Dietary Glycerol Monolaurate Effect on Growth Performance, Intestinal Barrier Function, and Gut Microbiota in Weaned Piglets Challenged by Lipopolysaccharide
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Experimental Design
2.2. Sample Collection
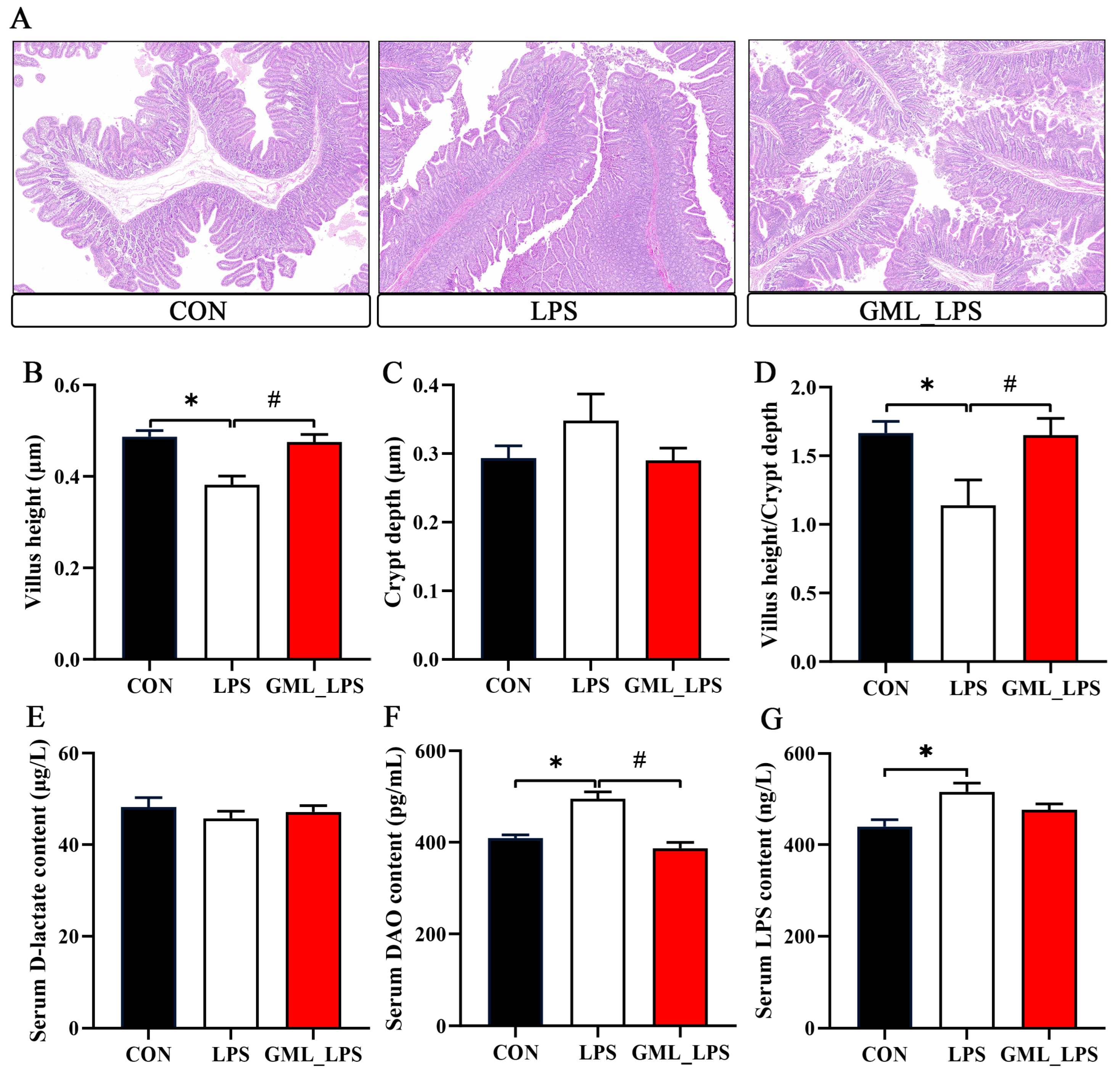
2.3. Intestinal Morphology in the Jejunum
2.4. Analysis of Serum Indicators
2.5. Real-Time PCR
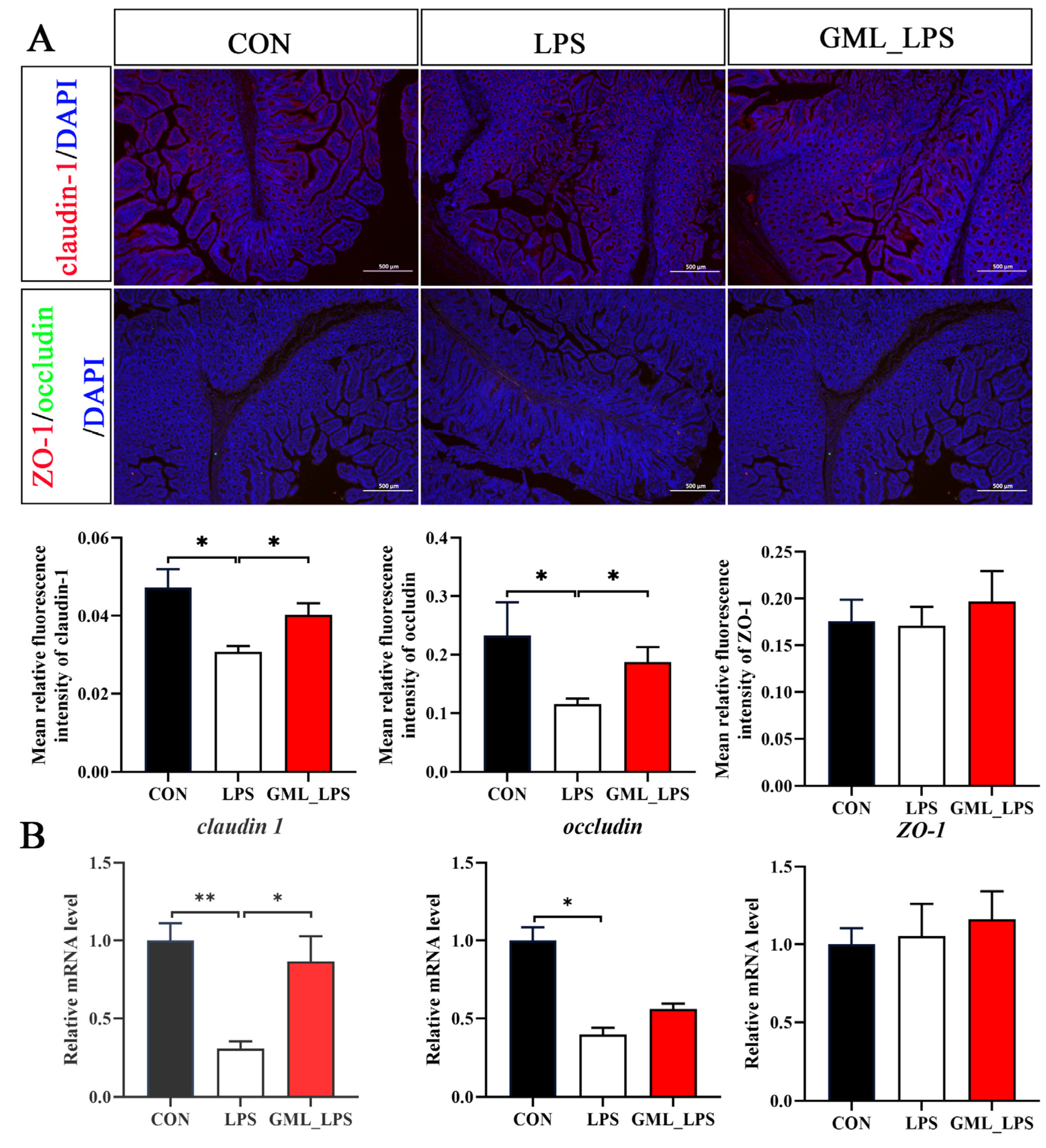
2.6. Immunofluorescence Staining
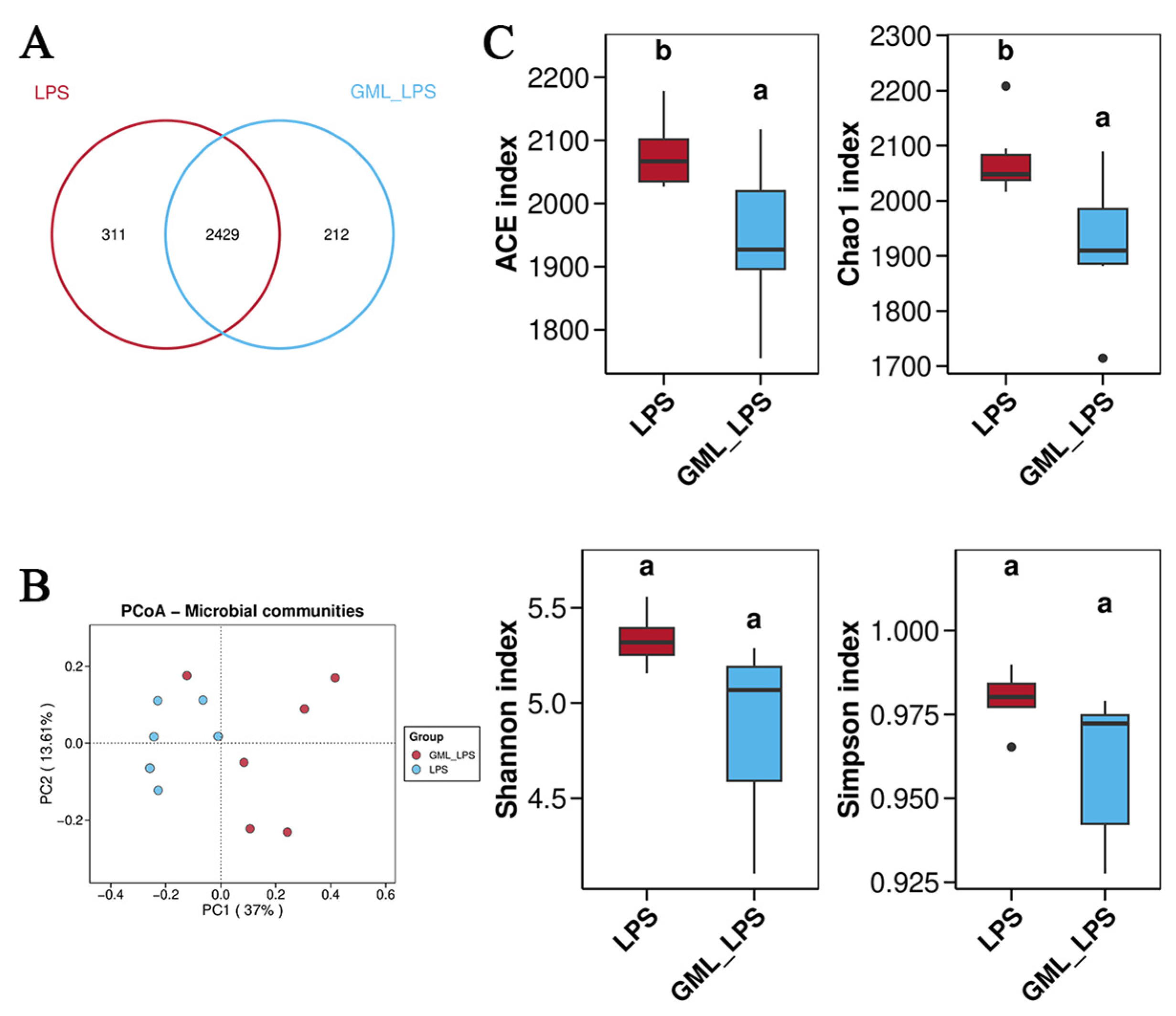
2.7. Gut Microbiota Analysis
2.8. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Intestinal Morphology
3.3. Tight Junction Protein
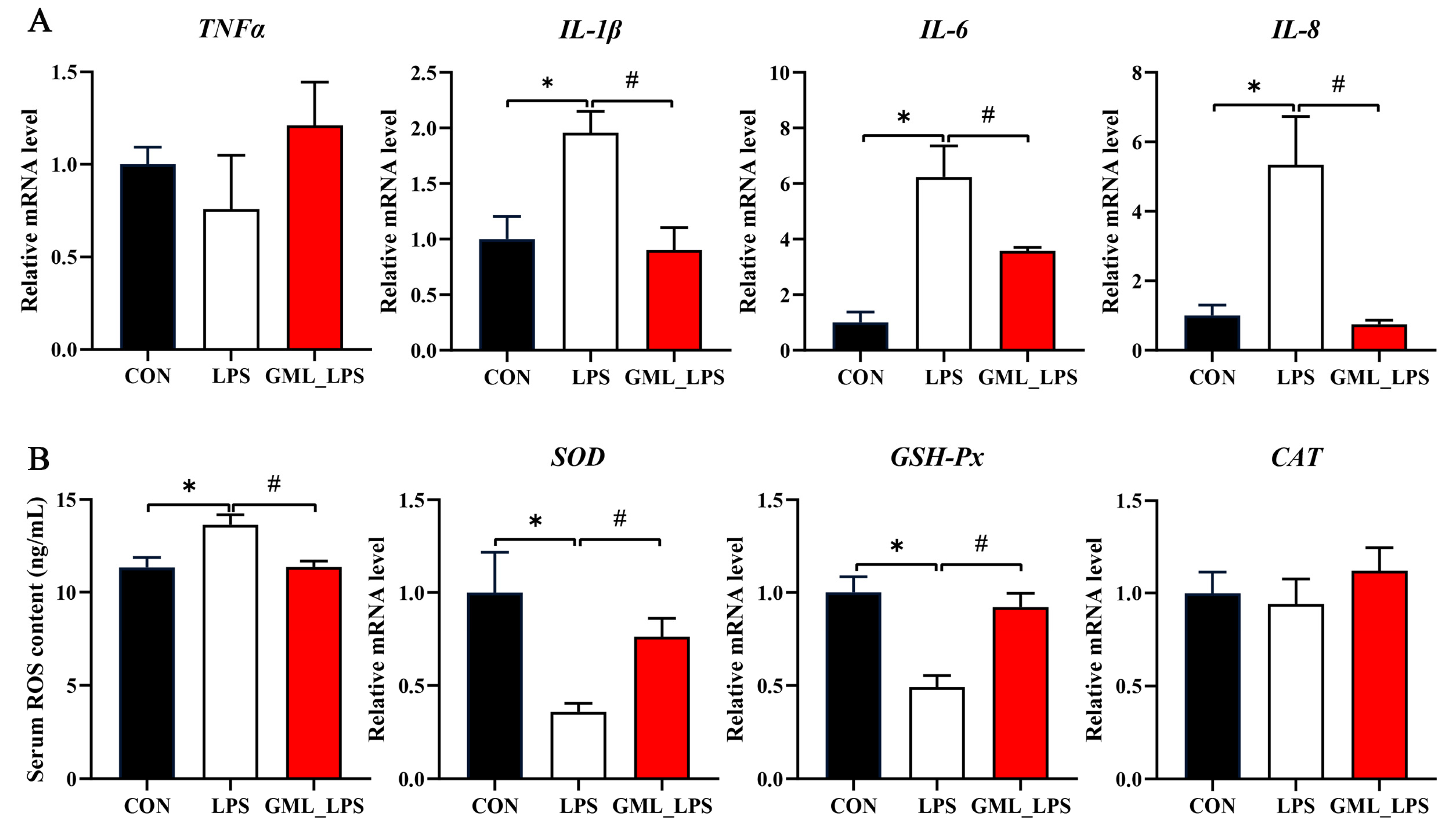
3.4. Inflammatory Factors and Antioxidant Enzymes
3.5. Gut Microbiota Community
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADFI | Average daily feed consumption |
| ADG | Average daily gain |
| DAO | Diamine oxidase |
| G/F | Gain-to-feed ratio |
| GML | Glycerol monolaurate |
| GRAS | Generally recognized as safe |
| ROS | Reactive oxygen |
| H&E | Hematoxylin and eosin |
| LPS | Lipopolysaccharide |
References
- Shao, Y.; Xiong, X.; Wang, K.; Cheng, P.; Zou, L.; Zhou, J.; Qi, M.; Yin, Y. Early weaning leads to the remodeling of lipid profile in piglet jejunal crypt cells during post-weaning days. Anim. Nutr. 2022, 11, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.; Offersen, S.M.; Brunse, A.; Pirolo, M.; Kar, S.K.; Guadabassi, L.; Thymann, T. Effects of early postnatal gastric and colonic microbiota transplantation on piglet gut health. J. Anim. Sci. Biotechnol. 2023, 14, 158. