Regional Prevalence and Molecular Detection of Enterocytozoon hepatopenaei in Coastal Shellfish from Korea
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. PCR and qPCR Assays for EHP Detection and Prevalence
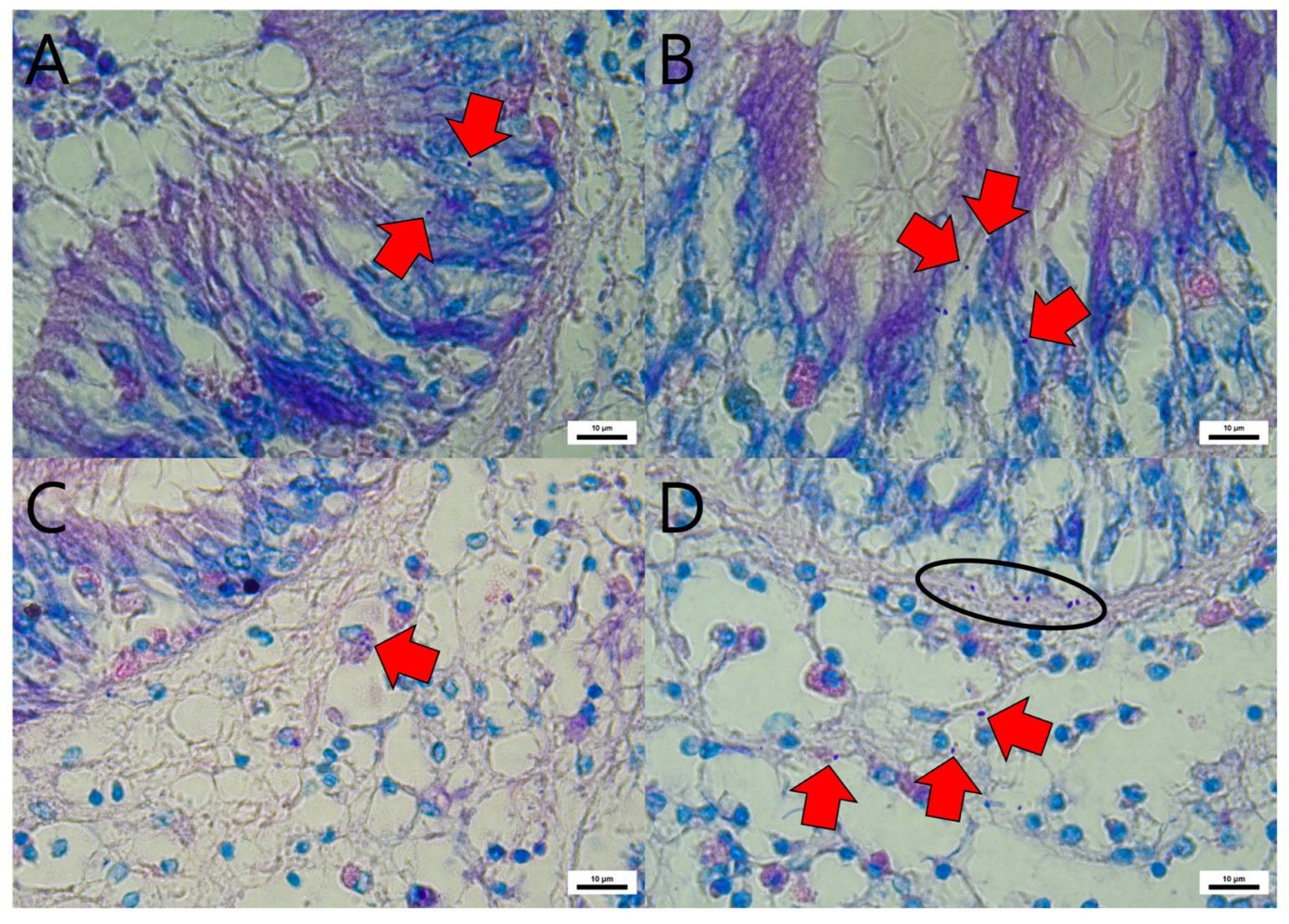
2.3. Histopathological Analysis of EHP-Containing Manila Clam
2.4. Transmission of EHP to L. vannamei After Feeding on EHP-Exposed Manila Clams
3. Results
3.1. Regional Sampling Results of SWP-PCR and qPCR
3.2. Histopathological Analysis of EHP-Exposed Manila Clams
3.3. Transmission of EHP L. vannamei After Feeding on EHP-Containing Manila Clams
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shinn, A.P.; Pratoomyot, J.; Griffiths, D.; Trong, T.Q.; Vu, N.T.; Jiravanichpaisal, P.; Briggs, M. Asian shrimp production and the economic costs of disease. Asian Fish. Sci. 2018, 31, 29–58. [Google Scholar] [CrossRef]
- Patil, P.K.; Geetha, R.; Ravisankar, T.; Avunje, S.; Solanki, H.G.; Abraham, T.J.; Vinoth, S.P.; Jithendran, K.P.; Alavandi, S.V.; Vijayan, K.K. Economic loss due to diseases in Indian shrimp farming with special reference to Enterocytozoon hepatopenaei (EHP) and white spot syndrome virus (WSSV). Aquaculture 2021, 533, 736231. [Google Scholar] [CrossRef]
- Kim, B.S.; Jang, G.I.; Kim, S.M.; Kim, Y.S.; Jeon, Y.G.; Oh, Y.K.; Hwang, J.Y.; Kwon, M.G. First report of Enterocytozoon hepatopenaei infection in Pacific Whiteleg Shrimp (Litopenaeus vannamei) cultured in Korea. Animals 2021, 11, 3150. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.I.; Kim, S.M.; Oh, Y.K.; Lee, S.J.; Hong, S.Y.; Lee, H.E.; Kwon, M.G.; Kim, B.S. First Report of Enterocytozoon hepatopenaei infection in Giant Freshwater Prawn (Macrobrachium rosenbergii de Man) cultured in the Republic of Korea. Animals 2022, 12, 3149. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, C.; Jeon, H.J.; Kim, B.K.; Lee, N.K.; Choi, S.K.; Han, J.E. First report on Enterocytozoon hepatopenaei (EHP) infection in Pacific white shrimp (Penaeus vannamei) cultured in Korea. Aquaculture 2022, 547, 737525. [Google Scholar] [CrossRef]
- Navaneeth Krishnan, A.; Jagadeesan, V.; Ezhil Praveena, P.; Bhuvaneswari, T.; Jithendran, K.P. A comparative analysis of different challenge routes against Enterocytozoon hepatopenaei infection in Penaeus vannamei. Aquac. Int. 2024, 32, 9415–9439. [Google Scholar] [CrossRef]
