Detection and Genetic Characterization of Enterocytozoon hepatopenaei in Giant Freshwater Prawn (Macrobrachium rosenbergii) Imported into South Korea
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction and EHP Monitoring
2.3. Sequence Analysis of SSU rRNA, SWP 1, and Internal Transcribed Spacer-1 Regions of EHP
2.4. Phylogenetic Tree Analysis
3. Results
3.1. EHP Monitoring in Imported M. rosenbergii
3.2. Sequence Analysis of SSU rRNA, SWP 1, and ITS-1 Regions of EHP
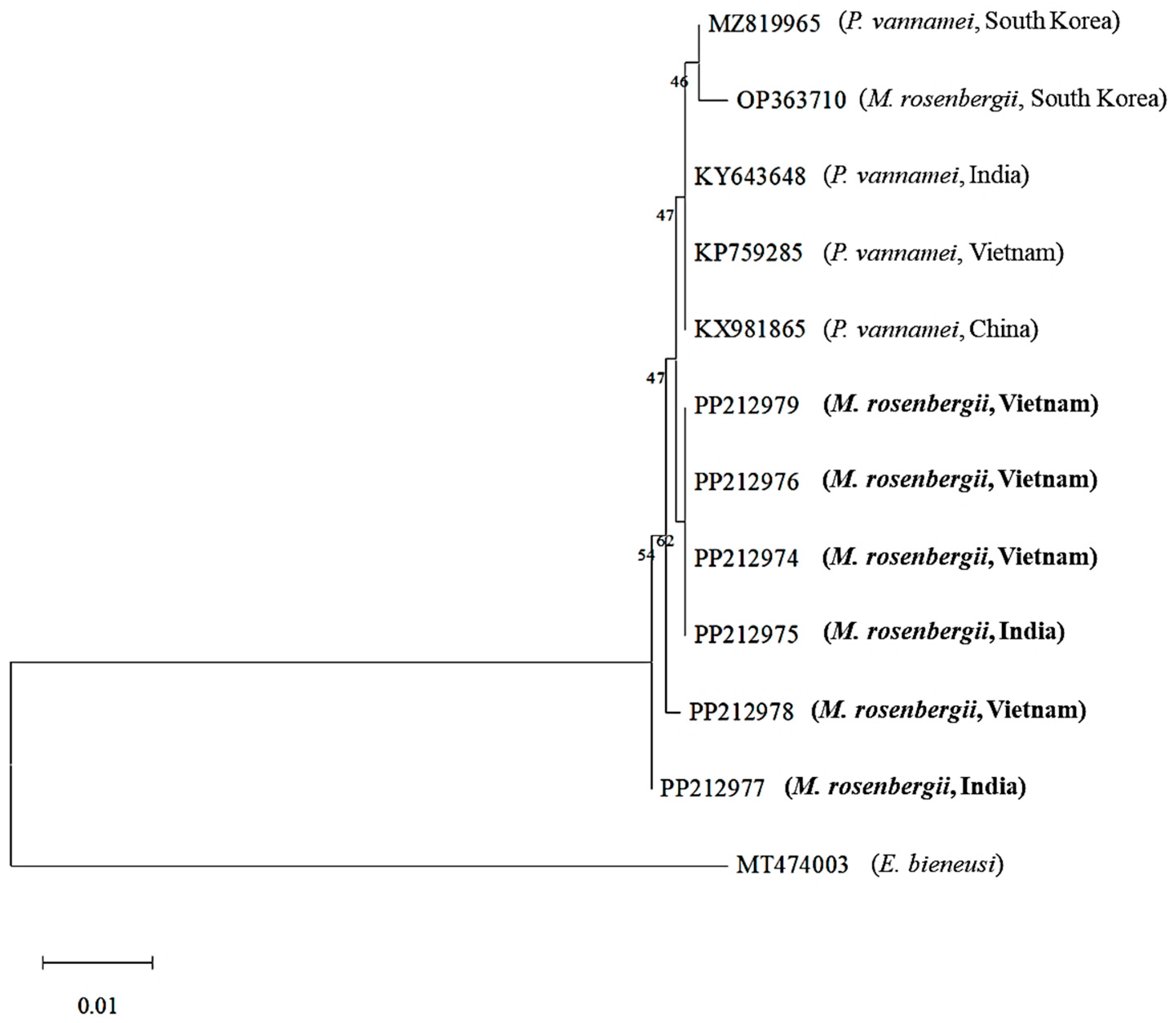
3.3. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Texier, C.; Vidau, C.; Viguès, B.; El Alaoui, H.; Delbac, F. Microsporidia: A model for minimal parasite–host interactions. Curr. Opin. Microbiol. 2010, 13, 443–449. [Google Scholar] [CrossRef]
- Dunn, A.M.; Smith, J.E. Microsporidian life cycles and diversity: The relationship between virulence and transmission. Microbes Infect. 2001, 3, 381–388. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, J.; Lv, Q.; Long, M.; Pan, G.; Zhou, Z. Germination of microsporidian spores: The known and unknown. J. Fungi 2023, 9, 774. [Google Scholar] [CrossRef]
- Stentiford, G.D.; Dunn, A.M. Microsporidia in aquatic invertebrates. In Microsporidia: Pathogens of Opportunity, 1st ed.; Louis, M.W., James, J.B., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2014; Chapter 23; pp. 579–604. [Google Scholar] [CrossRef]
- Geetha, R.; Avunje, S.; Solanki, H.G.; Priyadharshini, R.; Vinoth, S.; Anand, P.R.; Ravisankar, T.; Patil, P.K. Farm-level economic cost of Enterocytozoon hepatopenaei (EHP) to Indian Penaeus vannamei shrimp farming. Aquaculture 2022, 548, 737685. [Google Scholar] [CrossRef]
- Newman, S.G. Microsporidian Impacts Shrimp Production—Industry Efforts Address Control, Not Eradication; Global Aquaculture Advocate; The Global Seafood Alliance: Portsmouth, NH, USA, 2015; pp. 16–17, (March/April). [Google Scholar]
