Spatial and Environmental Drivers of Summer Growth Variability and Adaptive Mechanisms of Euphausia crystallorophias in the Amundsen Sea and Its Adjacent Regions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
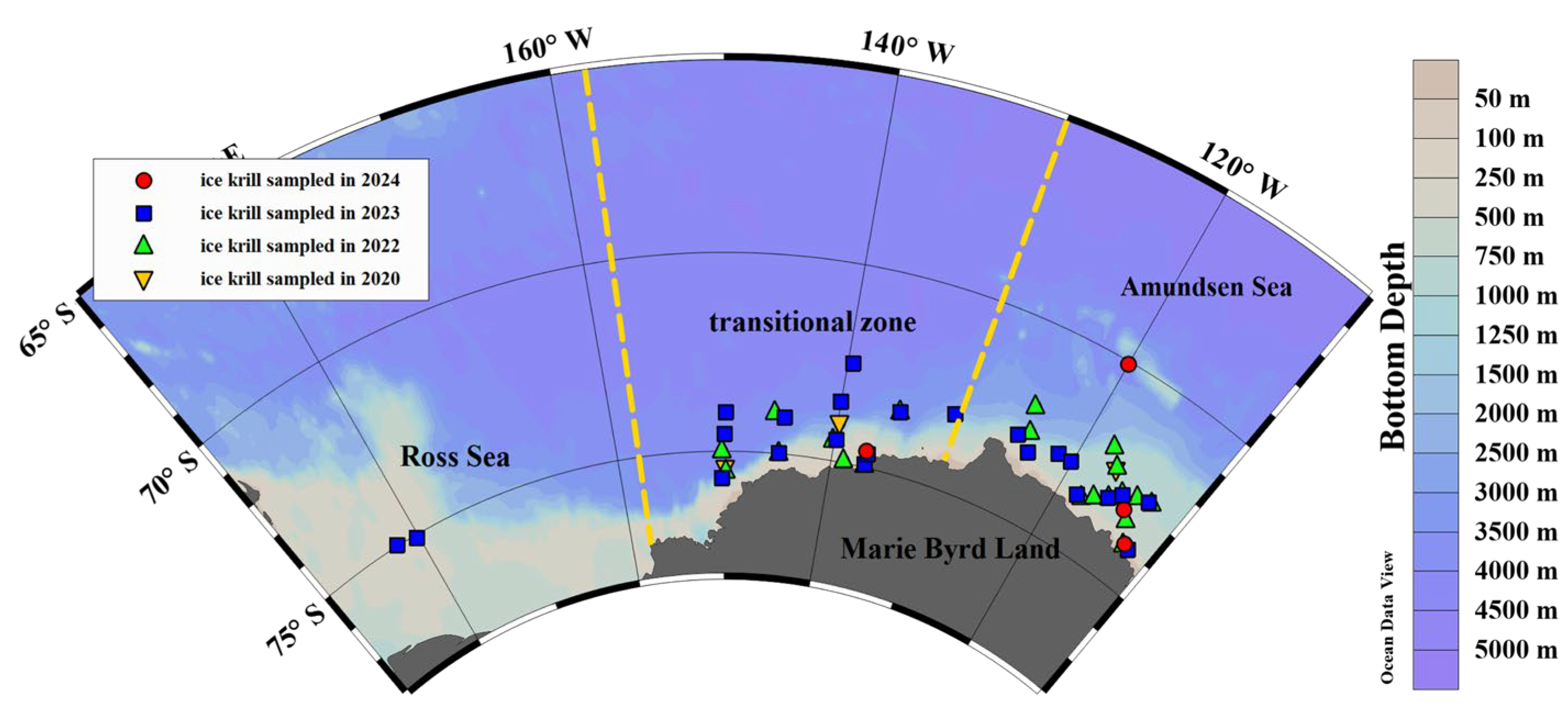
2.1. Study Area
2.2. Sample Collection and Preservation
2.3. Biological Measurements
2.4. Environmental Data Acquisition and Processing
2.5. Statistical Analyses
2.5.1. Length–Weight Relationship Modeling
2.5.2. Spatial Variation Analysis
2.5.3. Environmental Effects on Growth
3. Results
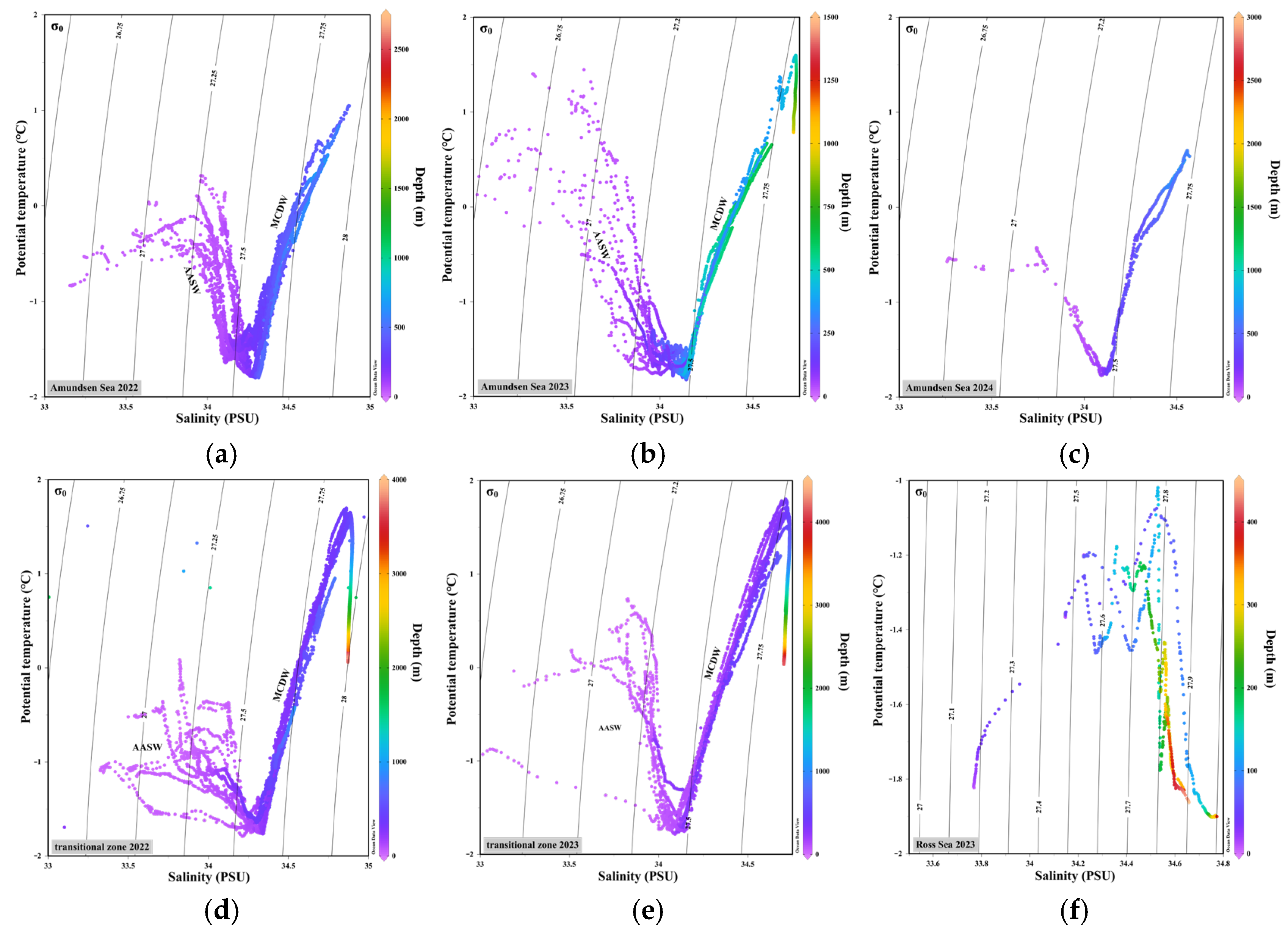
3.1. Environmental Characteristics in Survey Area
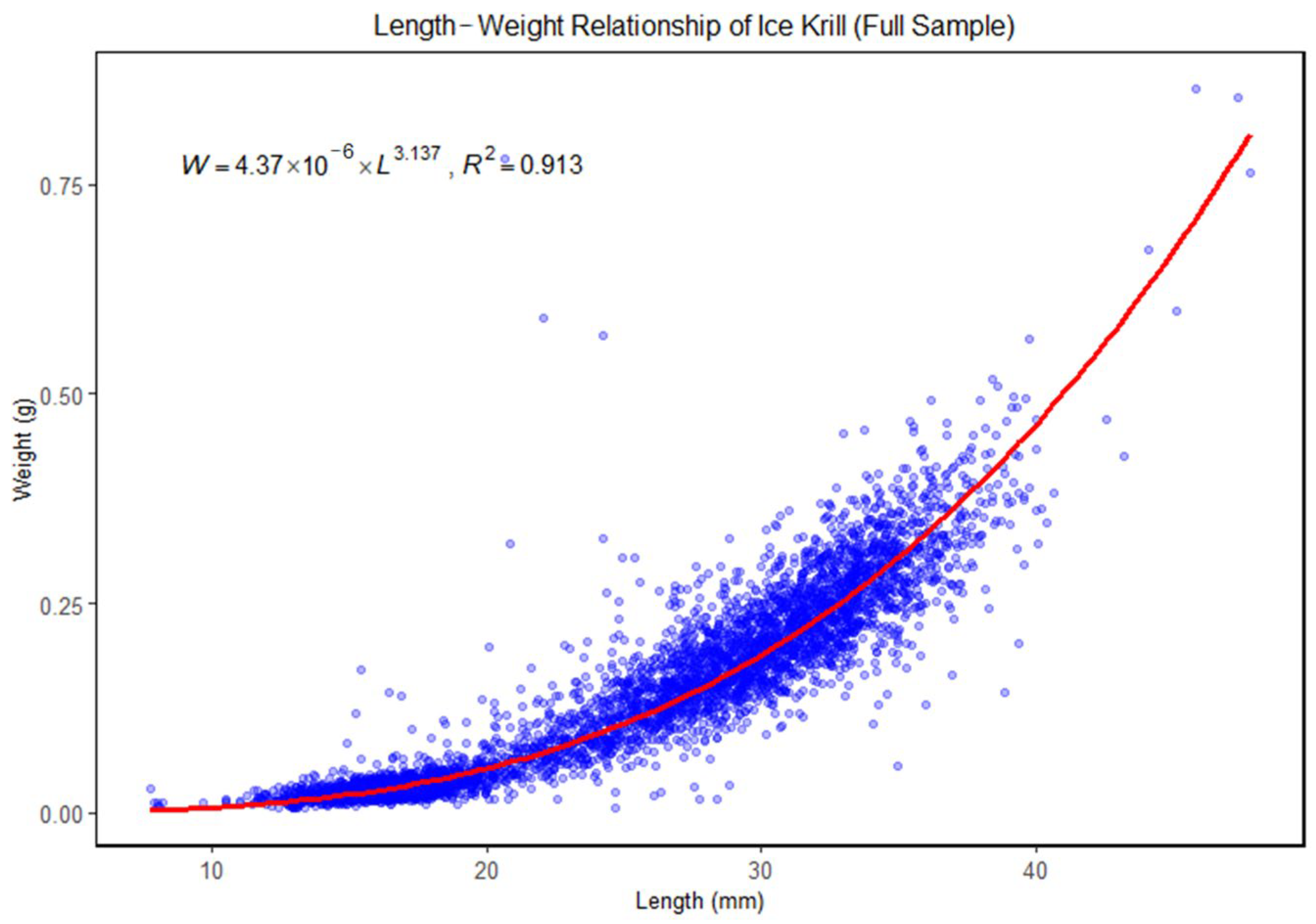
3.2. Length–Weight Relationship of Ice Krill
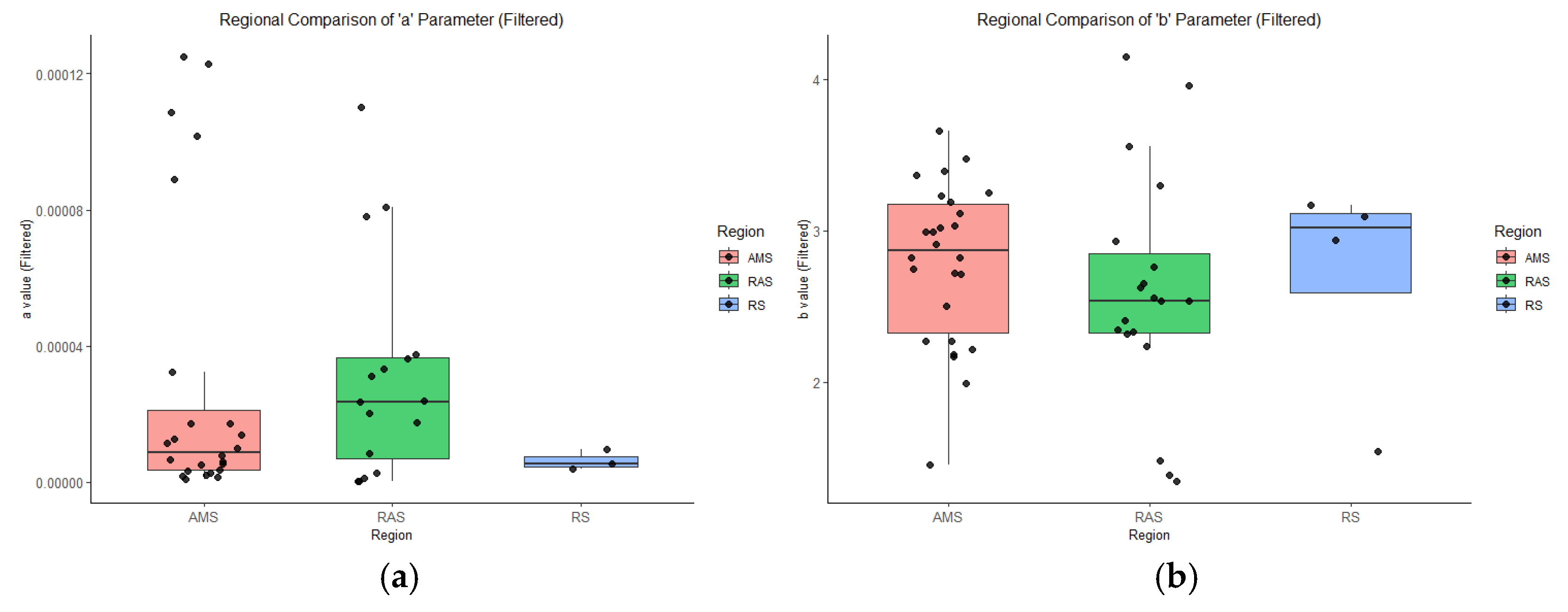
3.3. Regional Distributions of LWR Parameters Across Sampling Areas
3.4. RDA of LWR Parameters with Environmental and Spatial Variables
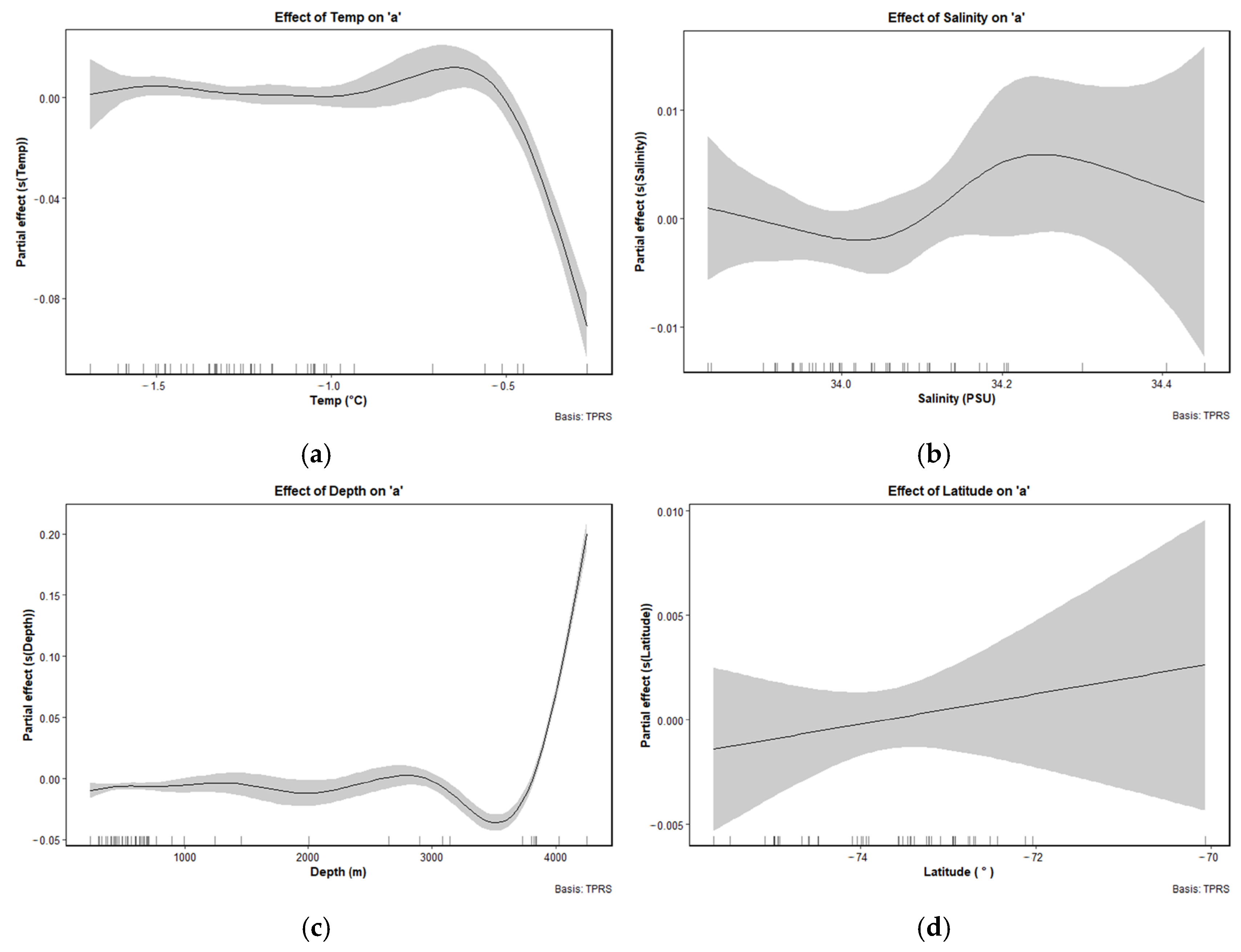
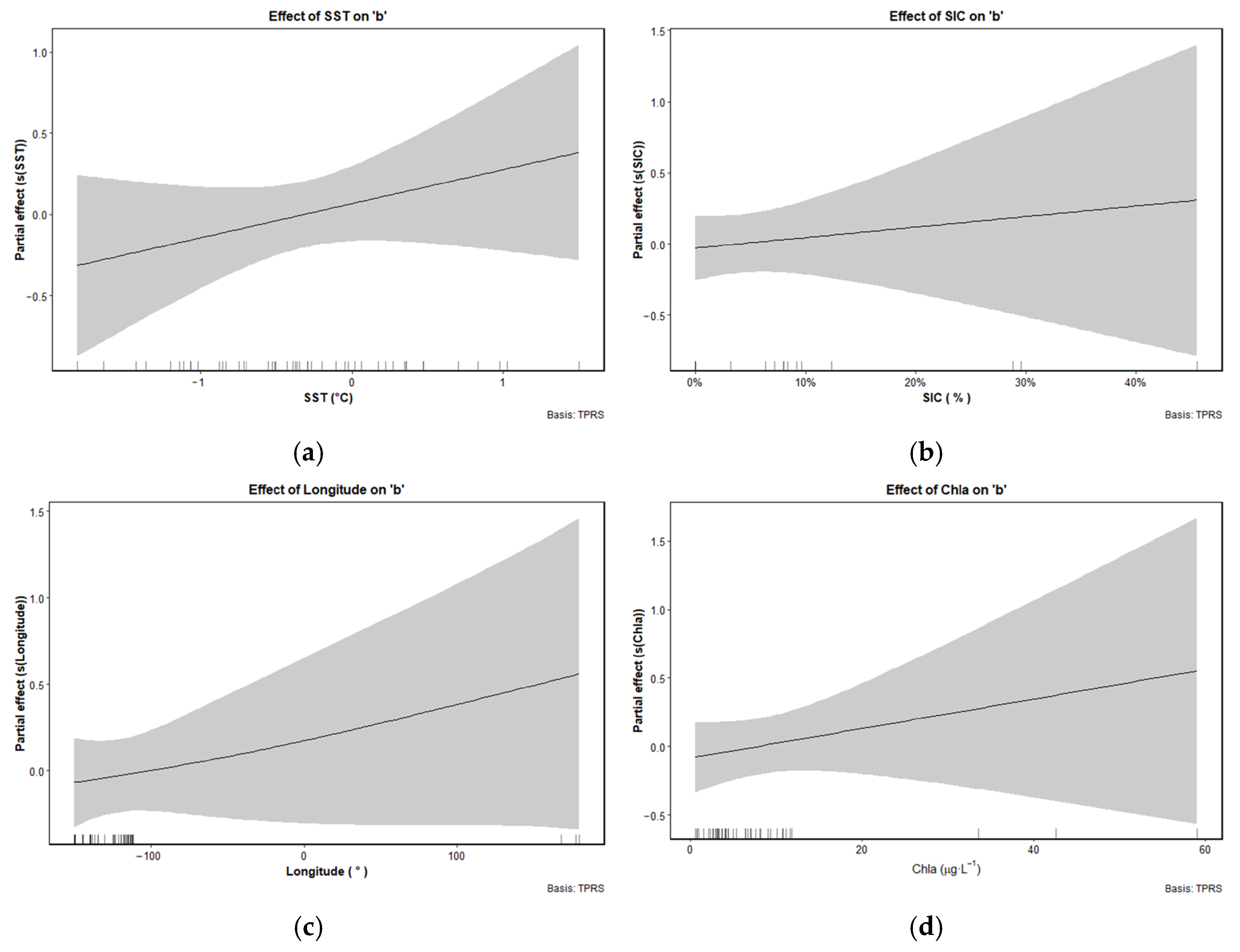