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhong, R.; Dang, G.; Xia, B.; Wu, W.; Tang, S.; Tang, L.; Liu, L.; Liu, Z.; Chen, L.; et al. Pectin supplementation ameliorates intestinal epithelial barrier function damage by modulating intestinal microbiota in lipopolysaccharide-challenged piglets. J. Nutr. Biochem. 2022, 109, 109107. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Zhen, W.; Bai, D.; Liu, K.; He, X.; Ito, K.; Liu, Y.; Li, Y.; Zhang, Y.; Zhang, B.; et al. Effects of dietary chlorogenic acid on intestinal barrier function and the inflammatory response in broilers during lipopolysaccharide-induced immune stress. Poult. Sci. 2023, 102, 102623. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Gong, J.G.; Li, J.H.; Hao, Y.-S.; Xu, H.-J.; Liu, Y.-C.; Feng, Z.-H. Dietary resveratrol supplementation on growth performance, immune function and intestinal barrier function in broilers challenged with lipopolysaccharide. Poult. Sci. 2023, 102, 102968. [Google Scholar] [CrossRef]
- Zhao, M.; Jiang, Z.; Cai, H.; Li, Y.; Mo, Q.; Deng, L.; Zhong, H.; Liu, T.; Zhang, H.; Kang, J.X.; et al. Modulation of the gut microbiota during high-dose glycerol monolaurate-mediated amelioration of obesity in mice fed a high-fat diet. mBio 2020, 11, e00190-20. [Google Scholar] [CrossRef]
- Peterson, M.L.; Schlievert, P.M. Glycerol monolaurate inhibits the effects of Gram-positive select agents on eukaryotic cells. Biochemistry 2006, 45, 2387–2397. [Google Scholar] [CrossRef]
