Effects of Macronutrients in Ten Different Plant Species on the Food Choice and Growth Performance of Achatina fulica
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Snail Rearing and Plant Species
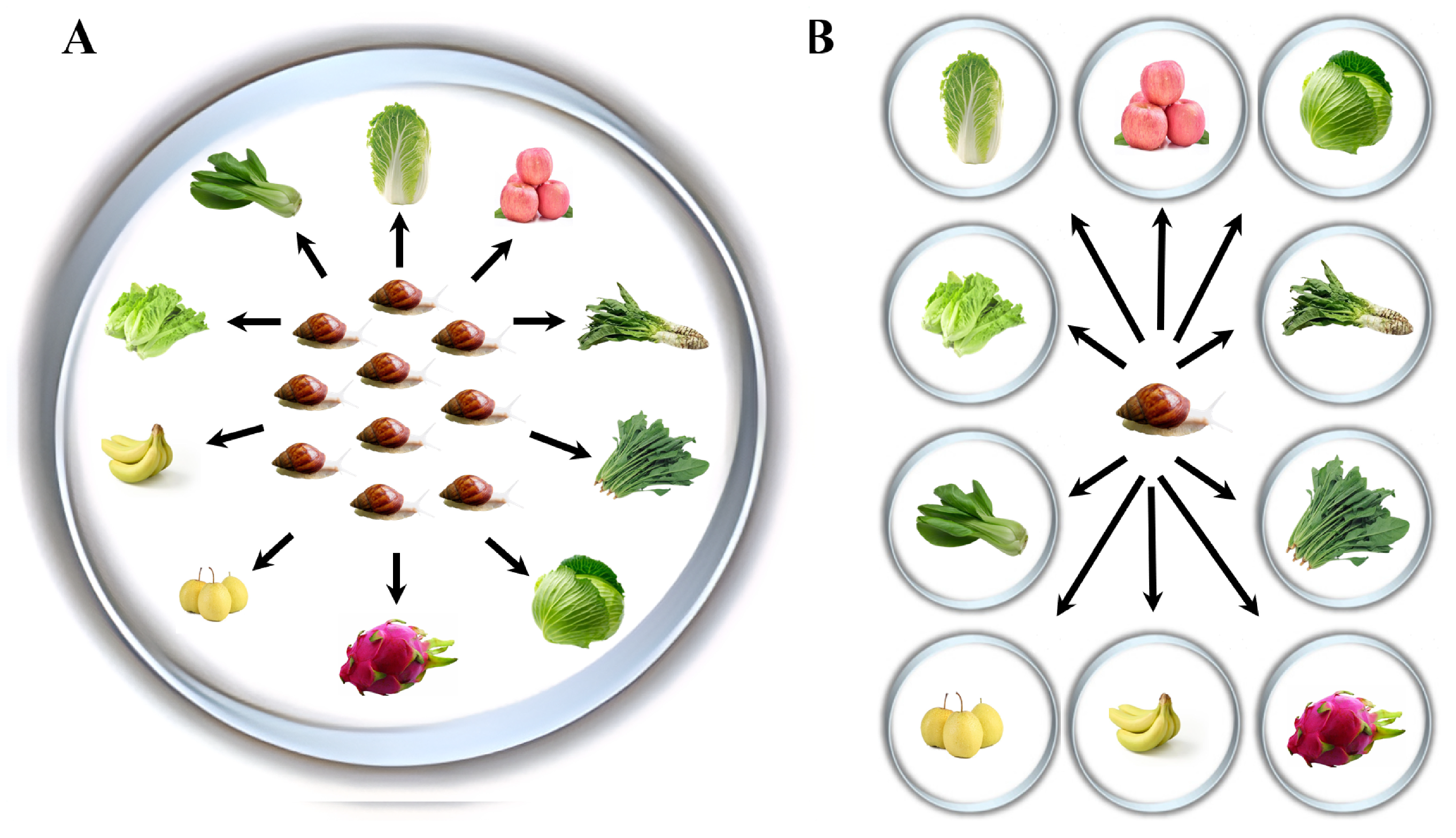
2.2. Feeding Selectivity of Snails Regarding 10 Plant Species
2.3. Feeding and Growth Performance of Snails
2.4. Analysis of Protein and Carbohydrate Contents of 10 Plant Species
2.5. Data Analysis
3. Results
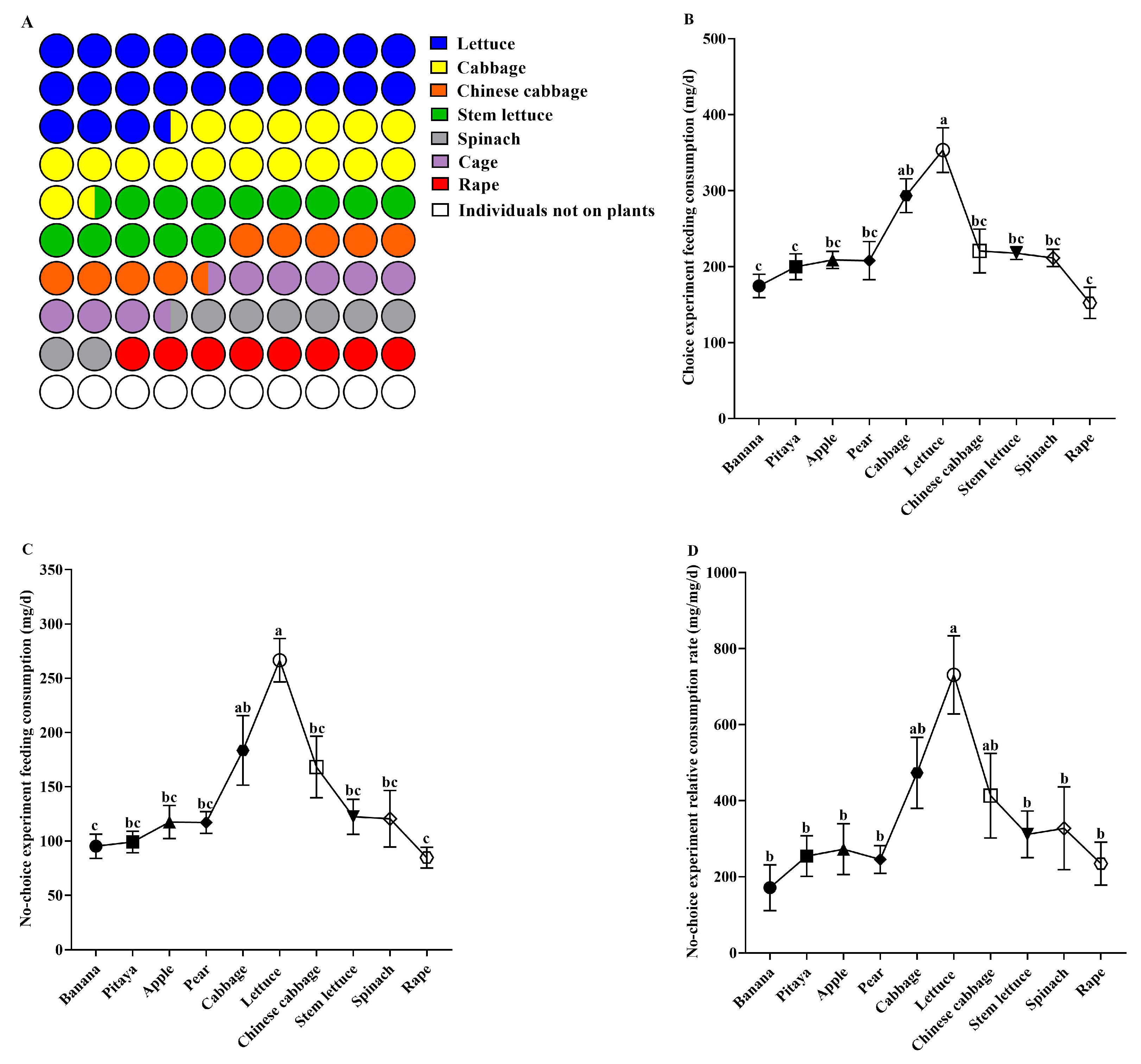
3.1. Feeding Selectivity of Snails Regarding 10 Plant Species in Choice Experiment
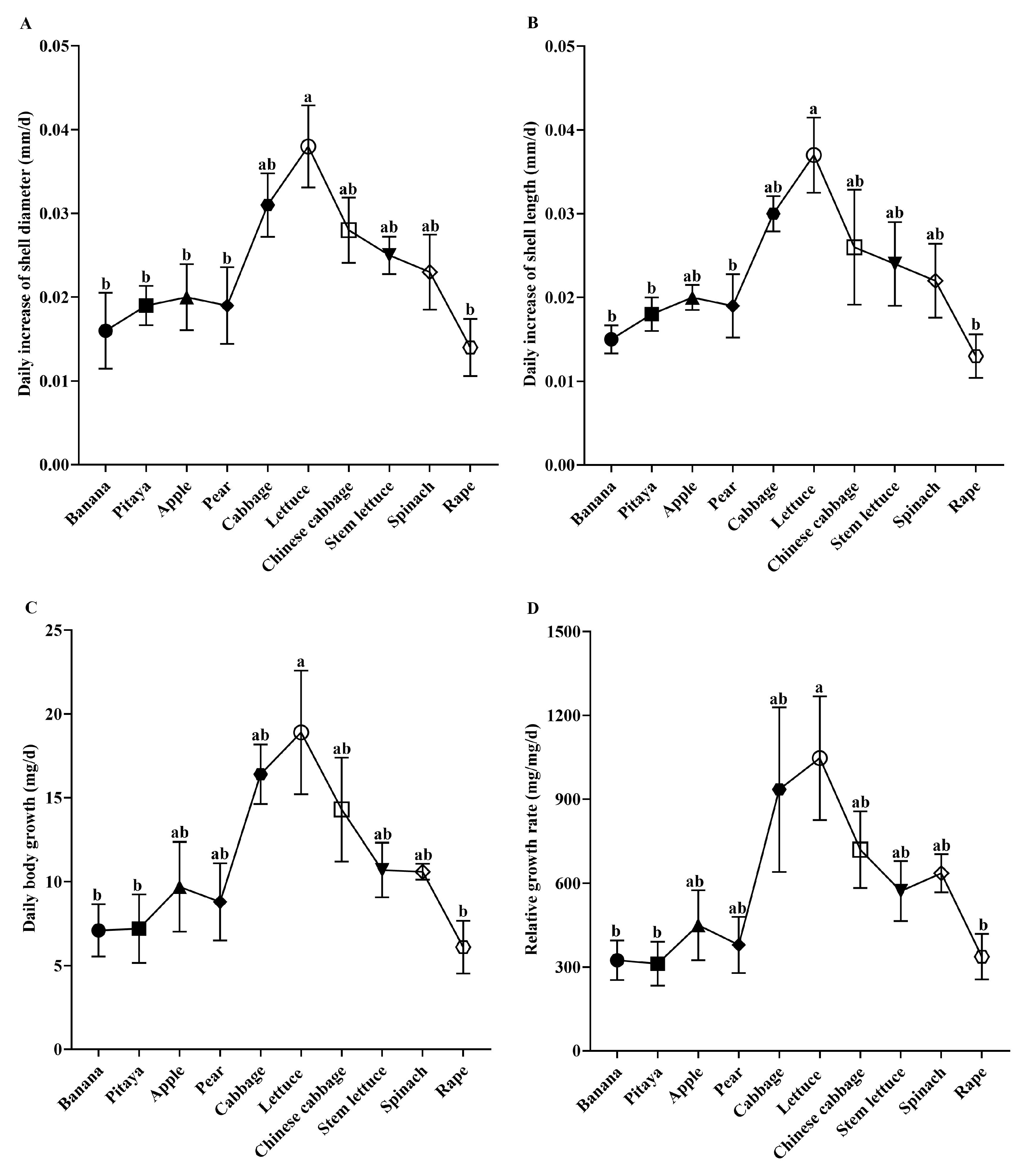
3.2. Feeding and Growth Performance of Snails in No-Choice Experiment
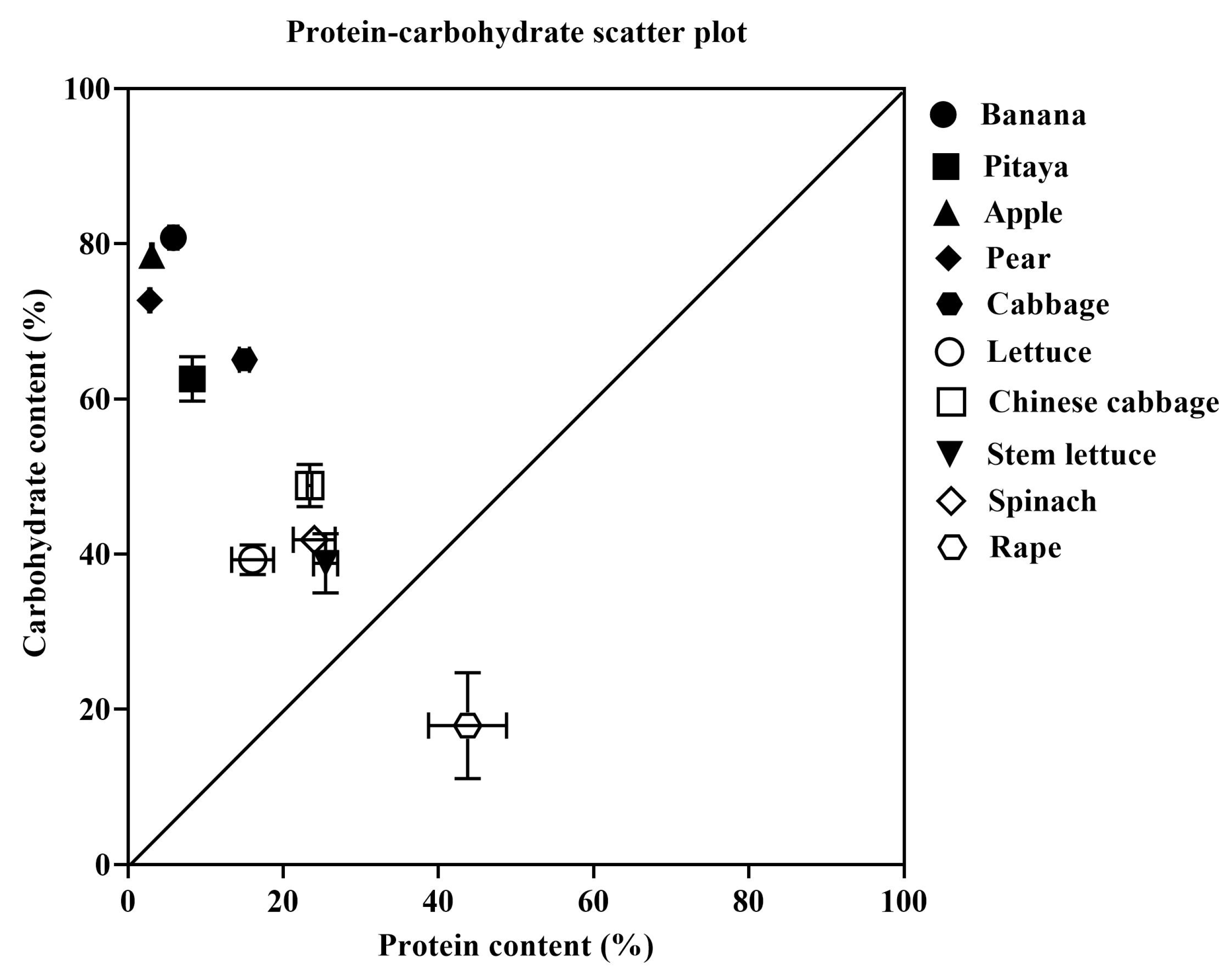
3.3. Protein and Carbohydrate Contents of 10 Plant Species
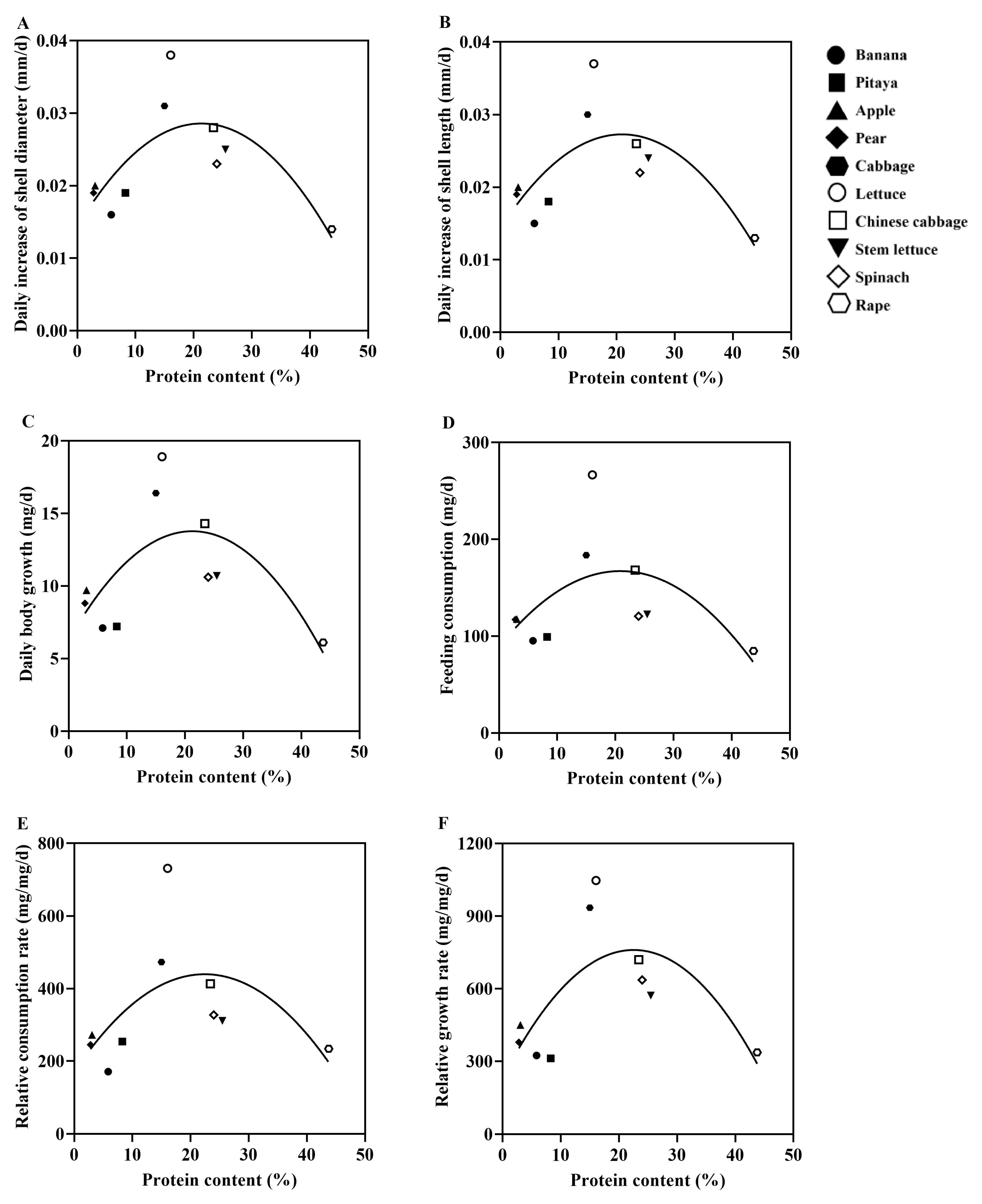
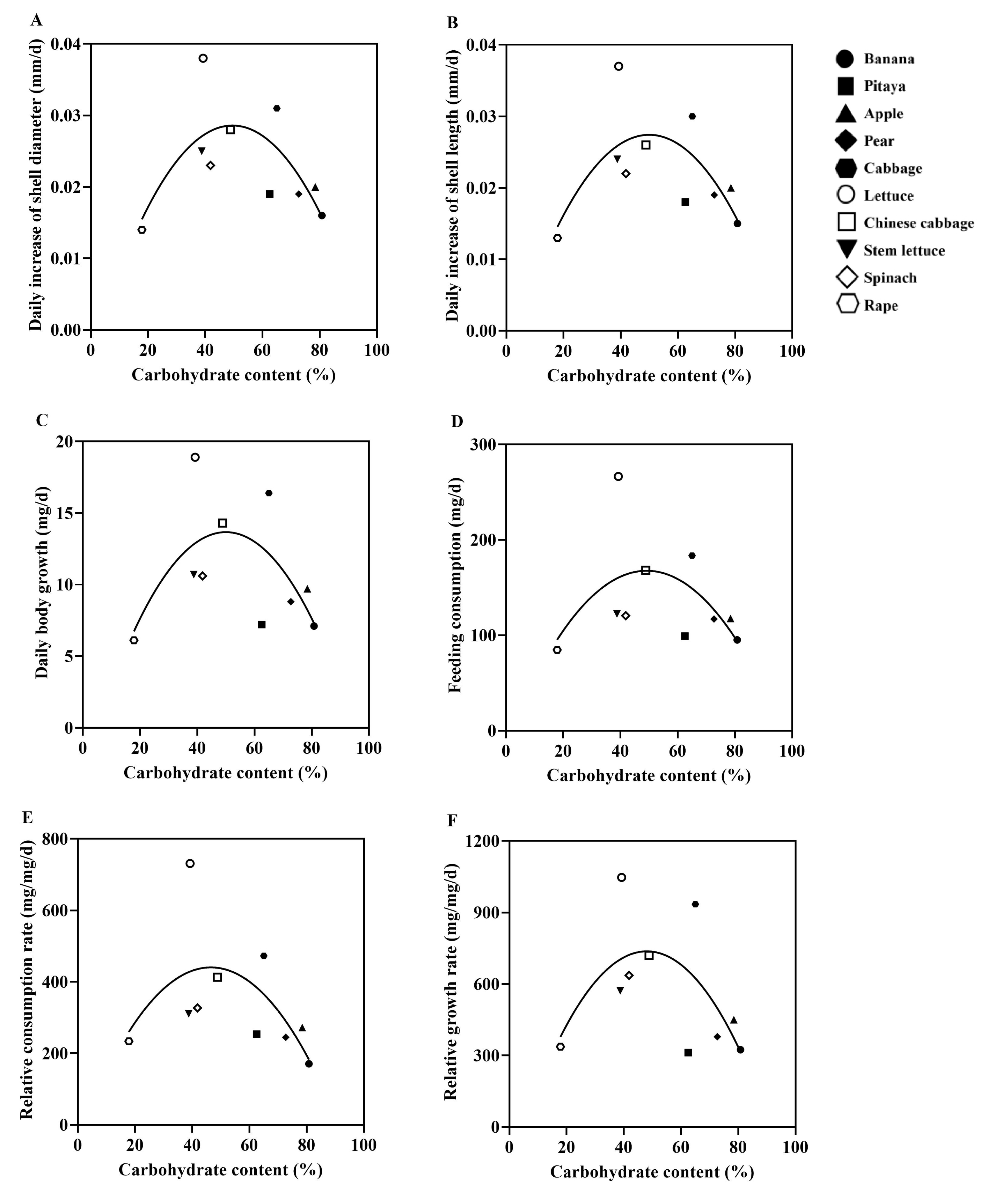
3.4. Correlation Between Protein/Carbohydrate Contents of 10 Plant Species and Growth Performance of the Giant African Snails
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luque, G.M.; Bellard, C.; Bertelsmeier, C.; Bonnaud, E.; Genovesi, P.; Simberloff, D.; Courchamp, F. The 100th of the world’s worst invasive alien species. Biol. Invasions 2014, 16, 981–985. [Google Scholar] [CrossRef]
- Raut, S.K.; Barker, G.M. Achatina fulica Bowdich and other Achatinidae as pests in tropical agriculture. In Molluscs as Crop Pests; CAB International: Wallingford, UK, 2002; pp. 55–114. [Google Scholar] [CrossRef]
