Comparative Study on Growth Performance and Meat Production Traits of Reciprocal Crosses Between Guizhou Recessive White Chickens and Qiandongnan Xiaoxiang Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Exprimental Material
2.2. Body Weight Measurement
2.3. Measurement of Body Size and Slaughter Indicators
2.4. Meat Quality Measurement
2.5. Growth Curve Model Fitting
2.6. Statistics Analysis
- Wt1—The weight from the prior measurement in grams (g);
- Wt0—The weight from the most recent measurement in grams (g);
- t0—The age at the time of the prior measurement in days (w);
- t1—The age at the time of the most recent measurement in days (w);
3. Results and Discussion
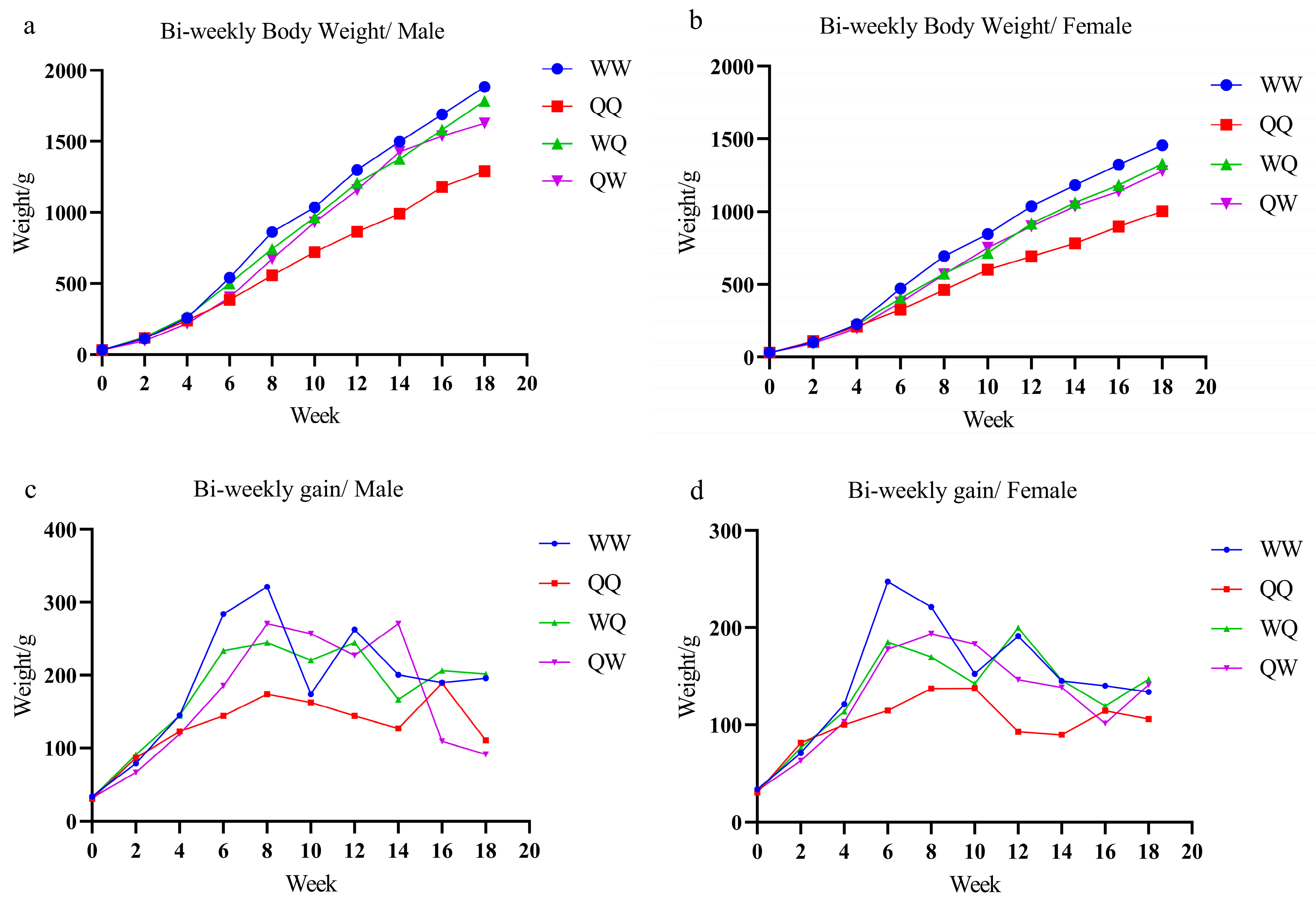
3.1. Body Weight
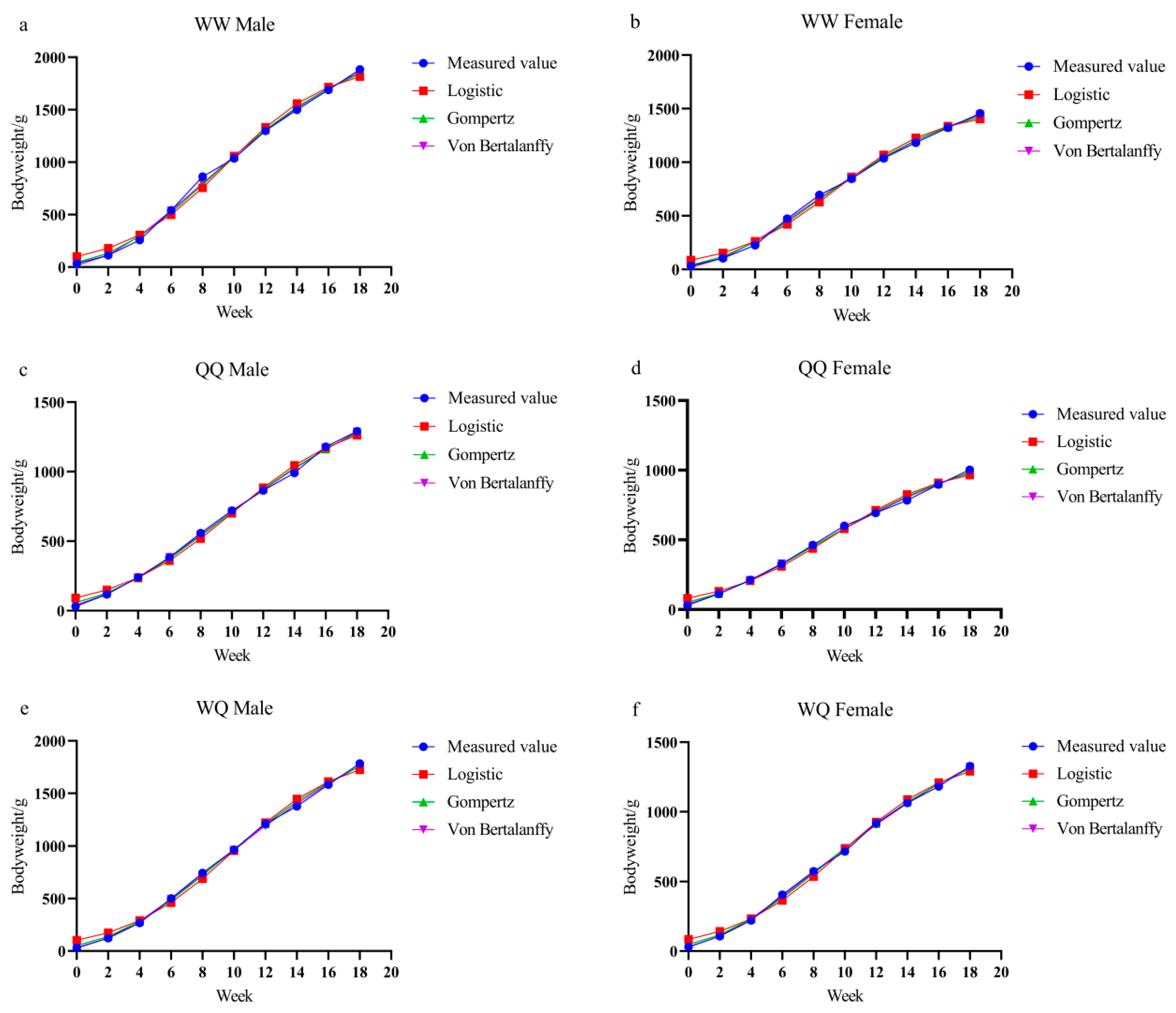
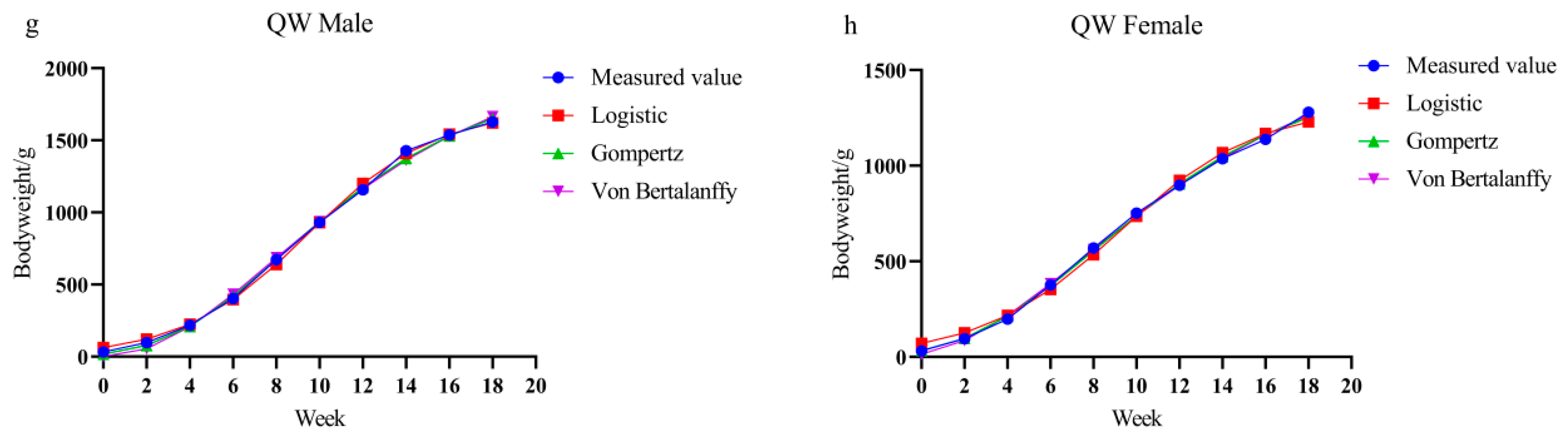
3.2. Growth Curve Modeling and Parameter Estimation
3.3. Body Size Traits
3.4. Slaughter Performance
3.5. Meat Quality
3.6. Application Strategies for Scaling the WQ Hybrid in Local Poultry Industry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mottet, A.; Tempio, G. Global poultry production: Current state and future outlook and challenges. World’s Poult. Sci. J. 2017, 73, 245–256. [Google Scholar] [CrossRef]