- Li, Q.; Estes, J.D.; Schlievert, P.M.; Duan, L.; Brosnahan, A.J.; Southern, P.J.; Reilly, C.S.; Peterson, M.L.; Schultz-Darken, N.; Brunner, K.G.; et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature 2009, 458, 1034–1038. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Zhang, N.; Zhang, T.; Ma, Y. Effects of α-glycerol monolaurate on intestinal morphology, nutrient digestibility, serum profiles, and gut microbiota in weaned piglets. J. Anim. Sci. 2022, 100, skac046. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 11th revised ed.; National Academy Press: Washington, DC, USA, 2012. [Google Scholar]
- Cui, C.; Wei, Y.; Wang, Y.; Ma, W.; Zheng, X.; Wang, J.; Ma, Z.; Wu, C.; Chu, L.; Zhang, S.; et al. Dietary supplementation of benzoic acid and essential oils combination enhances intestinal resilience against LPS stimulation in weaned piglets. J. Anim. Sci. Biotechnol. 2024, 15, 4. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Zhan, K.; Ma, Q.; Xu, L.; Li, Y.; Liu, Y. Vitamin K2 alleviates dextran sulfate sodium-induced colitis via inflammatory responses, gut barrier integrity, and the gut microbiota in mice. Int. J. Biol. Macromol. 2024, 280 Pt 4, 136091. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, X.; Wassie, T.; Wang, H.-H.; Li, T.; Xie, C.; Wu, X. Enteromorpha prolifera polysaccharide-zinc complex modulates the immune response and alleviates LPS-induced intestinal inflammation via inhibiting the TLR4/NF-κB signaling pathway. Food Funct. 2022, 13, 52–63. [Google Scholar] [CrossRef]
- Liu, T.; Li, C.; Zhong, H.; Feng, F. Dietary medium-chain α-monoglycerides increase BW, feed intake, and carcass yield in broilers with muscle composition alteration. Poult. Sci. 2021, 100, 186–195. [Google Scholar] [CrossRef]