- Fontanilla, I.K.C.; Sta Maria, I.M.P.; Garcia, J.R.M.; Ghate, H.; Naggs, F.; Wade, C.M. Restricted genetic variation in populations of Achatina (Lissachatina) fulica outside of East Africa and the Indian Ocean Islands points to the Indian Ocean Islands as the earliest known common source. PLoS ONE 2014, 9, e105151. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.E.; Wu, R.S.; Zhao, B.L.; Yang, H.R. Overview and perspective on the current research status for the giant snail Achatina fulica in China. J. South Agric. 2015, 46, 626–630. [Google Scholar]
- Diagne, C.; Leroy, B.; Vaissière, A.C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.M.; Bradshaw, C.J.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef]
- Barney, J.N.; Tekiela, D.R.; Barrios-Garcia, M.N.; Dimarco, R.D.; Hufbauer, R.A.; Leipzig-Scott, P.; Nuñez, M.A.; Pauchard, A.; Pyšek, P.; Vítková, M.; et al. Global Invader Impact Network (GIIN): Toward standardized evaluation of the ecological impacts of invasive plants. Ecol. Evol. 2015, 5, 2878–2889. [Google Scholar] [CrossRef]
- Colautti, R.I.; Lau, J.A. Contemporary evolution during invasion: Evidence for differentiation natural selection and local adaptation. Mol. Ecol. 2015, 24, 1999–2017. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, J.A.; Bamisile, B.S.; Khan, M.M.; Islam, W.; Hafeez, M.; Bodlah, I.; Xu, Y. Impact of invasive ant species on native fauna across similar habitats under global environmental changes. Environ. Sci. Pollut. Res. 2021, 28, 54362–54382. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; He, P.Y.; Liu, T.X.; Jing, X.F.; Zhang, S.Z. Comparative studies of ovipositional preference, larval feeding selectivity, and nutritional indices of Spodoptera frugiperda (Lepidoptera: Noctuidae) on 6 crops. J. Econ. Entomol. 2023, 116, 790–797. [Google Scholar] [CrossRef]
