Phylogeny and Body Size Predict Distress Call Divergence in Bats: A Comparative Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species and Sites
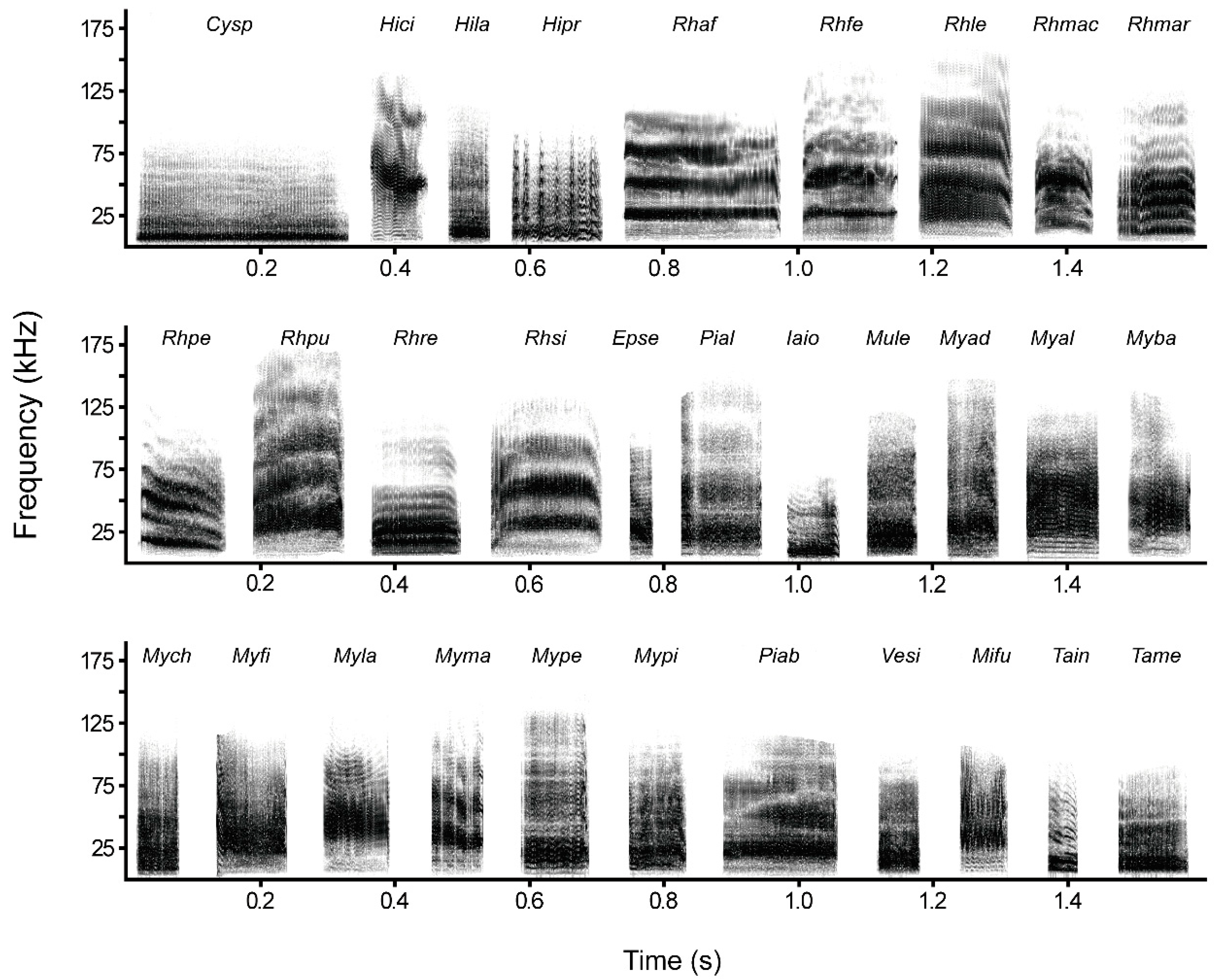
2.2. Recording and Analysis of Calls
2.3. Data Collection
2.4. Statistical Analyses
3. Results
3.1. Incidence of Distress Calling in Bats
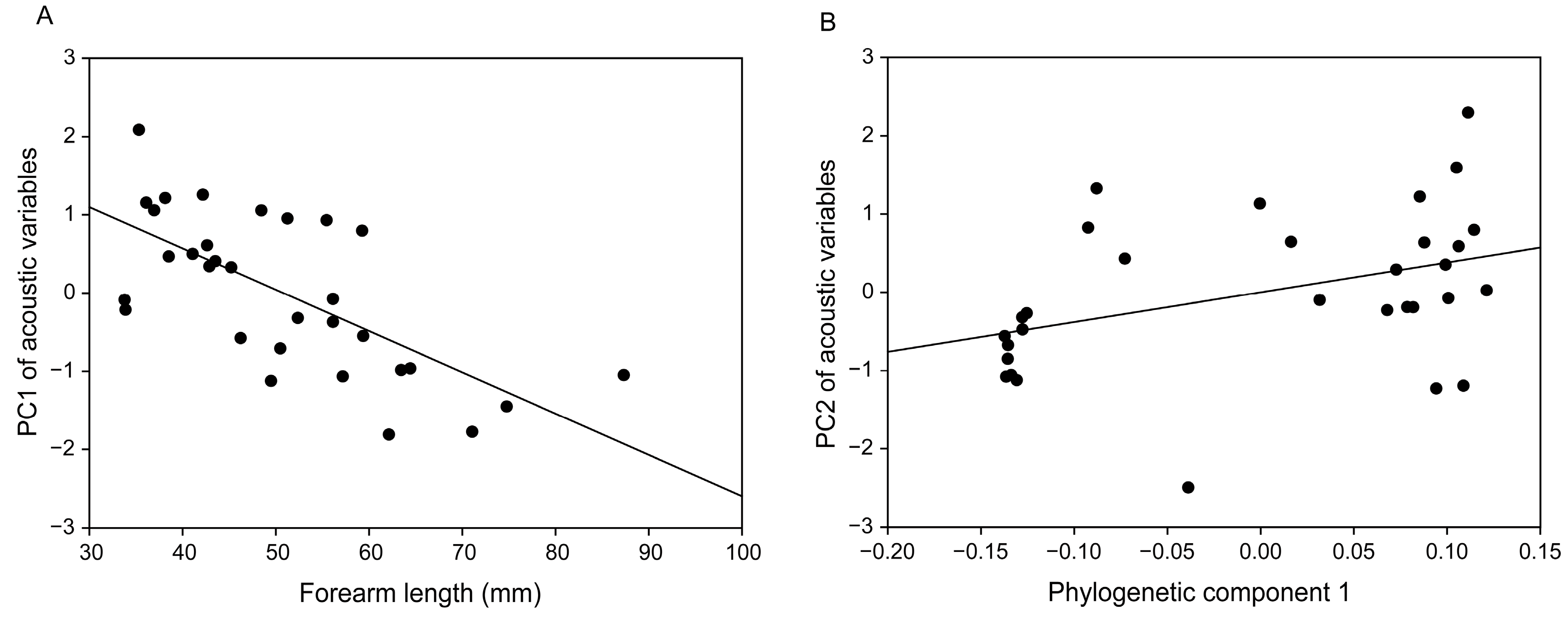
3.2. Distress Call Structure
4. Discussion
4.1. Incidence of Distress Calling
4.2. Structure of Distress Calls
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Mello Bezerra, A.; de Carvalho-e-Silva, S.P.; Gonzaga, L.P. Evolution of acoustic signals in Neotropical leaf frogs. Anim. Behav. 2021, 181, 41–49. [Google Scholar] [CrossRef]
- Russo, D.; Nagy, M.; Visnakova, I.; Wuntke, B.; Pfalzer, G.; Georgiakakis, P.; Knörnschild, M. Social vocalizations show stronger phylogenetic conservatism than echolocation calls in closely related pipistrelle bats. Anim. Behav. 2025, 227, 123283. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, J.Y.; Noh, H.J.; Kim, M.S.; Kim, K.H.; Yoo, J.C. Habitats matter: The incidence of and response to fear screams in a habitat generalist, the vinous-throated parrotbill Paradoxornis webbianus. Behav. Ecol. Sociobiol. 2015, 69, 1575–1584. [Google Scholar] [CrossRef]
- Romanow, C.A.; Riede, T.; Lingle, S. Vocal characteristics of distress and reproductive vocalizations in North American wapiti. Curr. Zool. 2025, 71, zoaf006. [Google Scholar] [CrossRef]
- Rajan, K.E. Olfactory learning and memory in the greater short-nosed fruit bat Cynopterus sphinx: The influence of conspecifics distress calls. J. Comp. Physiol. A 2021, 207, 667–679. [Google Scholar] [CrossRef]
- Huang, X.; Metzner, W.; Zhang, K.; Wang, Y.; Luo, B.; Sun, C.; Jiang, T.; Feng, J. Acoustic similarity elicits responses to heterospecific distress calls in bats (Mammalia: Chiroptera). Anim. Behav. 2018, 146, 143–154. [Google Scholar] [CrossRef]
- González-Palomares, E.; López-Jury, L.; Wetekam, J.; Kiai, A.; García-Rosales, F.; Hechavarria, J.C. Male Carollia perspicillata bats call more than females in a distressful context. R. Soc. Open Sci. 2021, 8, 202336. [Google Scholar] [CrossRef]
- Fan, B.; Wang, Y.; Huang, X.; Zhang, X.; Yang, J.; Jiang, T. The potential to encode detailed information about parasites in the acoustic signals of Chinese horseshoe bats (Rhinolophus sinicus). Front. Ecol. Evol. 2022, 10, 908209. [Google Scholar] [CrossRef]