3.5. Response of LWR Factors to Environmental and Spatial Gradients by GAM
4. Discussion
4.1. Regional Variation in the LWR of Ice Krill and Its Ecological Interpretation
4.2. Regional Differentiation in Growth Strategies of Ice Krill
4.3. Environmental and Spatial Drivers of Growth Strategy Divergence in Ice Krill
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicol, S.; Foster, J. Recent trends in the fishery for Antarctic krill. Aquat. Living Resour. 2003, 16, 42–45. [Google Scholar] [CrossRef]
- Atkinson, A.; Siegel, V.; Pakhomov, E.; Rothery, P. Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 2006, 432, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Constable, A.J.; Melbourne-Thomas, J.; Corney, S.P.; Arrigo, K.R.; Barbraud, C.; Barnes, D.K.A.; Bindoff, N.L.; Boyd, P.W.; Brandt, A.; Costa, D.P.; et al. Climate change and Southern Ocean ecosystems I: How changes in physical habitats directly affect marine biota. Glob. Change Biol. 2014, 20, 3004–3025. [Google Scholar] [CrossRef]
- Hill, S.L.; Murphy, E.J.; Reid, K.; Trathan, P.N.; Constable, A.J. Modelling Southern Ocean ecosystems: Krill, the food-web, and the impacts of harvesting. Biol. Rev. 2006, 81, 581–608. [Google Scholar] [CrossRef]
- Tarling, G.A.; Klevjer, T.; Fielding, S.; Watkins, J.; Atkinson, A.; Murphy, E.; Korb, R.; Whitehouse, M.; Leaper, R. Variability and predictability of Antarctic krill swarm structure. Deep Sea Res. Part I Oceanogr. Res. Pap. 2009, 56, 1994–2012. [Google Scholar] [CrossRef]
- Froese, R. Cube law, condition factor and weight-length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Färber–Lorda, J. Length-weight relationships and coefficient of condition of Euphausia superba and Thysanoessa macrura (Crustacea: Euphausiacea) in southwest Indian Ocean during summer. Mar. Biol. 1994, 118, 645–650. [Google Scholar] [CrossRef]
- Turner, J.; Bracegirdle, T.J.; Phillips, T.; Marshall, G.J.; Hosking, J.S. An initial assessment of Antarctic sea ice extent in the CMIP5 models. J. Clim. 2016, 26, 1473–1484. [Google Scholar] [CrossRef]