- Albuquerque, F.D.; Peso-Aguiar, M.C.; Assuncao-Albuquerque, M.J.T. Distribution feeding behavior and control strategies of the exotic land snail Achatina fulica (Gastropoda: Pulmonata) in the northeast of Brazil. Braz. J. Biol. 2008, 68, 837–842. [Google Scholar] [CrossRef]
- Dickens, K.L.; Capinera, J.L.; Smith, T.R. Effects of density and food deprivation on growth, reproduction, and survival of Lissachatina fulica. Am. Malacol. Bull. 2018, 36, 57–61. [Google Scholar] [CrossRef]
- Wu, C.; Li, S.; Zhou, Y.; Hu, X.; Feng, J. High lability of global niche and range in the Giant African snail (Lissachatina fulica): Small niche expansions resulting in large range shifts. Ecol. Indic. 2023, 151, 110328. [Google Scholar] [CrossRef]
- Lee, K.P.; Simpson, S.J.; Clissold, F.J.; Brooks, R.; Ballard, J.W.O.; Taylor, P.W.; Soran, N.; Raubenheimer, D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. USA 2008, 105, 2498–2503. [Google Scholar] [CrossRef]
- Tanga, C.M.; Ekesi, S.; Govender, P.; Mohamed, S.A. Effect of six host plant species on the life history and population growth parameters of Rastrococcus iceryoides (Hemiptera: Pseudococcidae). Fla. Entomol. 2013, 96, 1030–1041. [Google Scholar] [CrossRef]
- Deans, C.; Sword, G.; Behmer, S.T. Nutrition as a neglected factor in insect herbivore susceptibility to Bt toxins. Curr. Opin. Insect Sci. 2016, 15, 97–103. [Google Scholar] [CrossRef]