- Wise, K.; Conover, M.; Knowlton, F. Response of coyotes to avian distress calls: Testing the startle-predator and predator-attraction hypotheses. Behaviour 1999, 136, 935–949. [Google Scholar] [CrossRef]
- Ruat, J.; Genewsky, A.J.; Heinz, D.E.; Kaltwasser, S.F.; Canteras, N.S.; Czisch, M.; Chen, A.; Wotjak, C.T. Why do mice squeak? Toward a better understanding of defensive vocalization. iScience 2022, 25, 104657. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Monachesi, M.R.; Labra, A. Complex distress calls sound frightening: The case of the weeping lizard. Anim. Behav. 2020, 165, 71–77. [Google Scholar] [CrossRef]
- Yoshino-Hashizawa, K.; Nishiuchi, Y.; Hiragochi, M.; Kihara, M.; Kobayasi, K.I.; Hiryu, S. The distress context of social calls evokes a fear response in the bat Pipistrellus abramus. J. Exp. Biol. 2023, 226, jeb246271. [Google Scholar] [CrossRef] [PubMed]
- Hörmann, D.; Tschapka, M.; Rose, A.; Knörnschild, M. Distress calls of nectarivorous bats (Glossophaga soricina) encode individual and species identity. Bioacoustics 2021, 30, 253–271. [Google Scholar] [CrossRef]
- Neudorf, D.L.; Sealy, S.G. Distress calls of birds in a neotropical cloud forest. Biotropica 2002, 34, 118–126. [Google Scholar] [CrossRef]
- Møller, A.; Nielsen, J. Fear screams and adaptation to avoid imminent death: Effects of genetic variation and predation. Ethol. Ecol. Evol. 2010, 22, 183–202. [Google Scholar] [CrossRef]
- Hödl, W.; Gollmann, G. Distress calls in Neotropical frogs. Amphibia-Reptilia 1986, 7, 11–21. [Google Scholar] [CrossRef]
- Hogstedt, G. Adaptation unto death: Function of fear screams. Am. Nat. 1983, 121, 562–570. [Google Scholar] [CrossRef]
- Greig-Smith, P. Distress calling by woodland birds. Anim. Behav. 1982, 30, 299–301. [Google Scholar] [CrossRef]
- Luo, B.; Huang, X.; Li, Y.; Lu, G.; Zhao, J.; Zhang, K.; Zhao, H.; Liu, Y.; Feng, J. Social call divergence in bats: A comparative analysis. Behav. Ecol. 2017, 28, 533–540. [Google Scholar] [CrossRef]
- Hardt, B.; Benedict, L. Can you hear me now? A review of signal transmission and experimental evidence for the acoustic adaptation hypothesis. Bioacoustics 2021, 30, 716–742. [Google Scholar] [CrossRef]
- Snell-Rood, E.C. The effect of climate on acoustic signals: Does atmospheric sound absorption matter for bird song and bat echolocation? J. Acoust. Soc. Am. 2012, 131, 1650–1658. [Google Scholar] [CrossRef]
