <0.098 |
| ethinylestradiol | 5 | <0.098 | 0.2200 | 0.3200 | 0.3300 | <0.098 | N.D. | <0.098 | <0.098 |
| ethinylestradiol | 7 | <0.098 | <0.098 | <0.098 | <0.098 | <0.098 | N.D. | <0.098 | 0.1200 |
| ethinylestradiol | 9 | <0.098 | <0.098 | <0.098 | <0.098 | <0.098 | N.D. | <0.098 | <0.098 |
| progesterone | 1 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | <0.098 | N.D. |
| progesterone | 3 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| progesterone | 5 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| progesterone | 7 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| progesterone | 9 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| methylparaben | 1 | 1.0400 | 1.1000 | N.D. | N.D. | 2.3227 | 0.8000 | N.D. | N.D. |
| methylparaben | 3 | 0.1200 | 0.4500 | N.D. | N.D. | N.D. | 0.3900 | N.D. | N.D. |
| methylparaben | 5 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| methylparaben | 7 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| methylparaben | 9 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| propylparaben | 1 | 0.4924 | 0.4999 | 1.0016 | 1.0727 | 0.9729 | 0.4186 | 4.5851 | 0.7415 |
| propylparaben | 3 | 0.3900 | 0.2500 | N.D. | 0.1300 | N.D. | 0.2700 | 0.2600 | 0.5300 |
| propylparaben | 5 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| propylparaben | 7 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| propylparaben | 9 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| Collection Time | Water Tanks | Chlorophyll A | Phaeophytin A |
|---|---|---|---|
| µg/I | µg/I | ||
| 1st week of exposition | collective sample from all control tanks | 1.28 | 2.08 |
| collective sample from all experimental tanks | 17.32 | 3.22 | |
| 9th week of exposition | RA (C1-C4) mean value (±): SD | 143.29 (±): 144.81 | 24.58 (±): 15.95 |
| RT (C1-4) mean value (±): SD | 33.05 (±): 29.61 | 28.27 (±): 18.87 | |
| RA (E1-4) mean value (±): SD | 202.88 (±): 45.52 | 55.61 (±): 8.89 | |
| RT (E1-4) mean value (±): SD | 257.71 (±): 146.03 | 73.62 (±): 28.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frątczak, M.; Kaczmarski, M.; Szkudelska, K.; Abdelmajeed, A.Y.A.; Jankowiak, Ł.; Maliński, T.; Myczko, Ł.; Ostaszewska, M.; Przybylska-Balcerek, A.; Rozenblut-Kościsty, B.; et al. Investigating Sexual Characteristics in Two Frog Species Under Exposure to River Water Polluted with Endocrine Disruptors. Animals 2025, 15, 3364. https://doi.org/10.3390/ani15233364
Frątczak M, Kaczmarski M, Szkudelska K, Abdelmajeed AYA, Jankowiak Ł, Maliński T, Myczko Ł, Ostaszewska M, Przybylska-Balcerek A, Rozenblut-Kościsty B, et al. Investigating Sexual Characteristics in Two Frog Species Under Exposure to River Water Polluted with Endocrine Disruptors. Animals. 2025; 15(23):3364. https://doi.org/10.3390/ani15233364
Chicago/Turabian StyleFrątczak, Martyna, Mikołaj Kaczmarski, Katarzyna Szkudelska, Abdallah Yussuf Ali Abdelmajeed, Łukasz Jankowiak, Tomasz Maliński, Łukasz Myczko, Monika Ostaszewska, Anna Przybylska-Balcerek, Beata Rozenblut-Kościsty, and et al. 2025. "Investigating Sexual Characteristics in Two Frog Species Under Exposure to River Water Polluted with Endocrine Disruptors" Animals 15, no. 23: 3364. https://doi.org/10.3390/ani15233364
APA StyleFrątczak, M., Kaczmarski, M., Szkudelska, K., Abdelmajeed, A. Y. A., Jankowiak, Ł., Maliński, T., Myczko, Ł., Ostaszewska, M., Przybylska-Balcerek, A., Rozenblut-Kościsty, B., Siekiera, J., Stuper-Szablewska, K., & Tryjanowski, P. (2025). Investigating Sexual Characteristics in Two Frog Species Under Exposure to River Water Polluted with Endocrine Disruptors. Animals, 15(23), 3364. https://doi.org/10.3390/ani15233364