- Sun, S.L.; Abudisilimu, N.; Yi, H.; Li, S.L.; Liu, X.T.; Jing, X.F. Understanding nutritive need in Harmonia axyridis larvae: Insights from nutritional geometry. Insect Sci. 2022, 29, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Amobi, M.I.; Ezewudo, B.I.; Okpoko, V.O.; Ugokwe, C.U.; Okereke, H.N. Effects of three leafy vegetables on the growth performance of Giant African snail Achatina (Lissachatina) fulica. J. Agric. Rural Dev. Trop. 2019, 120, 15–20. [Google Scholar] [CrossRef]
- Seuffert, M.E.; Martín, P.R. Juvenile growth and survival of the apple snail Pomacea canaliculata (Caenogastropoda: Ampullariidae) reared at different constant temperatures. SpringerPlus 2013, 2, 312. [Google Scholar] [CrossRef] [PubMed]
- Sperfeld, E.; Wagner, N.D.; Halvorson, H.M.; Malishev, M.; Raubenheimer, D. Bridging ecological stoichiometry and nutritional geometry with homeostasis concepts and integrative models of organism nutrition. Funct. Ecol. 2017, 31, 286–296. [Google Scholar] [CrossRef]
- Hosking, C.J.; Raubenheimer, D.; Charleston, M.A.; Simpson, S.J.; Senior, A.M. Macronutrient intakes and the lifespan-fecundity trade-off: A geometric framework agent-based model. J. R. Soc. Interface 2019, 16, 20180733. [Google Scholar] [CrossRef]
- Wibawa, C.; Huang, Y.; Patterson, D.H.; Feng, Z.; Serventi, L. Carbohydrates for energy. In Sustainable Food Innovation; Springer International Publishing: Cham, Switzerland, 2023; pp. 13–28. [Google Scholar] [CrossRef]
- Sun, S.L.; Yang, Z.; Ren, J.C.; Liu, T.X.; Jing, X.F. Fitness of nutrition regulation in a caterpillar pest Mythimna separata (Walker): Insights from the geometric framework. Insects 2023, 14, 937. [Google Scholar] [CrossRef]