- Shen, H.; Fan, X.; Qiao, Y.; Jiang, G.; Wan, X.; Cheng, J.; Li, H.; Dou, Y.; Li, H.; Wang, L.; et al. The links among Enterocytozoon hepatopenaei infection, growth retardation and intestinal microbiota in different sized shrimp Penaeus vannamei. Aquac. Rep. 2021, 21, 100888. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Yin, M.; Ying, N.; Xiang, Y.; Liu, W.; Ye, J.; Li, X.; Fang, W.; Tan, H. A modification of nested PCR method for detection of Enterocytozoon hepatopenaei (EHP) in giant freshwater prawn Macrobrachium rosenbergii. Front. Cell Infect. Microbiol. 2022, 12, 1013016. [Google Scholar] [CrossRef]
- Chayaburakul, K.; Nash, G.; Pratanpipat, P.; Sriurairatana, S.; Withyachumnarnkul, B. Multiple pathogens found in growth-retarded black tiger shrimp Penaeus monodon cultivated in Thailand. Dis. Aquat. Organ. 2004, 60, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Aranguren, L.F.; Han, J.E.; Tang, K.F. Enterocytozoon hepatopenaei (EHP) is a risk factor for acute hepatopancreatic necrosis disease (AHPND) and septic hepatopancreatic necrosis (SHPN) in the Pacific white shrimp Penaeus vannamei. Aquaculture 2017, 471, 37–42. [Google Scholar] [CrossRef]
- Tangprasittipap, A.; Srisala, J.; Chouwdee, S.; Somboon, M.; Chuchird, N.; Limsuwan, C.; Srisuvan, T.; Flegel, T.W.; Sritunyalucksana, K. The microsporidian Enterocytozoon hepatopenaei is not the cause of white feces syndrome in whiteleg shrimp Penaeus (Litopenaeus) vannamei. BMC Vet. Res. 2013, 9, 139. [Google Scholar] [CrossRef]
- Tang, K.F.; Pantoja, C.R.; Redman, R.M.; Han, J.E.; Tran, L.H.; Lightner, D.V. Development of in situ hybridization and PCR assays for the detection of Enterocytozoon hepatopenaei (EHP), a microsporidian parasite infecting penaeid shrimp. J. Invertebr. Pathol. 2015, 130, 37–41. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Na, Y.; Li, X.C.; Zhou, J.F.; Fang, W.H.; Tan, H.X. Ecytonucleospora hepatopenaei n. gen. et comb. (Microsporidia: Enterocytozoonidae): A redescription of the Enterocytozoon hepatopenaei (Tourtip et al., 2009), a microsporidian infecting the widely cultivated shrimp Penaeus vannamei. J. Invertebr. Pathol. 2023, 201, 107988. [Google Scholar] [CrossRef]
- Jang, G.I.; Kim, S.M.; Oh, Y.K.; Lee, S.J.; Hong, S.Y.; Lee, H.E.; Kwon, M.G.; Kim, B.S. First Report of Enterocytozoon hepatopenaei Infection in Giant Freshwater Prawn (Macrobrachium rosenbergii de Man) Cultured in the Republic of Korea. Animals 2022, 12, 3149. [Google Scholar] [CrossRef]