- Krishnan, A.N.; Kannappan, S.; Aneesh, P.T.; Praveena, P.E.; Jithendran, K.P. Polychaete worm—A passive carrier for Enterocytozoon hepatopenaei in shrimp. Aquaculture 2021, 545, 737187. [Google Scholar] [CrossRef]
- Mondal, S.; Deepika, A.; Hundare, S.; Poojary, N.; Abraham, T.; Sreedharan, K.; Rajendran, K. A study on the natural host range of Enterocytozoon hepatopenaei in different species of shrimp and co-habiting aquatic fauna. Indian J. Agric. Res. 2023, 70, 98–106. [Google Scholar] [CrossRef]
- Tanpichai, P.; Charoenwai, O.; Sataporn, C.; Srisuwatanasagul, S.; Chothirunpanit, A.; Suksumran, N.; Piamsomboon, P. Experimental infection of Enterocytozoon hepatopenaei (EHP) by water and sediment transfer between Pacific white shrimp (Penaeus vannamei) and Green Mud Crab (Scylla paramamosain). Pak. Vet. J. 2025, 45. [Google Scholar] [CrossRef]
- Munkongwongsiri, N.; Thepmanee, O.; Lertsiri, K.; Vanichviriyakit, R.; Itsathitphaisarn, O.; Sritunyalucksana, K. False mussels (Mytilopsis leucophaeata) can be mechanical carriers of the shrimp microsporidian Enterocytozoon hepatopenaei (EHP). J. Invertebr. Pathol. 2022, 187, 107690. [Google Scholar] [CrossRef] [PubMed]
- Jaroenlak, P.; Sanguanrut, P.; Williams, B.A.P.; Stentiford, G.D.; Flegel, T.W.; Sritunyalucksana, K.; Itsathitphaisarn, O. A nested PCR assay to avoid false positive detection of the microsporidian Enterocytozoon hepatopenaei (EHP) in environmental samples in shrimp farms. PLoS ONE 2016, 11, e0166320. [Google Scholar] [CrossRef] [PubMed]
- Chaijarasphong, T.; Munkongwongsiri, N.; Stentiford, G.D.; Aldama-Cano, D.J.; Thansa, K.; Flegel, T.W.; Sritunyalucksana, K.; Itsathitphaisarn, O. The shrimp microsporidian Enterocytozoon hepatopenaei (EHP): Biology, pathology, diagnostics and control. J. Invertebr. Pathol. 2021, 186, 107458. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Qiu, L.; Sheng, A.Z.; Wan, X.Y.; Cheng, D.Y.; Huang, J. Quantitative detection method of Enterocytozoon hepatopenaei using TaqMan probe real-time PCR. J. Invertebr. Pathol. 2018, 151, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Sathish, K.T.; Praveena, P.E.; Sivaramakrishnan, T.; Rajan, J.J.S.; Makesh, M.; Jithendran, K.P. Effect of Enterocytozoon hepatopenaei (EHP) infection on physiology, metabolism, immunity, and growth of Penaeus vannamei. Aquaculture 2022, 553, 738105. [Google Scholar] [CrossRef]