- Maria, B.; Tonini, J.F.; Rebouças, R.; Toledo, L.F. Hidden shifts in allometry scaling between sound production and perception in anurans. PeerJ 2023, 11, e16322. [Google Scholar] [CrossRef]
- Friis, J.I.; Sabino, J.; Santos, P.; Dabelsteen, T.; Cardoso, G.C. Ecological adaptation and birdsong: How body and bill sizes affect passerine sound frequencies. Behav. Ecol. 2022, 33, 798–806. [Google Scholar] [CrossRef]
- López-Cuamatzi, I.L.; Vega-Gutiérrez, V.H.; Cabrera-Campos, I.; Ruiz-Sanchez, E.; Ayala-Berdon, J.; Saldaña-Vázquez, R.A. Does body mass restrict call peak frequency in echolocating bats? Mammal Rev. 2020, 50, 304–313. [Google Scholar] [CrossRef]
- Gingras, B.; Mohandesan, E.; Boko, D.; Fitch, W.T. Phylogenetic signal in the acoustic parameters of the advertisement calls of four clades of anurans. BMC Evol. Biol. 2013, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Thinh, V.N.; Hallam, C.; Roos, C.; Hammerschmidt, K. Concordance between vocal and genetic diversity in crested gibbons. BMC Evol. Biol. 2011, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Arato, J.; Fitch, W.T. Phylogenetic signal in the vocalizations of vocal learning and vocal non-learning birds. Philos. Trans. R. Soc. B 2021, 376, 20200241. [Google Scholar] [CrossRef]
- Pollard, K.A.; Blumstein, D.T. Social group size predicts the evolution of individuality. Curr. Biol. 2011, 21, 413–417. [Google Scholar] [CrossRef]
- Jurisevic, M.A.; Sanderson, K.J. A comparative analysis of distress call structure in Australian passerine and non-passerine species: Influence of size and phylogeny. J. Avian Biol. 1998, 29, 61–71. [Google Scholar] [CrossRef]
- Martin, J.P.; Doucet, S.M.; Knox, R.C.; Mennill, D.J. Body size correlates negatively with the frequency of distress calls and songs of Neotropical birds. J. Field Ornithol. 2011, 82, 259–268. [Google Scholar] [CrossRef]
- Frankenberg, E. Distress calls of gekkonid lizards from Israel and Sinai. Isr. J. Ecol. Evol. 1975, 24, 43–53. [Google Scholar]
- Liu, Y.; Geng, Y.; Huang, Z.; Feng, J.; Jiang, T. Pest suppression services and dietary niche differentiation of bats in Chinese smallholder farming systems: Implications for integrated pest management. J. Pest Sci. 2024, 97, 1587–1603. [Google Scholar] [CrossRef]
- Arévalo, M.; Leticia, R.; Amador, L.I.; Almeida, F.C.; Giannini, N.P. Evolution of body mass in bats: Insights from a large supermatrix phylogeny. J. Mamm. Evol. 2020, 27, 123–138. [Google Scholar] [CrossRef]