- New, M.B. History and Global Status of Freshwater Prawn Farming. In Freshwater Prawn Farming: The Farming of Macrobrachium rosenbergii.; New, M.B., Valenti, W.C., Eds.; Blackwell Science Ltd.: Oxford, UK, 2000; pp. 1–11. [Google Scholar]
- New, M.B.; Nair, C.M. Global scale of freshwater prawn farming. Aquac. Res. 2012, 43, 960–969. [Google Scholar] [CrossRef]
- Pillai, B.R.; Ponzoni, R.W.; Das Mahapatra, K.; Panda, D. Genetic improvement of giant freshwater prawn Macrobrachium rosenbergii: A review of global status. Rev. Aquac. 2022, 14, 1285–1299. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Global Production by Production Source Quantity (1950–2020). 2022. Available online: https://www.fao.org/fishery/statistics-query/en/global_production/global_production_quantity (accessed on 12 November 2022).
- National Fishery Products Quality Management Service (NFQS). 2024. Available online: https://www.nfqs.go.kr/hpmg/data/actionExportQuarantineStatisticsForm.do?menuId=M0000225 (accessed on 29 February 2024).
- Jaroenlak, P.; Sanguanrut, P.; Williams, B.A.P.; Stentiford, G.D.; Flegel, T.W.; Sritunyalucksana, K.; Itsathitphaisarn, O. A nested PCR assay to avoid false positive detection of the microsporidian Enterocytozoon hepatopenaei (EHP) in environmental samples in shrimp farms. PLoS ONE 2016, 11, e0166320. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeon, H.J.; Seo, S.; Lee, C.; Kim, B.; Kwak, D.M.; Rhee, M.H.; Piamsomboon, P.; Nuraini, Y.L.; Je, C.U.; et al. The use of the internal transcribed spacer region for phylogenetic analysis of the microsporidian parasite Enterocytozoon hepatopenaei infecting whiteleg shrimp (Penaeus vannamei) and for the development of a nested PCR as its diagnostic tool. J. Microbiol. Biotechnol. 2024, 34, 1146. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Sritunyalucksana, K.; Sanguanrut, P.; Salachan, P.V.; Thitamadee, S.; Flegel, T.W. Urgent Appeal to Control Spread of the Shrimp Microsporidian Parasite Enterocytozoon hepatopenaei (EHP); Network of Aquaculture Centres in Asia-Pacific (NACA): Bangkok, Thailand, 2014; pp. 4–6. Available online: https://enaca.org/?id=101 (accessed on 24 November 2014).
