Lactobacillus plantarum 17-5 Alleviates Escherichia coli Mastitis by Inhibiting the cGAS-STING Pathway
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animal Management and Group Assignment
2.2. Bacteria and the Culture Conditions
2.3. Cell Culture and Treatment
2.4. Colorimetric and ELISA Assays
2.5. Microbial DNA Extraction and 16S rRNA Sequencing
2.6. Cell Counting Kit-8 Assay
2.7. Western Blot
2.8. Real-Time Quantitative PCR1
2.9. Annexin V-FITC/PI Staining
2.10. Immunofluorescence
2.11. Hematoxylin and Eosin Staining
2.12. Statistical Analysis
3. Results
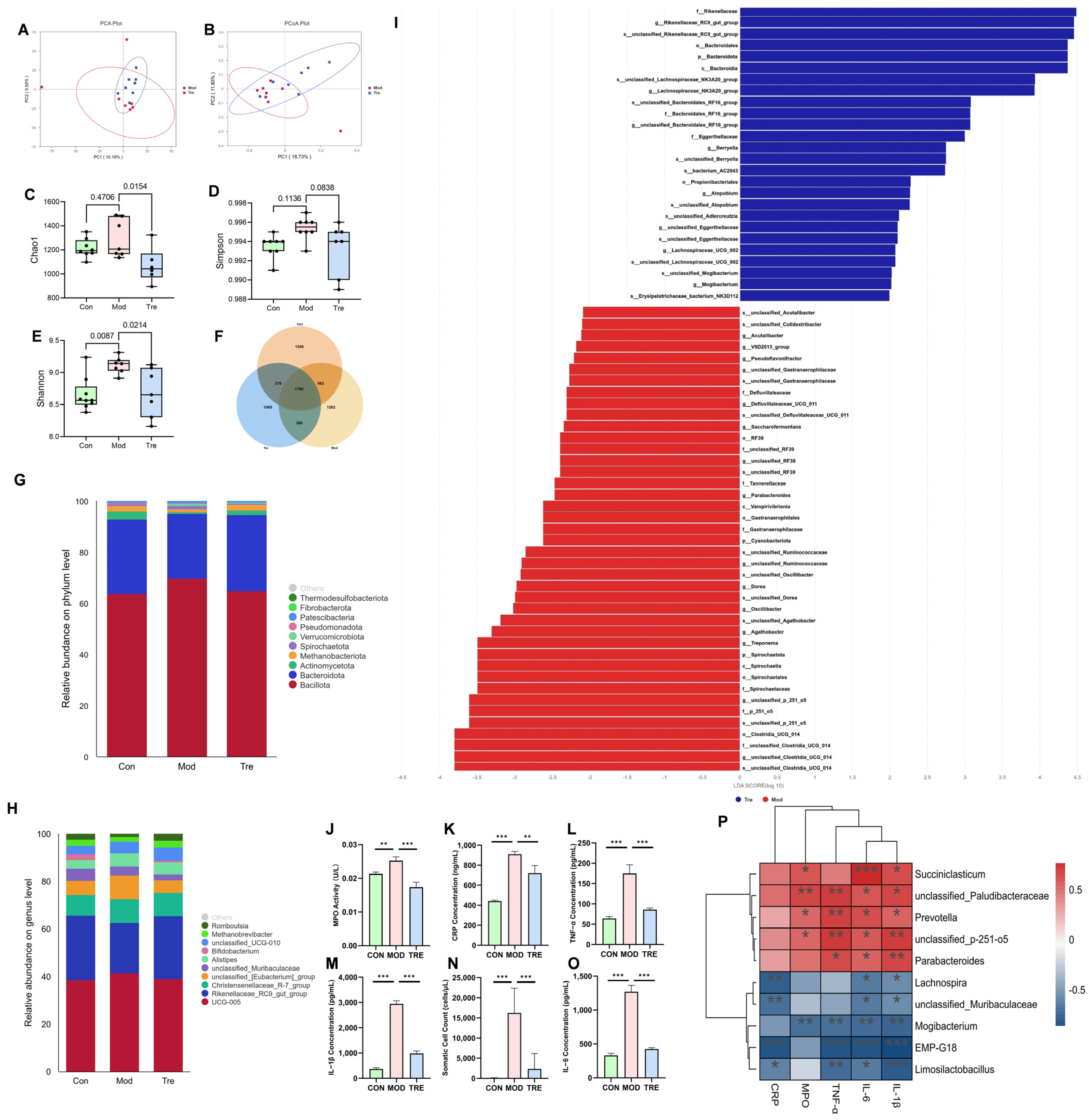
3.1. LP Significantly Alleviates Mastitis and Modulates the Gut Microbiota in Dairy Cows
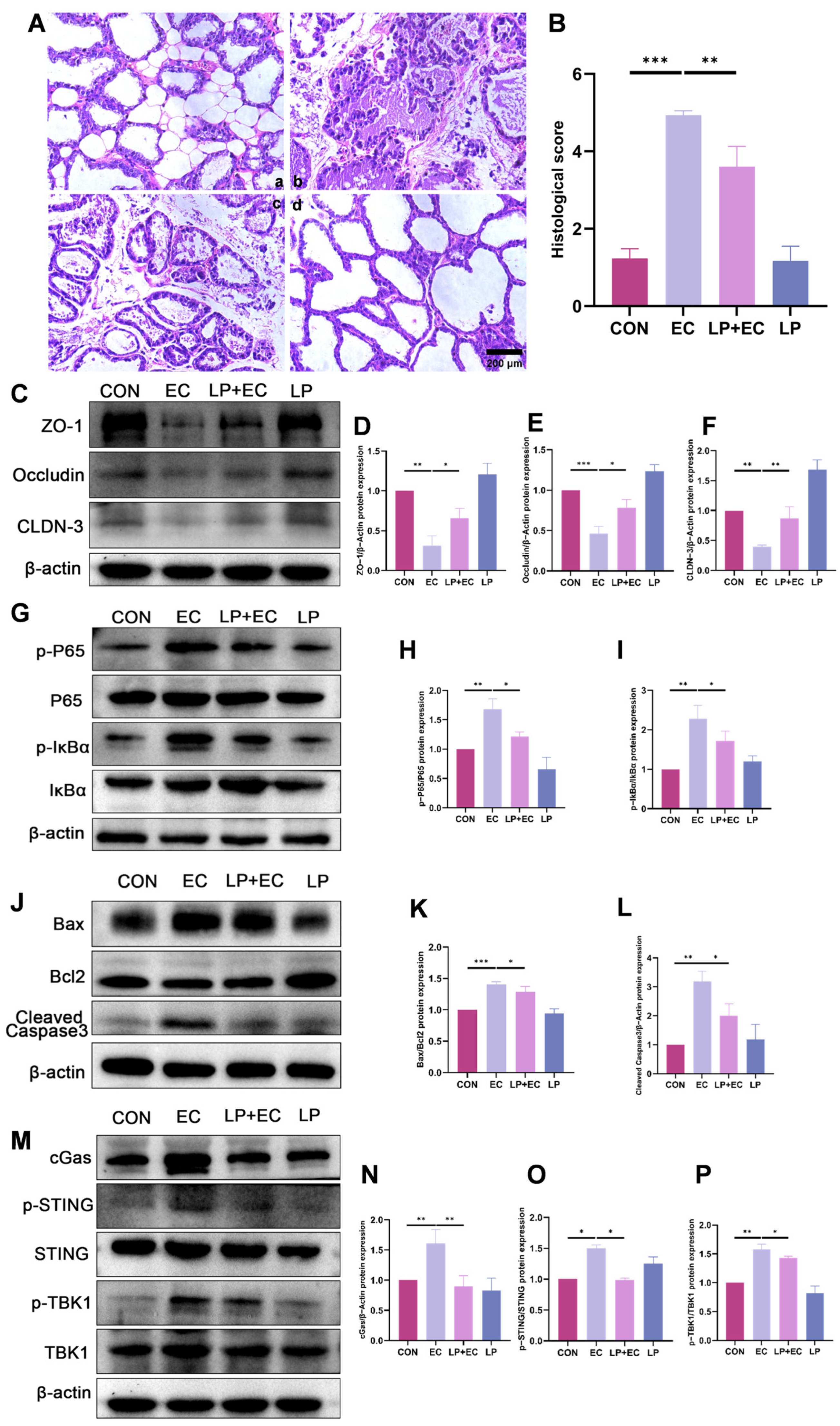
3.2. LP Significantly Alleviated Inflammatory Injury in the Mammary Gland of Mice
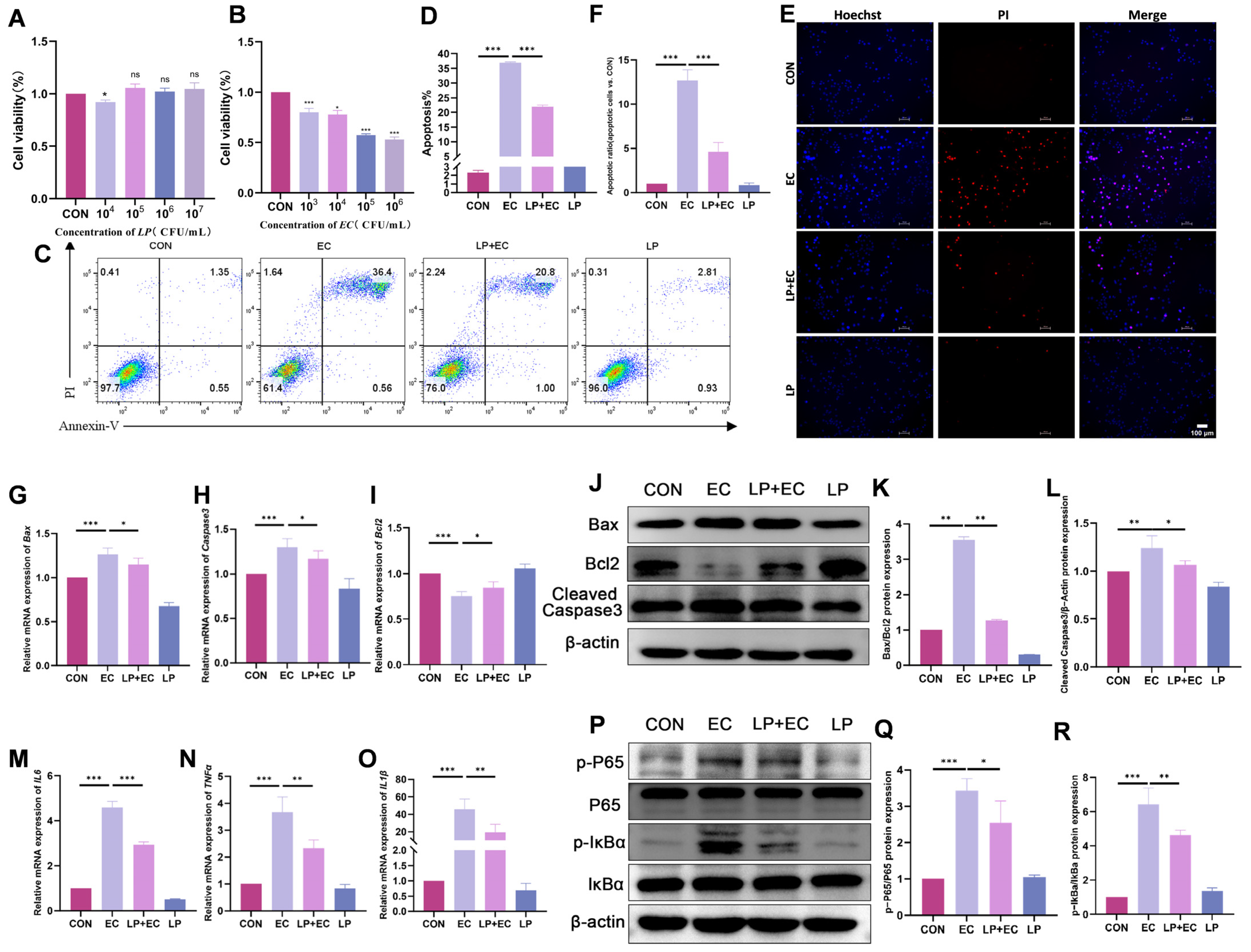
3.3. LP Alleviates E. coli-Induced Apoptosis and Inflammatory Response in BMECs
3.4. LP Mitigates E. coli-Induced Damage to Tight Junctions
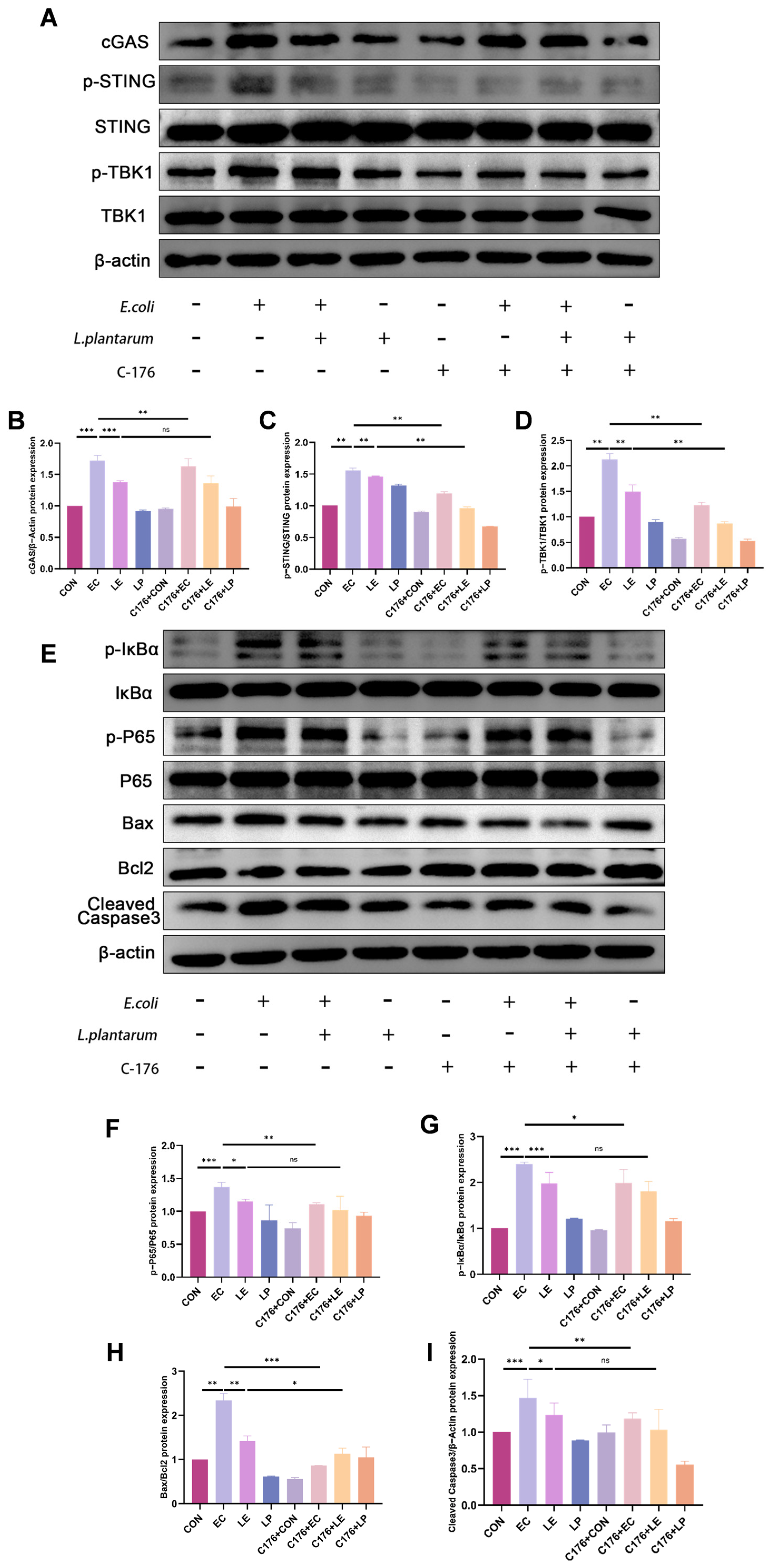
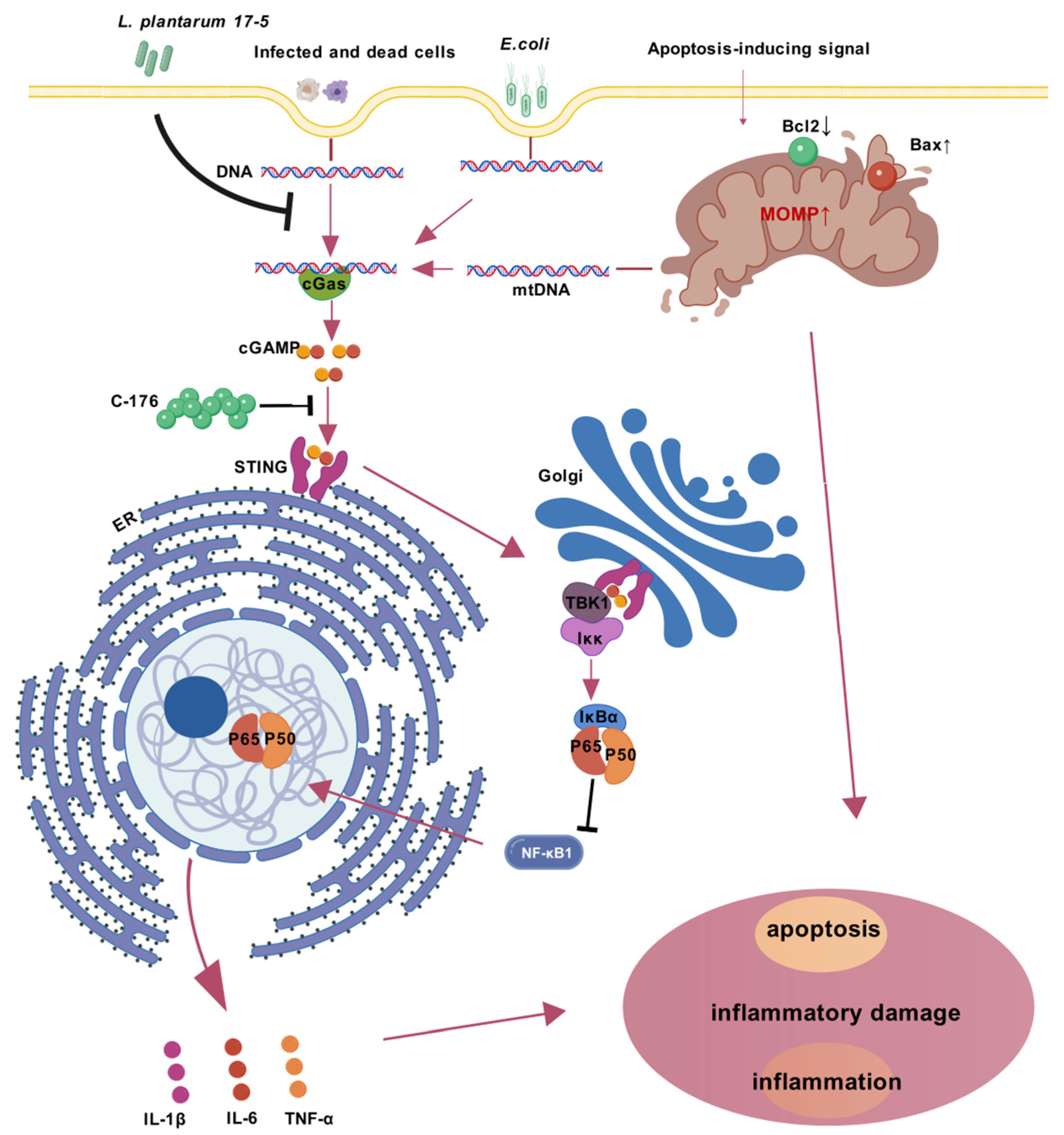
3.5. LP Exerts a Protective Effect by Inhibiting the cGAS-STING Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sinha, M.K.; Thombare, N.N.; Mondal, B. Subclinical mastitis in dairy animals: Incidence, economics, and predisposing factors. Sci. World J. 2014, 2014, 523984. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.A.; Cullimore, C.; Hutchison, W.D.; Grimsrud, A.; Nobrega, D.; De Buck, J.; Barkema, H.W.; Wilson, E.; Pickett, B.E.; Erickson, D.L. Genes associated with fitness and disease severity in the pan-genome of mastitis-associated Escherichia coli. Front. Microbiol. 2024, 15, 1452007. [Google Scholar] [CrossRef]