- Flores, H.; Atkinson, A.; Kawaguchi, S.; Krafft, B.A.; Milinevsky, G.; Nicol, S.; Reiss, C.; Tarling, G.A.; Werner, R.; Rebolledo, E.B.; et al. Impact of climate change on Antarctic krill. Mar. Ecol. Prog. Ser. 2012, 458, 1–19. [Google Scholar] [CrossRef]
- Siegel, V. Biology and Ecology of Antarctic Krill. Advances in Polar Ecology, 1st ed.; Spinger: Berlin, Germany, 2016; pp. 1–266. [Google Scholar]
- Cavan, E.L.; Belcher, A.; Atkinson, A.; Hill, S.L.; Kawaguchi, S.; McCormack, S.; Meyer, B.; Nicol, S.; Ratnarajah, L.; Schmidt, K.; et al. The importance of Antarctic krill in biogeochemical cycles. Nat. Commun. 2019, 10, 4742. [Google Scholar] [CrossRef]
- Nakayama, Y.; Menemenlis, D.; Zhang, H.; Schodlok, M.; Rignot, E. Origin of Circumpolar Deep Water intruding onto the Amundsen and Bellingshausen Sea continental shelves. Nat. Commun. 2018, 9, 3403. [Google Scholar] [CrossRef]