- Zhou, J.; Jiang, F.; Ding, F. The preliminary analysis of the reasons for the poor growth of Macrobrachium rosenbergii in pond. J. Shanghai Ocean Univ. 2017, 26, 853–861. [Google Scholar]
- Sonthi, M.; Kasikidpongpan, N. Histopathological changes in the hepatopancreas (Macrobrachium rosenbergii) infected with microsporidian (Enterocytozoon hepatopenaei). J. Agric. Ext. 2018, 35, 1015–1020. [Google Scholar]
- Chaijarasphong, T.; Munkongwongsiri, N.; Stentiford, G.D.; Aldama-Cano, D.J.; Thansa, K.; Flegel, T.W.; Sritunyalucksana, K.; Itsathitphaisarn, O. The shrimp microsporidian Enterocytozoon hepatopenaei (EHP): Biology, pathology, diagnostics and control. J. Invertebr. Pathol. 2021, 186, 107458. [Google Scholar] [CrossRef]
- Han, J.E.; Lee, S.C.; Park, S.C.; Jeon, H.J.; Kim, K.Y.; Lee, Y.S.; Park, S.; Han, S.H.; Kim, J.H.; Choi, S.K. Molecular detection of Enterocytozoon hepatopenaei and Vibrio parahaemolyticus-associated acute hepatopancreatic necrosis disease in Southeast Asian Penaeus vannamei shrimp imported into Korea. Aquaculture 2020, 517, 734812. [Google Scholar] [CrossRef]
- National Fishery Products Quality Management Service (NFQS). Quarantine of Imported and Exported Aquatic Organisms. 2024. Available online: https://www.nfqs.go.kr/hpmg/quar/actionImportExportQuarantineForm.do?menuId=M0000192 (accessed on 7 March 2024).
- Gresoviac, S.J.; Khattra, J.S.; Nadler, S.A.; Kent, M.L.; Devlin, R.H.; Vivares, C.P.; De La Fuente, E.; Hedrick, R.P. Comparison of small subunit ribosomal RNA gene and internal transcribed spacer sequences among isolates of the intranuclear microsporidian Nucleospora salmonis. J. Eukaryot. Microbiol. 2000, 47, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Haro, M.; Del Aguila, C.; Fenoy, S.; Henriques-Gil, N. Intraspecies Genotype Variability of the Microsporidian Parasite Encephalitozoon hellem. J. Clin. Microbiol. 2003, 41, 4166–4171. [Google Scholar] [CrossRef] [PubMed]
- Cendales, Y.G.; Schofield, P.J.; Morahan, B.; McQuinn, T.; Starkey, C.; Gross, K.; Dhar, A.K. Impact of commercial freezing on transmission of Enterocytozoon hepatopenaei. J. Invertebr. Pathol. 2025, 211, 108293. [Google Scholar] [CrossRef] [PubMed]


| Sample ID | Country | Collection Month/Year | Length (cm) | Weight (g) | EHP Detection 1 |
|---|---|---|---|---|---|
| 23-026C5-1-VIE | Vietnam | May 2023 | 20.0–23.0 | 121.1–157.7 | − |
| 23-026C6-1-VIE | Vietnam | June 2023 | 14.0–18.3 | 33.0–60.0 | + |
| 23-026C6-2-INDIA | India | June 2023 | 20.5–23.0 | 93.6–153.4 | + |
| 23-026C7-1-VIE | Vietnam | July 2023 | 15.0–18.6 | 49.5–89.4 | + |
| 23-026C7-2-INDIA | India | July 2023 | 19.0–22.5 | 105.5–183.6 | + |
| 23-026C7-3-VIE | Vietnam | July 2023 | 14.4–18.0 | 31.4–75.8 | + |
| 23-026C8-1-INDIA | India | August 2023 | 20.9–26.1 | 95.1–199.4 | − |
| 23-026C8-2-VIE | Vietnam | August 2023 | 14.7–16.3 | 51.8–80.5 | − |
| 23-026C9-1-INDIA | India | September 2023 | 19.0–22.8 | 92.6–130.7 | − |