- Zou, W.; Liang, H.; Wu, P.; Luo, B.; Zhou, D.; Liu, W.; Wu, J.; Fang, L.; Lei, Y.; Feng, J. Correlated evolution of wing morphology and echolocation calls in bats. Front. Ecol. Evol. 2022, 10, 1031548. [Google Scholar] [CrossRef]
- Liu, Y.; Si, M.; Huang, Z.; Feng, J.; Jiang, T. Bats are sentinels for invasive pest surveillance based on DNA metabarcoding. Ecol. Indic. 2023, 152, 110354. [Google Scholar] [CrossRef]
- Bernardy, J.V.; Llusia, D.; Maciel, N.M.; De Marco, P.; Bastos, R.P. Do body size and habitat shape call frequencies of Brazilian hylids (Amphibia: Anura)? J. Ethol. 2024, 42, 197–207. [Google Scholar] [CrossRef]
- Kanwal, J.S.; Matsumura, S.; Ohlemiller, K.; Suga, N. Analysis of acoustic elements and syntax in communication sounds emitted by mustached bats. J. Acoust. Soc. Am. 1994, 96, 1229–1254. [Google Scholar] [CrossRef]
- Safi, K.; Meiri, S.; Jones, K.E.; Smith, F.; Lyons, K. Evolution of body size in bats. In Animal Body Size: Linking Pattern and Process Across Space, Time, and Taxonomic Group; Smith, F.A., Lyons, S.K., Eds.; University of Chicago Press: Chicago, IL, USA, 2013; pp. 95–151. [Google Scholar]
- Denzinger, A.; Schnitzler, H.-U. Bat guilds, a concept to classify the highly diverse foraging and echolocation behaviors of microchiropteran bats. Front. Physiol. 2013, 4, 164. [Google Scholar] [CrossRef]
- The IUCN Red List of Threatened Species. 2023-2. Available online: https://www.iucnredlist.org/en (accessed on 13 May 2024).
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Storz, J.F.; Bhat, H.R.; Kunz, T.H. Social structure of a polygynous tent-making bat, Cynopterus sphinx (Megachiroptera). J. Zool. 2000, 251, 151–165. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Grothendieck, G.; Green, P.; Bolker, M.B. Package ‘lme4’. Convergence 2015, 12, 2. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Package ‘vegan’. In Community Ecology Package, Version; CRAN: Vienna, Austria, 2013; Volume 2, pp. 1–295. [Google Scholar]
- Revelle, W.; Revelle, M.W. Package ‘psych’. In The Comprehensive R Archive Network; CRAN: Vienna, Austria, 2015; Volume 337, pp. 161–165. [Google Scholar]
- Bartoń, K. R Package, version 1.46.0. MuMIn: Multi-Model Inference. In The R Project for Statistical Computing; CRAN: Vienna, Austria, 2022. [Google Scholar]