- Meyer, B.; Fuentes, V.; Guerra, C.; Schmidt, K.; Atkinson, A.; Spahic, S.; Cisewski, B.; Freier, U.; Olariaga, A.; Bathmann, U. Physiology, growth, and development of larval krill (Euphausia superba) in autumn and winter in the Lazarev Sea, Antarctica. Limnol. Oceanogr. 2009, 54, 1595–1614. [Google Scholar] [CrossRef]
- Bahlburg, D.; Thorpe, S.E.; Meyer, B.; Berger, U.; Murphy, E.J. An intercomparison of models predicting growth of Antarctic krill (Euphausia superba): The importance of recognizing model specificity. PLoS ONE 2023, 18, e0286036. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Li, S.; Li, L.Z.; Zhao, G.Q.; Rao, X.; Huang, H.L. Age group of Euphausia crystallorophias population in the Amundsen Sea and its surrounding waters in summer based on distribution mixture analysis from length frequency. Mar. Fish. 2024, 46, 702–712. [Google Scholar] [CrossRef]
- Yang, J.L.; Li, S.; Li, L.Z.; Rao, X.; Chen, S.; Huang, H.L. Response of ice krill (Euphausia crystallorophias) density to environmental changes in the Amundsen Sea Coastal Polynya, Antarctica. Deep Sea Res. Part I Oceanogr. Res. Pap. 2024, 205, 104250. [Google Scholar] [CrossRef]
- Cuzin–Roudy, J.; Irisson, J.O.; Penot, F.; Kawaguchi, S.; Vallet, C. Southern Ocean Euphausiids. In Biogeographic Atlas of the Southern Ocean, 1st ed.; De Broyer, C., Koubbi, P., Griffiths, H.J., Raymond, B., Udekem d’Acoz, C., Van de Putte, A.P., Danis, B., David, B., Grant, S., Gutt, J., et al., Eds.; Scientific Committee on Antarctic Research: Cambridge, UK, 2014; pp. 309–320. [Google Scholar]
- Atkinson, A.; Shreeve, R.S.; Hirst, A.G.; Rothery, P.; Tarling, G.A.; Pond, D.W.; Korb, R.E.; Murphy, E.J.; Watkins, J.L. Natural growth rates in Antarctic krill (Euphausia superba): II. Predictive models based on food, temperature, body length, sex, and maturity stage. Limnol. Oceanogr. 2006, 51, 973–987. [Google Scholar] [CrossRef]
- Community Ecology Package. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 29 January 2025).