- Ramdwar, M.; Ganpat, W.; Harripersad, J.; Isaac, W.; Palmer, D. The preferential feeding habits of Achatina (Lissachatina) fulica (Bowdich) on selected crops grown and weeds found in Trinidad, West Indies. Cogent Food Agric. 2018, 4, 1492360. [Google Scholar] [CrossRef]
- Tchowan, G.M.; Zangho, J.; Kenmogne, M.K.; Ngoula, F.; Tchoumboué, J. Production performance of Giant African Land snails (Archachatina marginata) at the Sudano-Guinean highland zone of Cameroon. Heliyon 2022, 8, e11891. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lei, W.; Wen, L.; Hou, M. Silicon-mediated resistance in a susceptible rice variety to the rice leaf folder, Cnaphalocrocis medinalis Guenée (Lepidoptera: Pyralidae). PLoS ONE 2015, 10, e0120557. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Fu, W.Y.; Zhang, Z.F.; Fan, Y.L.; Liu, T.X. Effects of elevated CO2 concentration and temperature on some physiological characteristics of cotton (Gossypium hirsutum L.) leaves. Environ. Exp. Bot. 2017, 133, 108–117. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Lim, T.J. Effects of dietary protein and energy levels on growth and lipid composition of juvenile snail (Semisulcospira gottschei). J. Shellfish Res. 2005, 24, 99–102. [Google Scholar] [CrossRef]
- Simpson, S.J.; Raubenheimer, D. The nature of nutrition: A unifying framework. Aust. J. Zool. 2012, 59, 350–368. [Google Scholar] [CrossRef]
- Clissold, F.J.; Sanson, G.D.; Read, J.; Simpson, S.J. Gross vs net income: How plant toughness affects performance of an insect herbivore. Ecology 2009, 90, 3393–3405. [Google Scholar] [CrossRef]