- Cao, Z.; Chen, C.; Wang, C.; Li, T.; Chang, L.; Si, L.; Yan, D. Enterocytozoon hepatopenaei (EHP) infection alters the metabolic processes and induces oxidative stress in Penaeus vannamei. Animals 2023, 13, 3661. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhu, B.; Xiong, J.; Chen, J. The pathogenic mechanism of Enterocytozoon hepatopenaei in Litopenaeus vannamei. Microorganisms 2024, 12, 1208. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Dou, Y.; Li, H.; Qiao, Y.; Jiang, G.; Wan, X.; Cheng, J.; Fan, X.; Li, H.; Wang, L.; et al. Changes in the intestinal microbiota of Pacific white shrimp (Litopenaeus vannamei) with different severities of Enterocytozoon hepatopenaei infection. J. Invertebr. Pathol. 2022, 191, 107763. [Google Scholar] [CrossRef] [PubMed]
| Province | Site | Organism | Sampling Date | Shell Length (cm) | Weight (g) | Number of Sampled Individuals |
|---|---|---|---|---|---|---|
| Gyeonsangnam-do | Goseong-gun | Bay scallop | March 2024. | 6.7 ± 0.6 | 43.9 ± 9.1 | 10 |
| Pacific oyster | March 2024. | 12.4 ± 0.9 | 125.9 ± 21.5 | 10 | ||
| Tongyeong-si | Bay scallop | February 2024. | 6.7 ± 0.7 | 37.4 ± 11.6 | 10 | |
| Pacific oyster | February 2024. | 10.0 ± 1.5 | 63.6 ± 24.6 | 10 | ||
| Jeollanam-do | Goheung-gun | Manila clam | February 2024. | 4.7 ± 0.3 | 11.5 ± 2.6 | 10 |
| Yeosu-si | Mediterranean mussel | March 2024. | 6.6 ± 0.6 | 14.6 ± 4.4 | 10 | |
| Chungcheongnam-do | Boryeong-si | Pacific oyster | March 2024. | 9.5 ± 0.9 | 77.1 ± 19.8 | 10 |
| Province | Site | Species | Method | Organs | Average Prevalence (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mantle | Digestive Gland | Gill | Stomach | Intestine | |||||
| Gyeonsangnam-do | Goseong-gun a | Bay scallop | SWP-PCR | 40% (4/10) | 10% (1/10) | 70% (7/10) | 20% (2/10) | 70% (7/10) | 67.4% (37.9~91.3%) |
| qPCR | - (0/10) | - (0/10) | 3.0 × 102 copies (2/10) | - (0/10) | 2.2 × 102 copies (2/10) | ||||
| Pacific oyster | SWP-PCR | 30% (3/10) | 30% (3/10) | 20% (2/10) | 20% (2/10) | 30% (3/10) | 32.2% (8.7~60.9%) | ||