- Walsh, C.; Mac Nally, R.; Walsh, M.C. Package ‘hier.part’. In R Project for Statistical Computing; CRAN: Vienna, Austria, 2020. [Google Scholar]
- Nally, R.M.; Walsh, C.J. Hierarchical partitioning public-domain software. Biodivers. Conserv. 2004, 13, 659–660. [Google Scholar] [CrossRef]
- Huang, X.; Kanwal, J.S.; Jiang, T.; Long, Z.; Luo, B.; Yue, X.; Gu, Y.; Feng, J. Situational and age-dependent decision making during life threatening distress in Myotis macrodactylus. PLoS ONE 2015, 10, e0132817. [Google Scholar] [CrossRef]
- Skok, J.; Povše, M.P.; Škorjanc, D. A case of thanatosis in domestic sheep. Behaviour 2025, 162, 569–578. [Google Scholar] [CrossRef]
- Lima, S.L.; O’Keefe, J.M. Do predators influence the behaviour of bats? Biol. Rev. 2013, 88, 626–644. [Google Scholar] [CrossRef] [PubMed]
- Danilovich, S.; Krishnan, A.; Lee, W.-J.; Borrisov, I.; Eitan, O.; Kosa, G.; Moss, C.F.; Yovel, Y. Bats regulate biosonar based on the availability of visual information. Curr. Biol. 2015, 25, R1124–R1125. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Q.; Wei, J.K.; Li, B.; Wang, M.S.; Wu, R.Q.; Rizak, J.D.; Zhong, L.; Wang, L.; Xu, F.Q.; Shen, Y.Y. Divergence of dim-light vision among bats (order: Chiroptera) as estimated by molecular and electrophysiological methods. Sci. Rep. 2015, 5, 11531. [Google Scholar] [CrossRef]
- Cap, H.; Deleporte, P.; Joachim, J.; Reby, D. Male vocal behavior and phylogeny in deer. Cladistics 2008, 24, 917–931. [Google Scholar] [CrossRef]
- Kamilar, J.M.; Cooper, N. Phylogenetic signal in primate behaviour, ecology and life history. Philos. Trans. R. Soc. B. 2013, 368, 20120341. [Google Scholar] [CrossRef]
- Forstmeier, W.; Burger, C.; Temnow, K.; Derégnaucourt, S. The genetic basis of zebra finch vocalizations. Evolution 2009, 63, 2114–2130. [Google Scholar] [CrossRef]
- Hechavarría, J.C.; Jerome Beetz, M.; García-Rosales, F.; Kössl, M. Bats distress vocalizations carry fast amplitude modulations that could represent an acoustic correlate of roughness. Sci. Rep. 2020, 10, 7332. [Google Scholar] [CrossRef]
- Morton, E.S. On the occurrence and significance of motivation-structural rules in some bird and mammal sounds. Am. Nat. 1977, 111, 855–869. [Google Scholar] [CrossRef]