- Clements, K.D.; Raubenheimer, D.; Choat, J.H. Nutritional ecology of marine herbivorous fishes: Ten years on. Funct. Ecol. 2009, 23, 79–92. [Google Scholar] [CrossRef]
- Cease, A.J.; Trumper, E.V.; Medina, H.; Bazán, F.C.; Franc, J.; Harrison, J.; Joaquin, N.; Learned, J.; Roca, M.; Rojas, J.E.; et al. Field bands of marching locust juveniles show carbohydrate, not protein, limitation. Curr. Res. Insect Sci. 2023, 4, 100069. [Google Scholar] [CrossRef]
- Đukić, N.; Radonjić, A.; Popović, B.; Kljajić, P.; Pražič-Golić, M.; Andrić, G. The impact of the protein-carbohydrate ratio in animal feed and the initial insect population density on the development of the red flour beetle, Tribolium castaneum. J. Stored Prod. Res. 2022, 97, 101983. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Wang, D.M.; Liu, J.X.; Liu, H.Y.; Wu, Z.G. Effect of dietary phosphorus content on milk production and phosphorus excretion in dairy cows. J. Anim. Sci. Biotechnol. 2014, 5, 23. [Google Scholar] [CrossRef]
- Roeder, K.A.; Behmer, S.T. Lifetime consequences of food protein-carbohydrate content for an insect herbivore. Funct. Ecol. 2014, 28, 1135–1143. [Google Scholar] [CrossRef]
- Heras, H.; Garin, C.F.; Pollero, R.J. Biochemical composition and energy sources during embryo development and in early juveniles of the snail Pomacea canaliculata (Mollusca: Gastropoda). J. Exp. Zool. 1998, 280, 375–383. [Google Scholar] [CrossRef]
- Rodríguez, M.; Álvarez, B.; Loy, I. Hunger and satiety determine foraging decisions in land snails: Evidence from the invasive species Theba pisana. Behav. Processes 2019, 164, 230–236. [Google Scholar] [CrossRef]