- Cai, K.; Liu, R.; Wei, L.; Wang, X.; Cui, H.; Luo, N.; Wen, J.; Chang, Y.; Zhao, G. Genome-wide association analysis identifies candidate genes for feed efficiency and growth traits in Wenchang chickens. BMC Genom. 2024, 25, 645. [Google Scholar] [CrossRef]
- Chumngoen, W.; Tan, F.J. Relationships between descriptive sensory attributes and physicochemical analysis of broiler and Taiwan native chicken breast meat. Asian-Australas. J. Anim. Sci. 2015, 28, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Lee, S.Y.; Park, J.Y.; Jung, S.; Jo, C.; Nam, K.C. Comparison of functional compounds and micronutrients of chicken breast meat by breeds. Food Sci. Anim. Resour. 2019, 39, 632–640. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Toppi, V.; Syiffah, L.A. comparative review on Ayam Cemani chicken—A comparison with the most common chicken species in terms of nutritional values, LCA, price and consumer acceptance. Trop. Anim. Health Prod. 2024, 56, 161. [Google Scholar] [CrossRef] [PubMed]
- Tunim, S.; Phasuk, Y.; Aggrey, S.E.; Duangjinda, M. Increasing fat deposition via upregulation of peroxisome proliferator-activated receptor gamma in native crossbred chickens. Animals 2021, 11, 90. [Google Scholar] [CrossRef]