- Naumann, R.T.; Kanwal, J.S. Basolateral amygdala responds robustly to social calls: Spiking characteristics of single unit activity. J. Neurophysiol. 2011, 105, 2389–2404. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, L.; Christensen-Dalsgaard, J.; Juhl, P.M.; Elemans, C.P. How loud can you go? Physical and physiological constraints to producing high sound pressures in animal vocalizations. Front. Ecol. Evol. 2021, 9, 657254. [Google Scholar] [CrossRef]
- Friis, J.I.; Dabelsteen, T.; Cardoso, G.C. Contingency and determinism in the evolution of bird song sound frequency. Sci. Rep. 2021, 11, 11600. [Google Scholar] [CrossRef]
- Gingras, B.; Böckle, M.; Herbst, C.; Fitch, W.T. Call acoustics reflect body size across four clades of anurans. J. Zool. 2013, 289, 143–150. [Google Scholar] [CrossRef]
- Bowling, D.L.; Garcia, M.; Dunn, J.C.; Ruprecht, R.; Stewart, A.; Frommolt, K.-H.; Fitch, W.T. Body size and vocalization in primates and carnivores. Sci. Rep. 2017, 7, 41070. [Google Scholar] [CrossRef]
- McCracken, K.G.; Sheldon, F.H. Avian vocalizations and phylogenetic signal. Proc. Natl. Acad. Sci. USA 1997, 94, 3833–3836. [Google Scholar] [CrossRef]
- Morton, E.S. Ecological sources of selection on avian sounds. Am. Nat. 1975, 109, 17–34. [Google Scholar] [CrossRef]
- Boncoraglio, G.; Saino, N. Habitat structure and the evolution of bird song: A meta-analysis of the evidence for the acoustic adaptation hypothesis. Funct. Ecol. 2007, 21, 134–142. [Google Scholar] [CrossRef]
- Gillam, E.H.; Chaverri, G. Strong individual signatures and weaker group signatures in contact calls of Spix’s disc-winged bat, Thyroptera tricolor. Anim. Behav. 2012, 83, 269–276. [Google Scholar] [CrossRef]
- Düpjan, S.; Tuchscherer, A.; Langbein, J.; Schön, P.-C.; Manteuffel, G.; Puppe, B. Behavioural and cardiac responses towards conspecific distress calls in domestic pigs (Sus scrofa). Physiol. Behav. 2011, 103, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Manteuffel, G.; Puppe, B.; Schön, P.C. Vocalization of farm animals as a measure of welfare. Appl. Anim. Behav. Sci. 2004, 88, 163–182. [Google Scholar] [CrossRef]


| Parameters | Models | Intercept | AICc | ΔAICc | w |
|---|---|---|---|---|---|
| Incidence of distress calls | PCO2 | 2.33 | 8.00 | 0.00 | 0.558 |
| PC1 of acoustic variables | Forearm length + PCO1 + PCO2 | 2.81 | 78.70 | 0.00 | 0.218 |
| Forearm length + PCO1 + PCO2 + Colony size | 1.66 | 79.20 | 0.53 | 0.167 | |
| Forearm length + PCO1 + Colony size | 1.53 | 79.60 | 0.940 | 0.136 | |
| Forearm length + PCO1 + PCO2 + Annual Precipitation | 3.45 | 80.60 | 1.86 | 0.086 | |