- Kumar, P.; Yang, Z.; Fatima, H.; Mitchell, T. Hydroxyproline increases inflammation and Uropathogenic E. coli (UPEC) infection in female rats. Sci. Rep. 2024, 14, 22237. [Google Scholar] [CrossRef]
- Vangroenweghe, F.; Duchateau, L.; Burvenich, C. Short communication: J-5 Escherichia coli vaccination does not influence severity of an Escherichia coli intramammary challenge in primiparous cows. J. Dairy Sci. 2020, 103, 6692–6697. [Google Scholar] [CrossRef]
- Croxen, M.A.; Finlay, B.B. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 2010, 8, 26–38. [Google Scholar] [CrossRef]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Iqbal Yatoo, M.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in therapeutic and managemental approaches of bovine mastitis: A comprehensive review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef]
- Hodzhev, V.; Dzhambazov, K.; Sapundziev, N.; Encheva, M.; Todorov, S.; Youroukova, V.; Benchev, R.; Nikolov, R.; Bogov, B.; Momekov, G.; et al. High-dose Probiotic Mix of Lactobacillus spp., Bifidobacterium spp., Bacillus coagulans, and Saccharomyces boulardii to Prevent Antibiotic-associated Diarrhea in Adults: A Multicenter, Randomized, Double-blind, Placebo-controlled Trial (SPAADA). Open Forum Infect. Dis. 2024, 11, ofae615. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, D.; Ding, H.; Li, Q.; Yuan, S.; Li, H.; Guan, W.; Zhang, S. Lactobacillus amylovorus extracellular vesicles mitigate mammary gland ferroptosis via the gut–mammary gland axis. NPJ Biofilms Microbiomes 2025, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ran, X.; Han, J.; Ding, H.; Wang, X.; Li, Y.; Guo, W.; Li, X.; Guo, W.; Fu, S.; et al. Astragalus polysaccharide alleviates mastitis disrupted by Staphylococcus aureus infection by regulating gut microbiota and SCFAs metabolism. Int. J. Biol. Macromol. 2025, 286, 138422. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Bangar, S.P.; Echegaray, N.; Suri, S.; Tomasevic, I.; Manuel Lorenzo, J.; Melekoglu, E.; Rocha, J.M.; Ozogul, F. The Impacts of Lactiplantibacillus plantarum on the Functional Properties of Fermented Foods: A Review of Current Knowledge. Microorganisms 2022, 10, 826. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, Q.; de Haan, B.J.; Zhang, H.; Faas, M.M.; de Vos, P. Identification of TLR2/TLR6 signalling lactic acid bacteria for supporting immune regulation. Sci. Rep. 2016, 6, 34561. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Q.; Ren, K.; Wu, P.; Wang, Y.; Lv, C. ALDH2 mitigates LPS-induced cardiac dysfunction, inflammation, and apoptosis through the cGAS/STING pathway. Mol. Med. 2023, 29, 171. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Huang, Y.Y.; Tsai, S.Y.; Kuo, Y.W.; Lin, J.H.; Ho, H.H.; Chen, J.F.; Hsia, K.C.; Sun, Y. Efficacy of Probiotic Supplements on Brain-Derived Neurotrophic Factor, Inflammatory Biomarkers, Oxidative Stress and Cognitive Function in Patients with Alzheimer’s Dementia: A 12-Week Randomized, Double-Blind Active-Controlled Study. Nutrients 2023, 16, 16. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Wu, W.; Zhang, H.; Yang, Y.; Usman, M.; Aernouts, B.; Loor, J.J.; Xu, C. Inflammatory Signaling via PEIZO1 Engages and Enhances the LPS-Mediated Apoptosis during Clinical Mastitis. J. Agric. Food Chem. 2024, 72, 20321–20330. [Google Scholar] [CrossRef]
- Qiu, M.; Ye, C.; Zhao, X.; Zou, C.; Tang, R.; Xie, J.; Liu, Y.; Hu, Y.; Hu, X.; Zhang, N.; et al. Succinate exacerbates mastitis in mice via extracellular vesicles derived from the gut microbiota: A potential new mechanism for mastitis. J. Nanobiotechnology 2024, 22, 712. [Google Scholar] [CrossRef]
- Zhao, C.; Hu, X.; Qiu, M.; Bao, L.; Wu, K.; Meng, X.; Zhao, Y.; Feng, L.; Duan, S.; He, Y.; et al. Sialic acid exacerbates gut dysbiosis-associated mastitis through the microbiota-gut–mammary axis by fueling gut microbiota disruption. Microbiome 2023, 11, 78. [Google Scholar] [CrossRef]
- Derakhshani, H.; Fehr, K.B.; Sepehri, S.; Francoz, D.; De Buck, J.; Barkema, H.W.; Plaizier, J.C.; Khafipour, E. Invited review: Microbiota of the bovine udder: Contributing factors and potential implications for udder health and mastitis susceptibility. J. Dairy Sci. 2018, 101, 10605–10625. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nan, X.; Zhao, Y.; Jiang, L.; Wang, M.; Wang, H.; Zhang, F.; Xue, F.; Hua, D.; Liu, J.; et al. Rumen microbiome structure and metabolites activity in dairy cows with clinical and subclinical mastitis. J. Anim. Sci. Biotechnol. 2021, 12, 36. [Google Scholar] [CrossRef]