- Fortuoso, B.F.; Dos Reis, J.H.; Gebert, R.R.; Barreta, M.; Griss, L.G.; Casagrande, R.A.; de Cristo, T.G.; Santiani, F.; Campigotto, G.; Rampazzo, L.; et al. Glycerol monolaurate in the diet of broiler chickens replacing conventional antimicrobials: Impact on health, performance and meat quality. Microb. Pathog. 2019, 129, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Tang, J.; Feng, F. Glycerol monolaurate improves performance, intestinal development, and muscle amino acids in yellow-feathered broilers via manipulating gut microbiota. Appl. Microbiol. Biotechnol. 2020, 104, 10279–10291. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Chen, G.; Cao, G.; Tang, J.; Li, Q.; Zhang, B.; Yang, C. Effects of α-glyceryl monolaurate on growth, immune function, volatile fatty acids, and gut microbiota in broiler chickens. Poult. Sci. 2021, 100, 100875. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.L.; Woodworth, J.C.; Tokach, M.D.; Dritz, S.S.; DeRouchey, J.M.; Goodband, R.D.; Williams, H.E.; Hartman, A.R.; Mellick, D.J.; McKilligan, D.M.; et al. Evaluation of different blends of medium-chain fatty acids, lactic acid, and monolaurin on nursery pig growth performance. Transl. Anim. Sci. 2020, 4, txaa024. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, X.; Hou, Z.; Liao, S.; Qi, M.; Zha, A.; Yang, Z.; Zuo, G.; Liao, P.; Chen, Y.; et al. Low-Protein Diet Supplemented with Medium-Chain Fatty Acid Glycerides Improves the Growth Performance and Intestinal Function in Post-Weaning Piglets. Animals 2020, 10, 1852. [Google Scholar] [CrossRef]