- Bassey, O.A.; Akpan, U.; Ikeobi, C.O.N.; Adebambo, O.A.; Idowu, O. Egg production and quality assessment of Nigerian indigenous chicken genotypes and their crosses with Marshall. Niger. J. Anim. Prod. 2021, 43, 28–36. [Google Scholar] [CrossRef]
- El-Tahawy, W.S.; Habashy, W.S. Genetic effects on growth and egg production traits in two-way crosses of Egyptian and commercial layer chickens. South Afr. J. Anim. Sci. 2021, 51, 349–354. [Google Scholar] [CrossRef]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Longman: Harlow, UK, 1996. [Google Scholar]
- Ni, A.; Calus, M.P.; Bovenhuis, H.; Yuan, J.; Wang, Y.; Sun, Y.; Chen, J. Genetic parameters, reciprocal cross differences, and age-related heterosis of egg-laying performance in chickens. Genet. Sel. Evol. 2023, 55, 87. [Google Scholar] [CrossRef]
- Ahmed Soliman, M.; Hassan Khalil, M.; El-Sabrout, K.; Kamel Shebl, M. Crossing effect for improving egg production traits in chickens involving local and commercial strains. Vet. World 2020, 13, 407–412. [Google Scholar] [CrossRef]
- Barbato, G.F.; Vasilatos-Younken, R. Sex-linked and maternal effects on growth in chickens. Poult. Sci. 1991, 70, 709–718. [Google Scholar] [CrossRef]