- Zhong, Y.; Xue, M.; Liu, J. Composition of Rumen Bacterial Community in Dairy Cows With Different Levels of Somatic Cell Counts. Front. Microbiol. 2018, 9, 3217. [Google Scholar] [CrossRef] [PubMed]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, C.; Zhang, J.; Xu, C.; Ma, L.; Chang, Y.; Hussain, M.A.; Ma, J.; Hou, J.; Jiang, Z. Lactobacillus plantarum 69-2 combined with α-lactalbumin hydrolysate alleviates DSS-induced ulcerative colitis through the TLR4/NF-κB inflammatory pathway and the gut microbiota in mice. Food Funct. 2024, 15, 10987–11004. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Fang, J. Progress on the mechanisms of Lactobacillus plantarum to improve intestinal barrier function in ulcerative colitis. J. Nutr. Biochem. 2024, 124, 109505. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Han, Z.; Meng, M.; Shi, X.; Wang, L.; Chen, M.; Chang, G.; Shen, X. iE-DAP Induced Inflammatory Response and Tight Junction Disruption in Bovine Mammary Epithelial Cells via NOD1-Dependent NF-κB and MLCK Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 6263. [Google Scholar] [CrossRef] [PubMed]
- Wellnitz, O.; Bruckmaier, R.M. Invited review: The role of the blood-milk barrier and its manipulation for the efficacy of the mammary immune response and milk production. J. Dairy Sci. 2021, 104, 6376–6388. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Fang, Z.; Duan, H.; Dong, W.; Xiao, L. Ginsenoside Rg1 Alleviates Blood-Milk Barrier Disruption in Subclinical Bovine Mastitis by Regulating Oxidative Stress-Induced Excessive Autophagy. Antioxidants 2024, 13, 1446. [Google Scholar] [CrossRef]