| 23-026C9-2-VIE | Vietnam | September 2023 | 15.0–16.4 | 45.7–77.8 | + |
| 23-026C10-1-INDIA | India | October 2023 | 19.5–22.8 | 90.6–140.2 | − |
| 23-026C11-1-VIE | Vietnam | October 2023 | 15.8–18.5 | 43.4–79.0 | + |
| 24-004C2-1-INDIA | India | February 2024 | 18.9–21.4 | 96.1–161.6 | − |
| 24-004C2-2-INDIA | India | February 2024 | 19.0–21.4 | 97.4–159.0 | − |
| 24-004C3-1-INDIA | India | March 2024 | 20.2–22.7 | 110.6–159.4 | − |
| 24-004C3-2-INDIA | India | March 2024 | 19.2–22.9 | 101.3–137.6 | − |
| 24-004C4-1-INDIA | India | April 2024 | 18.4–22.1 | 76.9–139.6 | − |
| 24-004C4-2-INDIA | India | April 2024 | 18.9–21.9 | 87.5–143.9 | − |
| 24-004C4-3-INDIA | India | April 2024 | 23.3–26.6 | 185.0–289.9 | − |
| 24-004C5-1-INDIA | India | May 2024 | 22.5–25.5 | 95.5–143.0 | − |
| 24-004C5-2-INDIA | India | May 2024 | 19.4–21.9 | 86.8–134.5 | − |
| 24-004C6-1-INDIA | India | June 2024 | 22.2–25.2 | 109.56–148.15 | − |
| Target | Primer | Sequence (5′ to 3′) | Amplicon Size (bp) | Reference |
|---|---|---|---|---|
| SSU rRNA 1 | 510-F | GCCTGAGAGATGGCTCCCACGT | 510 | [12] |
| 510-R | GCGTACTATCCCCAGAGCCCGA | |||
| SSU rRNA 2 | 18S-F | CACCAGGTTGATTCTGCCTGA | 1146 | [12] |
| 18S-R | TCTGAAATAGTGACGGGCGG | |||
| SWP 1 | 1F (1st) | TTGCAGAGTGTTGTTAAGGGTTT | 514 | [20] |
| 1R (1st) | CACGATGTGTCTTTGCAATTTTC | |||
| 2F’ (2nd) | GCAGAGTGTTGTTAAGGGTTTAAG | 182 | [13] | |
| 2R’ (2nd) | GCTGTTTGTCWCCAACTGTATT | |||
| ITS-1 | ITS1-1F (1st) | CGCCCGTCACTATTTCAGAT | 603 | [21] |
| ITS1-1R (1st) | TACGTTCGTCATCGCTGCTA | |||
| ITS1-2F (2nd) | GAACCTGCTGTGGGATCATT | 400 | ||
| ITS1-2R (2nd) | AATTTTTGCTTGGCTCATTCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, H.J.; Kim, B.; Bang, S.Y.; Kim, Y.; Hwang, J.Y.; Piamsomboon, P.; Kim, J.H.; Han, J.E. Detection and Genetic Characterization of Enterocytozoon hepatopenaei in Giant Freshwater Prawn (Macrobrachium rosenbergii) Imported into South Korea. Animals 2025, 15, 3286. https://doi.org/10.3390/ani15223286
Jeon HJ, Kim B, Bang SY, Kim Y, Hwang JY, Piamsomboon P, Kim JH, Han JE. Detection and Genetic Characterization of Enterocytozoon hepatopenaei in Giant Freshwater Prawn (Macrobrachium rosenbergii) Imported into South Korea. Animals. 2025; 15(22):3286. https://doi.org/10.3390/ani15223286
Chicago/Turabian StyleJeon, Hye Jin, Bumkeun Kim, So Young Bang, Yukyung Kim, Jee Youn Hwang, Patharapol Piamsomboon, Ji Hyung Kim, and Jee Eun Han. 2025. "Detection and Genetic Characterization of Enterocytozoon hepatopenaei in Giant Freshwater Prawn (Macrobrachium rosenbergii) Imported into South Korea" Animals 15, no. 22: 3286. https://doi.org/10.3390/ani15223286
APA StyleJeon, H. J., Kim, B., Bang, S. Y., Kim, Y., Hwang, J. Y., Piamsomboon, P., Kim, J. H., & Han, J. E. (2025). Detection and Genetic Characterization of Enterocytozoon hepatopenaei in Giant Freshwater Prawn (Macrobrachium rosenbergii) Imported into South Korea. Animals, 15(22), 3286. https://doi.org/10.3390/ani15223286