- Jørgensen, L.L.; Primicerio, R.; Ingvaldsen, R.B.; Fossheim, M.; Anisimova, N.; Thangstad, T.H.; Manushin, I.; Zakharov, D. Impact of multiple stressors on sea bed fauna in a warming Arctic. Mar. Ecol. Prog. Ser. 2019, 608, 1–12. [Google Scholar] [CrossRef]
- Yang, G.; Li, C.L.; Wang, Y.Q. Fatty acid composition of Euphausia superba, Thysanoessa macrura and Euphausia crystallorophias collected from Prydz Bay, Antarctica. J. Ocean Univ. China 2016, 15, 297–302. [Google Scholar] [CrossRef]
- Hellessey, N.; Johnson, R.; Ericson, J.A.; Nichols, P.D.; Kawaguchi, S.; Nicol, S.; Hoem, N.; Virtue, P. Antarctic krill lipid and fatty acid content variability is associated with satellite-derived chlorophyll a and sea surface temperatures. Sci. Rep. 2020, 10, 6060. [Google Scholar] [CrossRef]
- Atkinson, A.; Siegel, V.; Pakhomov, E.; Rothery, P. Oceanic circumpolar habitats of Antarctic krill. Mar. Ecol. Prog. Ser. 2008, 362, 1–23. [Google Scholar] [CrossRef]
- Saba, G.K.; Fraser, W.R.; Saba, V.S.; Iannuzzi, R.A.; Coleman, K.E.; Doney, S.C.; Ducklow, H.W.; Martinson, D.G.; Miles, T.N.; Patterson–Fraser, D.L.; et al. Winter and spring controls on the summer food web of the coastal West Antarctic Peninsula. Nat. Commun. 2014, 5, 4318. [Google Scholar] [CrossRef]
- Fach, B.A.; Hofmann, E.E.; Murphy, E.J. Transport of Antarctic krill (Euphausia superba) across the Scotia Sea. Part II: Krill growth and survival. Deep Sea Res. Part I Oceanogr. Res. Pap. 2006, 53, 1011–1043. [Google Scholar] [CrossRef]
- Schmidt, K.; Atkinson, A.; Venables, H.J.; Pond, D.W. Early spawning of Antarctic krill in the Scotia Sea is fuelled by “superfluous” feeding on non-ice associated phytoplankton blooms. Deep Sea Res. Part II Top. Stud. Oceanogr. 2012, 59–60, 159–172. [Google Scholar] [CrossRef]
- Schmidt, K.; Atkinson, A.; Pond, D.W.; Ireland, L.C. Feeding and overwintering of Antarctic krill across its major habitats: The role of sea ice cover, water depth, and phytoplankton abundance. Limnol. Oceanogr. 2014, 59, 17–36. [Google Scholar] [CrossRef]
- Leonori, I.; Felice, A.D.; Canduci, G.; Costantini, I.; Biagiotti, I.; Giuliani, G.; Budillon, G. Krill distribution in relation to environmental parameters in mesoscale structures in the Ross Sea. J. Mar. Syst. 2017, 166, 159–171. [Google Scholar] [CrossRef]
- Katsanevakis, S. Modelling fish growth: Model selection, multi-model inference and model selection uncertainty. Fish. Res. 2006, 81, 229–235. [Google Scholar] [CrossRef]
- Atkinson, A.; Siegel, V.; Pakhomov, E.A.; Jessopp, M.J.; Loeb, V. A re-appraisal of the total biomass and annual production of Antarctic krill. Deep Sea Res. Part I Oceanogr. Res. Pap. 2009, 56, 727–740. [Google Scholar] [CrossRef]
- Tarling, G.A.; Hobbs, C.; Johnson, M.L.; Färber-Lorda, J. Comparative morphology of Southern Ocean Euphausia species. Polar Biol. 2017, 40, 2043–2058. [Google Scholar] [CrossRef]
- Smetacek, V.; Nicol, S. Polar ocean ecosystems in a changing world. Nature 2005, 437, 362–368. [Google Scholar] [CrossRef]
- Naito, Y.; Kawamura, A.; Ichii, T. Ecological studies of the Antarctic krill, Euphausia superba Dana, in the Indian Sector of the Southern Ocean. Mem. Natl. Inst. Polar Res. 1986, 44, 130–155. [Google Scholar]
- Pakhomov, E.A.; Froneman, P.W.; Perissinotto, R. Salp/krill interactions in the Southern Ocean: Spatial segregation and implications for the carbon flux. Deep Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 1881–1907. [Google Scholar] [CrossRef]
- Hunt, B.P.V.; Pakhomov, E.A.; Hosie, G.W.; Siegel, V.; Ward, P.; Bernard, K. Pteropods in Southern Ocean ecosystems. Prog. Oceanogr. 2008, 78, 192–221. [Google Scholar] [CrossRef]