- Wellnitz, O.; Zbinden, C.; Huang, X.; Bruckmaier, R.M. Short communication: Differential loss of bovine mammary epithelial barrier integrity in response to lipopolysaccharide and lipoteichoic acid. J. Dairy Sci. 2016, 99, 4851–4856. [Google Scholar] [CrossRef]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular permeability and tight junction regulation in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 417–432. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Zhao, B.; Chang, R.; Wu, W.; Zhang, H.; Usman, M.; Loor, J.J.; Xu, C. A Disintegrin and Metalloproteinase 17 Disrupts Bovine Macrophage MER Proto-Oncogene Tyrosine Kinase Integrity to Impede Apoptotic Cell Clearance and Promote Inflammation in Clinical Mastitis. J. Agric. Food Chem. 2025, 73, 549–561. [Google Scholar] [CrossRef]
- Ou, Q.; Huang, W.; Wang, B.; Niu, L.; Li, Z.; Mao, X.; Shi, S. Apoptotic Vesicles: Therapeutic Mechanisms and Critical Issues. J. Dent. Res. 2024, 103, 1057–1065. [Google Scholar] [CrossRef]
- Miyagishima, K.J.; Nadal-Nicolás, F.M.; Ma, W.; Li, W. Annexin-V binds subpopulation of immune cells altering its interpretation as an in vivo biomarker for apoptosis in the retina. Int. J. Biol. Sci. 2024, 20, 6073–6089. [Google Scholar] [CrossRef]
- Kumar, R.; Saneja, A.; Panda, A.K. An Annexin V-FITC-Propidium Iodide-Based Method for Detecting Apoptosis in a Non-Small Cell Lung Cancer Cell Line. Methods Mol. Biol. 2021, 2279, 213–223. [Google Scholar] [CrossRef]