| PC2 of acoustic variables | PCO1 + PCO2 + Annual Precipitation | 5.27 | 86.00 | 0.00 | 0.315 |
| PCO1 + PCO2 | −4.22 | 86.70 | 0.69 | 0.223 |
| Measurement | PC1 | PC2 |
|---|---|---|
| Syllable rate (syllable/sec) | 0.051 | 0.947 |
| Syllable duration (ms) | −0.024 | −0.944 |
| Fundamental frequency (kHz) | 0.912 | 0.08 |
| Peak frequency (kHz) | 0.925 | −0.107 |
| Minimum frequency (kHz) | 0.89 | −0.044 |
| Maximum frequency (kHz) | 0.925 | 0.102 |
| Bandwidth (kHz) | 0.777 | 0.144 |
| Eigenvalue | 3.962 | 1.818 |
| % Variance explained | 56.606 | 25.971 |
| Predictor Variables | Parameters | PC1 of Acoustic Variables | PC2 of Acoustic Variables |
|---|---|---|---|
| Forearm length | RVI | 1.00 | − |
| 95% CI | (−0.073, −0.037) | − | |
| IE (%) | 69.95 * | 1.48 | |
| Colony size | RVI | 0.50 | − |
| 95% CI | (0.051, 1.003) | − | |
| IE (%) | 11.54 | 3.72 | |
| Habitat type | RVI | − | − |
| 95% CI | − | − | |
| IE (%) | 1.63 | 21.89 | |
| Annual mean temperature | RVI | − | − |
| 95% CI | − | − | |
| IE (%) | 0.65 | 6.25 | |
| Annual precipitation | RVI | 0.14 | 0.59 |
| 95% CI | (−1.839, 1.414) | (−3.509, 0.125) | |
| IE (%) | 0.65 | 2.53 | |
| Phylogenetic component 1 | RVI | 1.00 | 1.00 |
| 95% CI | (−5.423, −0.853) | (2.282, 7.612) | |
| IE (%) | 10.69 | 58.18 * | |
| Phylogenetic component 2 | RVI | 0.78 | 1.00 |
| 95% CI | (−7.057, 1.960) | (2.392, 10.924) | |
| IE (%) | 4.90 | 5.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Huang, X.; Zhang, K.; Gong, L.; Gu, H.; Dai, W.; Feng, J.; Jiang, T. Phylogeny and Body Size Predict Distress Call Divergence in Bats: A Comparative Analysis. Animals 2025, 15, 3268. https://doi.org/10.3390/ani15223268
Wang Y, Huang X, Zhang K, Gong L, Gu H, Dai W, Feng J, Jiang T. Phylogeny and Body Size Predict Distress Call Divergence in Bats: A Comparative Analysis. Animals. 2025; 15(22):3268. https://doi.org/10.3390/ani15223268
Chicago/Turabian StyleWang, Yujuan, Xiaobin Huang, Kangkang Zhang, Lixin Gong, Hao Gu, Wentao Dai, Jiang Feng, and Tinglei Jiang. 2025. "Phylogeny and Body Size Predict Distress Call Divergence in Bats: A Comparative Analysis" Animals 15, no. 22: 3268. https://doi.org/10.3390/ani15223268
APA StyleWang, Y., Huang, X., Zhang, K., Gong, L., Gu, H., Dai, W., Feng, J., & Jiang, T. (2025). Phylogeny and Body Size Predict Distress Call Divergence in Bats: A Comparative Analysis. Animals, 15(22), 3268. https://doi.org/10.3390/ani15223268