- Cui, Z.W.; Wang, Z.L.; Shao, Q.; Raubenheimer, D.; Lu, J.Q. Macronutrient signature of dietary generalism in an ecologically diverse primate in the wild. Behav. Ecol. 2018, 29, 804–813. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Xie, S.W.; Guo, T.Y.; Liu, Z.L.; Fang, H.H.; Zheng, L.; Xie, J.J.; Tian, L.X.; Liu, Y.J.; Jin, N. High dietary starch inclusion impairs growth and antioxidant status, and alters liver organization and intestinal microbiota in largemouth bass (Micropterus salmoides). Aquacult. Nutr. 2020, 26, 1806–1821. [Google Scholar] [CrossRef]
- Jimoh, O.A.; Akinola, M.O.; Ayedun, E.S.; Oloruntola, O.D.; Daramola, O.T.; Ayodele, S.O.; Omoniyi, I.S. Effects of different protein sources on growth and carcass characteristics of African giant land snail (Archachatina marginata) in captivity. Anim. Res. Int. 2020, 17, 3682–3690. [Google Scholar]
- Oyeagu, C.E.; Udeh, F.U.; Uzochukwu, I.E.; Osita, C.O.; Ugwu, S.O.; Agugom, O.H. Effect of dietary Centrosema pubescens leaf meal on growth and reproductive traits of Archachatina marginata snails. J. Appl. Anim. Res. 2018, 46, 947–952. [Google Scholar] [CrossRef]
- Obakanurhe, O.; Irabor, A.E.; Okpara, O.; Jn Pierre, H.A. Feeding snails (Archachatina marginata) with leaves: Growth performance, carcass characteristics, nutrient digestibility, proximate composition and minerals contents of snail meat. Trop. Anim. Health Prod. 2024, 56, 270. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.T.T. Effects of Calcium Levels in Artificial Pellet Feed on the Growth and Survival Rate of Black Apple Snails (Pila polita). Vietnam J. Agric. Sci. 2019, 2, 387–396. [Google Scholar]