- Crowley, L.C.; Marfell, B.J.; Scott, A.P.; Waterhouse, N.J. Quantitation of Apoptosis and Necrosis by Annexin V Binding, Propidium Iodide Uptake, and Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, A.; Shu, X.; Huang, W.; Zhang, R.; Xu, Y.; Yang, C. Lactobacillus plantarum postbiotics trigger AMPK-dependent autophagy to suppress Salmonella intracellular infection and NLRP3 inflammasome activation. J. Cell. Physiol. 2023, 238, 1336–1353. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Rui, Y.; Wang, Y.; Su, J.; Yu, X.F. SARS-CoV-2, HIV, and HPV: Convergent evolution of selective regulation of cGAS-STING signaling. J. Med. Virol. 2023, 95, e28220. [Google Scholar] [CrossRef] [PubMed]
- Hopfner, K.P.; Hornung, V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat. Rev. Mol. Cell Biol. 2020, 21, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.T.H.; Abdul Rasid, S.Z.; Wan Ismail, W.K.; Tobechan, J.; Tan, E.T.Y.; Yusof, A.N.; Low, J.H. Willingness to Pay for National Health Insurance: A Contingent Valuation Study Among Patients Visiting Public Hospitals in Melaka, Malaysia. Appl. Health Econ. Health Policy 2022, 20, 255–267. [Google Scholar] [CrossRef]
- Riley, J.S.; Quarato, G.; Cloix, C.; Lopez, J.; O’Prey, J.; Pearson, M.; Chapman, J.; Sesaki, H.; Carlin, L.M.; Passos, J.F.; et al. Mitochondrial inner membrane permeabilisation enables mtDNA release during apoptosis. EMBO J. 2018, 37, e99238. [Google Scholar] [CrossRef]