- Liu, Y. Fatty acids, inflammation and intestinal health in pigs. J. Anim. Sci. Biotechnol. 2015, 6, 41. [Google Scholar] [CrossRef]
- Martín-Venegas, R.; Roig-Pérez, S.; Ferrer, R.; Moreno, J.J. Arachidonic acid cascade and epithelial barrier function during Caco-2 cell differentiation. J. Lipid Res. 2006, 47, 1416–1423. [Google Scholar] [CrossRef]
- Song, W.B.; Lv, Y.H.; Zhang, Z.S.; Li, Y.-N.; Xiao, L.-P.; Yu, X.-P.; Wang, Y.-Y.; Ji, H.-L.; Ma, L. Soluble intercellular adhesion molecule-1, D-lactate and diamine oxidase in patients with inflammatory bowel disease. World J. Gastroenterol. 2009, 15, 3916–3919. [Google Scholar] [CrossRef]
- Mishra, S.P.; Wang, B.; Jain, S.; Ding, J.; Rejeski, J.; Furdui, C.M.; Kitzman, D.W.; Taraphder, S.; Brechot, C.; Kumar, A.; et al. A mechanism by which gut microbiota elevates permeability and inflammation in obese/diabetic mice and human gut. Gut 2023, 72, 1848–1865. [Google Scholar] [CrossRef]
- Amer, S.A.; A-Nasser, A.; Al-Khalaifah, H.S.; AlSadek, D.M.M.; Fattah, D.M.A.; Roushdy, E.M.; Sherief, W.R.I.A.; Farag, M.F.M.; Altohamy, D.E.; Abdel-Wareth, A.A.A.; et al. Effect of Dietary Medium-Chain α-Monoglycerides on the Growth Performance, Intestinal Histomorphology, Amino Acid Digestibility, and Broiler Chickens’ Blood Biochemical Parameters. Animals 2020, 11, 57. [Google Scholar] [CrossRef]
- Mo, Q.; Fu, A.; Deng, L.; Zhao, M.; Li, Y.; Zhang, H.; Feng, F. High-dose Glycerol Monolaurate Up-Regulated Beneficial Indigenous Microbiota without Inducing Metabolic Dysfunction and Systemic Inflammation: New Insights into Its Antimicrobial Potential. Nutrients 2019, 11, 1981. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, Y.; Zhang, Y.; Xu, F.; Chen, J.; Duan, L.; Zhang, T.; Wang, J.; Zhang, F. Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress. Redox Biol. 2020, 32, 101500. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Wang, Z.; Xiao, C.; Zhu, Q.; Song, Z. Glycerol Monolaurate Ameliorated Intestinal Barrier and Immunity in Broilers by Regulating Intestinal Inflammation, Antioxidant Balance, and Intestinal Microbiota. Front. Immunol. 2021, 12, 713485. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tsai, T.; Deng, F.; Wei, X.; Chai, J.; Knapp, J.; Apple, J.; Maxwell, C.V.; Lee, J.A.; Li, Y.; et al. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome 2019, 7, 109. [Google Scholar] [CrossRef]
- Peghini, P.L.; Guccion, J.G.; Sharma, A. Improvement of chronic diarrhea after treatment for intestinal spirochetosis. Dig. Dis. Sci. 2000, 45, 1006–1010. [Google Scholar] [CrossRef]
- Valeriano, V.; Balolong, M.; Kang, D.K. Probiotic roles of Lactobacillus sp. in swine: Insights from gut microbiota. J. Appl. Microbiol. 2017, 122, 554–567. [Google Scholar] [CrossRef]
- Sánchez, B.; Ruiz, L.; Gueimonde, M.; Ruas-Madiedo, P.; Margolles, A. Adaptation of bifidobacteria to the gastrointestinal tract and functional consequences. Pharmacol. Res. 2013, 69, 127–136. [Google Scholar] [CrossRef]
- Lugli, G.A.; Milani, C.; Turroni, F.; Tremblay, D.; Ferrario, C.; Mancabelli, L.; Duranti, S.; Ward, D.V.; Ossiprandi, M.C.; Moineau, S.; et al. Prophages of the genus Bifidobacterium as modulating agents of the infant gut microbiota. Environ. Microbiol. 2016, 18, 2196–2213. [Google Scholar] [CrossRef]