- Deans, C.A.; Sword, G.A.; Lenhart, P.A.; Burkness, E.; Hutchison, W.D.; Behmer, S.T. Quantifying plant soluble protein and digestible carbohydrate content, using corn (Zea mays) as an exemplar. JoVE-J. Vis. Exp. 2018, 138, 58164. [Google Scholar] [CrossRef]
- Deans, C.A.; Sword, G.A.; Vogel, H.; Behmer, S.T. Quantity versus quality: Effects of diet protein-carbohydrate ratios and amounts on insect herbivore gene expression. Insect Biochem. Mol. Biol. 2022, 145, 103773. [Google Scholar] [CrossRef] [PubMed]
- Waldbauer, G.P.; Friedman, S. Self-selection of optimal diets by insects. Annu. Rev. Entomol. 1991, 36, 43–63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, K.; Zheng, Z.; Lu, X.; Han, Z.; Sun, S. Effects of Macronutrients in Ten Different Plant Species on the Food Choice and Growth Performance of Achatina fulica. Animals 2025, 15, 3333. https://doi.org/10.3390/ani15223333
Tang K, Zheng Z, Lu X, Han Z, Sun S. Effects of Macronutrients in Ten Different Plant Species on the Food Choice and Growth Performance of Achatina fulica. Animals. 2025; 15(22):3333. https://doi.org/10.3390/ani15223333
Chicago/Turabian StyleTang, Kelin, Zuohang Zheng, Xi Lu, Zhiqiang Han, and Shaolei Sun. 2025. "Effects of Macronutrients in Ten Different Plant Species on the Food Choice and Growth Performance of Achatina fulica" Animals 15, no. 22: 3333. https://doi.org/10.3390/ani15223333
APA StyleTang, K., Zheng, Z., Lu, X., Han, Z., & Sun, S. (2025). Effects of Macronutrients in Ten Different Plant Species on the Food Choice and Growth Performance of Achatina fulica. Animals, 15(22), 3333. https://doi.org/10.3390/ani15223333