- Cosentino, K.; García-Sáez, A.J. Bax and Bak Pores: Are We Closing the Circle? Trends Cell Biol. 2017, 27, 266–275. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Marques, J.T.; Yamashita, M.; Peters, K.L.; Smith, K.; Desai, A.; Williams, B.R.; Sen, G.C. Viral apoptosis is induced by IRF-3-mediated activation of Bax. EMBO J. 2010, 29, 1762–1773. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.-Z.; Li, M.-M.; Yu, X.-W.; Zhang, R.-N.; Zou, Q.; Deng, J.-C.; Zhao, F.-J.; Li, H.-Q.; Li, K.; Yan, Z.-G. Lactobacillus plantarum 17-5 Alleviates Escherichia coli Mastitis by Inhibiting the cGAS-STING Pathway. Animals 2025, 15, 3305. https://doi.org/10.3390/ani15223305
Han J-Z, Li M-M, Yu X-W, Zhang R-N, Zou Q, Deng J-C, Zhao F-J, Li H-Q, Li K, Yan Z-G. Lactobacillus plantarum 17-5 Alleviates Escherichia coli Mastitis by Inhibiting the cGAS-STING Pathway. Animals. 2025; 15(22):3305. https://doi.org/10.3390/ani15223305
Chicago/Turabian StyleHan, Jia-Ze, Meng-Meng Li, Xiao-Wen Yu, Rui-Ning Zhang, Qian Zou, Jun-Chi Deng, Fa-Jian Zhao, Han-Qing Li, Ke Li, and Zhen-Gui Yan. 2025. "Lactobacillus plantarum 17-5 Alleviates Escherichia coli Mastitis by Inhibiting the cGAS-STING Pathway" Animals 15, no. 22: 3305. https://doi.org/10.3390/ani15223305
APA StyleHan, J.-Z., Li, M.-M., Yu, X.-W., Zhang, R.-N., Zou, Q., Deng, J.-C., Zhao, F.-J., Li, H.-Q., Li, K., & Yan, Z.-G. (2025). Lactobacillus plantarum 17-5 Alleviates Escherichia coli Mastitis by Inhibiting the cGAS-STING Pathway. Animals, 15(22), 3305. https://doi.org/10.3390/ani15223305