- Lizotte, M.P. The contributions of sea ice algae to Antarctic marine primary production. Am. Zool. 2001, 41, 57–73. [Google Scholar] [CrossRef]
- Melnikov, I.A. The Arctic Sea Ice Ecosystem; Gordon and Breach Publishers: London, UK, 1997; pp. 1–221. [Google Scholar]
- Arrigo, K.R.; van Dijken, G.L.; Strong, A.L. Environmental controls of marine productivity hot spots around Antarctica. J. Geophys. Res. Ocean. 2015, 120, 5545–5565. [Google Scholar] [CrossRef]
- Cuzin-Roudy, J.; Labat, J.P. Early summer distribution of Antarctic krill sexual development in relation to environmental conditions in the Scotia-Weddell region. Polar Biol. 2004, 12, 65–74. [Google Scholar] [CrossRef]
- Hagen, W.; Kattner, G. Lipid metabolism of the Antarctic euphausiid Thysanmssa macrura and its ecological implications. Limnol. Oceanogr. 2003, 43, 1894–1901. [Google Scholar] [CrossRef]
- Mauchline, J. Groth of mysids and euphausiids. In Crustacean Issues 3 Factors in Adult Growth, 1st ed.; Wenner, A.M., Ed.; Routledge: London, UK, 1985; pp. 18–144. [Google Scholar]
- Ikeda, T. Metabolic rates of epipelagic marine zooplankton as a function of body mass and temperature. Mar. Biol. 1985, 85, 1–11. [Google Scholar] [CrossRef]
- Orsi, A.H.; Whitworth, T., III; Nowlin, W.D., Jr. On the meridional extent and fronts of the Antarctic Circumpolar Current. Deep Sea Res. Part I Oceanogr. Res. Pap. 1995, 42, 641–673. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.; Zhou, M. Assessment of water mass distribution and intrusions of Circumpolar Deep Water in the Amundsen Sea based on the ocean reanalysis product FOAM-GLOSEA5v13. Front. Mar. Sci. 2024, 11, 1358196. [Google Scholar] [CrossRef]



| Value | SST | SSS | Chl-a | SIC | Temp | Salinity | Depth | Latitude | Longitude |
|---|---|---|---|---|---|---|---|---|---|
| a | 1.684 | 2.662 | 1.324 | 1.505 | 2.069 | 3.637 | 1.906 | 1.616 | 2.999 |
| b | 1.655 | 2.766 | 1.311 | 1.515 | 2.127 | 3.531 | 1.755 | 1.634 | 3.108 |
| Factor | RDA1 | RDA2 |
|---|---|---|
| SST | 0.269 | 0.086 |
| SSS | −0.065 | 0.351 |
| Chl-a | 0.251 | 0.013 |
| SIC | 0.159 | 0.068 |
| Temp | −0.387 | 0.665 |
| Salinity | −0.184 | 0.109 |
| Depth | −0.460 | −0.207 |
| Latitude | −0.584 | −0.080 |
| Longitude | 0.242 | −0.086 |
| a | −0.849 | −0.589 |
| b | 1.093 | −0.457 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Li, L.; Li, S.; Zhao, G.; Rao, X.; Chen, S.; Liu, H.; Shen, F.; Huang, H.; Wang, Z. Spatial and Environmental Drivers of Summer Growth Variability and Adaptive Mechanisms of Euphausia crystallorophias in the Amundsen Sea and Its Adjacent Regions. Animals 2025, 15, 3345. https://doi.org/10.3390/ani15223345
Yang J, Li L, Li S, Zhao G, Rao X, Chen S, Liu H, Shen F, Huang H, Wang Z. Spatial and Environmental Drivers of Summer Growth Variability and Adaptive Mechanisms of Euphausia crystallorophias in the Amundsen Sea and Its Adjacent Regions. Animals. 2025; 15(22):3345. https://doi.org/10.3390/ani15223345
Chicago/Turabian StyleYang, Jialiang, Lingzhi Li, Shuai Li, Guoqing Zhao, Xin Rao, Shuai Chen, Hewei Liu, Fengyuan Shen, Hongliang Huang, and Ziyi Wang. 2025. "Spatial and Environmental Drivers of Summer Growth Variability and Adaptive Mechanisms of Euphausia crystallorophias in the Amundsen Sea and Its Adjacent Regions" Animals 15, no. 22: 3345. https://doi.org/10.3390/ani15223345
APA StyleYang, J., Li, L., Li, S., Zhao, G., Rao, X., Chen, S., Liu, H., Shen, F., Huang, H., & Wang, Z. (2025). Spatial and Environmental Drivers of Summer Growth Variability and Adaptive Mechanisms of Euphausia crystallorophias in the Amundsen Sea and Its Adjacent Regions. Animals, 15(22), 3345. https://doi.org/10.3390/ani15223345