| qPCR | 2.7 × 102 copies (1/10) | 7.8 × 102 copies (1/10) | 2.8 × 102 copies (1/10) | 1.5 × 102 copies (1/10) | 2.2 × 102 copies (2/10) | ||||
| Tongyeong-si b | Bay scallop | SWP-PCR | 0% (0/10) | 80% (8/10) | 80% (8/10) | 50% (5/10) | 50% (5/10) | 76.2% (47.8~96.2%) | |
| qPCR | - (0/10) | - (0/10) | - (0/10) | - (0/10) | - (0/10) | ||||
| Pacific oyster | SWP-PCR | 10% (1/10) | 50% (5/10) | 30% (3/10) | 60% (6/10) | 20% (2/10) | 58.8% (29.5~85.3%) | ||
| qPCR | - (0/10) | - (0/10) | - (0/10) | - (0/10) | - (0/10) | ||||
| Jeollanam-do | Goheung-gun a,b | Manilla clam | SWP-PCR | 70% (7/10) | 60% (6/10) | 60% (6/10) | 70% (7/10) | 80% (8/10) | 76.2% (47.8~96.2%) |
| qPCR | 4.0 × 102 copies (1/10) | 2.9 × 102 copies (1/10) | - (0/10) | - (0/10) | 2.2 × 102 copies (1/10) | ||||
| Yeosu-si b | Mediterranean mussel | SWP-PCR | 10% (1/10) | 40% (4/10) | 40% (4/10) | 40% (4/10) | 10% (1/10) | 41.4% (15.1~71.1%) | |
| qPCR | - (0/10) | - (0/10) | - (0/10) | - (0/10) | - (0/10) | ||||
| Chungcheongnam-do | Boryeong-si b | Pacific oyster | SWP-PCR | 30% (3/10) | 50% (5/10) | 80% (8/10) | 30% (3/10) | 50% (5/10) | 76.2% (47.8~96.2%) |
| qPCR | - (0/10) | - (0/10) | - (0/10) | - (0/10) | - (0/10) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.H.; Noh, E.B.; Choi, H.J.; Kwon, M.G.; Kim, B.S. Regional Prevalence and Molecular Detection of Enterocytozoon hepatopenaei in Coastal Shellfish from Korea. Animals 2025, 15, 3356. https://doi.org/10.3390/ani15223356
Lee BH, Noh EB, Choi HJ, Kwon MG, Kim BS. Regional Prevalence and Molecular Detection of Enterocytozoon hepatopenaei in Coastal Shellfish from Korea. Animals. 2025; 15(22):3356. https://doi.org/10.3390/ani15223356
Chicago/Turabian StyleLee, Beom Hee, Eul Bit Noh, Hee Jung Choi, Mun Gyeong Kwon, and Bo Seong Kim. 2025. "Regional Prevalence and Molecular Detection of Enterocytozoon hepatopenaei in Coastal Shellfish from Korea" Animals 15, no. 22: 3356. https://doi.org/10.3390/ani15223356
APA StyleLee, B. H., Noh, E. B., Choi, H. J., Kwon, M. G., & Kim, B. S. (2025). Regional Prevalence and Molecular Detection of Enterocytozoon hepatopenaei in Coastal Shellfish from Korea. Animals, 15(22), 3356. https://doi.org/10.3390/ani15223356