- Lim, H.J.; Shin, H.S. Antimicrobial and Immunomodulatory Effects of Bifidobacterium Strains: A Review. J. Microbiol. Biotechnol. 2020, 30, 1793–1800. [Google Scholar] [CrossRef]

| Ingredient | % | Nutrient Level b | |
|---|---|---|---|
| Corn | 65.06 | Digestible Energy b, MJ/kg | 16.20 |
| Soybean meal 46% | 14.00 | Crude protein, % | 19.31 |
| Extruded soybean | 6.00 | Calcium, % | 0.81 |
| Soy protein concentrate | 4.00 | Available Phosphorus, % | 0.40 |
| Fish meal | 2.00 | Lysine, % | 1.51 |
| Whey powder | 5.00 | Methionine, % | 0.43 |
| Calcium hydrogen phosphate | 1.15 | Threonine, % | 0.92 |
| Limestone | 1.00 | Tryptophan, % | 0.26 |
| Salt | 0.30 | ||
| Lysine 98.5% | 0.61 | ||
| Methionine 98.5% | 0.12 | ||
| Threonine | 0.20 | ||
| Tryptophan | 0.06 | ||
| Premix a | 0.50 | ||
| Total | 100 |
| Gene | Accession Number | Primer Sequences (5′→3′) |
|---|---|---|
| GAPDH | XM_047787748.1 | F:ACCCAGAAGACTGTGGATGG |
| R:AAGCAGGGATGATGTTCTGG | ||
| TNF-α | NM_214022.1 | F:TTGAGCATCAACCCTCTGGC |
| R:ATTGGCATACCCACTCTGCC | ||
| IL-1β | NM_001302388.2 | F:CCGCCAAGATATAACTGAC |
| R:GCAGCAACCATGTACCAA | ||
| IL-6 | NM_214399.1 | F:ACCGGTCTTGTGGAGTTTCA |
| R:GCATTTGTGGTGGGGTTAGG | ||
| IL-8 | NM_213867.1 | F:TTCCAAACTGGCTGTTGCCT |
| R:ACAGTGGGGTCCACTCTCAA | ||
| claudin-1 | NM_001244539.1 | F:GGTGACAACATTGTGACGGC |
| R:TTACCATCAAGGCACGGGTT | ||
| occludin | XM_005672525.3 | F:TAATGGGCGTCAACCCAACA |
| R:GTAGAGTCCAGTCACCGCAG | ||
| ZO-1 | XM_047766890.1 | F:GCCATCCACTCCTGCCTAT |
| R:CGGGACCTGCTCATAACTTC | ||
| 16S rRNA | 341F:CCTACGGGNGGCWGCAG | |
| 806R:GGACTACHVGGGTWTCTAAT |
| Item | CON | LPS | GML_LPS | SEM | p-Value |
|---|---|---|---|---|---|
| BW 0 d (kg) | 7.55 | 7.54 | 7.51 | 0.12 | 0.99 |
| BW 21 d (kg) | 13.90 | 13.82 | 14.27 | 0.42 | 0.91 |
| ADG (g/d) | 302 | 299 | 322 | 16.23 | 0.84 |
| ADFI (g/d) | 483 | 498 | 503 | 16.56 | 0.89 |
| G/F ratio | 1.63 | 1.70 | 1.59 | 0.04 | 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Ma, R.; Qi, R.; Liu, Z.; Li, Y.; Wu, X.; Zhao, C.; Ma, Q.; Zhan, K. Dietary Glycerol Monolaurate Effect on Growth Performance, Intestinal Barrier Function, and Gut Microbiota in Weaned Piglets Challenged by Lipopolysaccharide. Animals 2025, 15, 3263. https://doi.org/10.3390/ani15223263
Wang H, Ma R, Qi R, Liu Z, Li Y, Wu X, Zhao C, Ma Q, Zhan K. Dietary Glycerol Monolaurate Effect on Growth Performance, Intestinal Barrier Function, and Gut Microbiota in Weaned Piglets Challenged by Lipopolysaccharide. Animals. 2025; 15(22):3263. https://doi.org/10.3390/ani15223263
Chicago/Turabian StyleWang, Huakai, Ruiyu Ma, Renrong Qi, Zhen Liu, Yinghao Li, Xudong Wu, Chunfang Zhao, Qiugang Ma, and Kai Zhan. 2025. "Dietary Glycerol Monolaurate Effect on Growth Performance, Intestinal Barrier Function, and Gut Microbiota in Weaned Piglets Challenged by Lipopolysaccharide" Animals 15, no. 22: 3263. https://doi.org/10.3390/ani15223263
APA StyleWang, H., Ma, R., Qi, R., Liu, Z., Li, Y., Wu, X., Zhao, C., Ma, Q., & Zhan, K. (2025). Dietary Glycerol Monolaurate Effect on Growth Performance, Intestinal Barrier Function, and Gut Microbiota in Weaned Piglets Challenged by Lipopolysaccharide. Animals, 15(22), 3263. https://doi.org/10.3390/ani15223263