- Atallah, J.; Lott, S.E. Evolution of maternal and zygotic mRNA complements in the early Drosophila embryo. PLoS Genet. 2018, 14, e1007838. [Google Scholar] [CrossRef]
- Bergero, R.; Gardner, J.; Bader, B.; Yong, L.; Charlesworth, D. Exaggerated heterochiasmy in a fish with sex-linked male coloration polymorphisms. Proc. Natl. Acad. Sci. USA 2019, 116, 6924–6931. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Li, H.; Yang, S.; Chen, X.; Long, S.; Yang, S.; Yang, Y.; Wang, Z. Metabolic and inflammatory linkage of the chicken cecal microbiome to growth performance. Front. Microbiol. 2023, 14, 1060458. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, N.; Kang, K.; Zhang, R.; Hao, M.; Song, P.; Wang, Q.; Xie, Y.; Li, C. Dietary guanidineacetic acid supplementation ameliorated meat quality and regulated muscle metabolism of broilers subjected to pre-slaughter transport stress by metabolomics analysis. Poult. Sci. 2022, 101, 101739. [Google Scholar] [CrossRef]
- Tian, Y.; Du, Y.; Jiang, Y.; Liu, Q.; Zhou, X.; Wu, S.; Zhao, X.; Zhang, F. Study on the Growth and Development, Meat Performance and Their Correlation Analysis of Guizhou Recessive White-feather Chickens. China Anim. Husb. Vet. Med. 2025, 52, 2208–2218. (In Chinese) [Google Scholar]
- Tian, Y. Purification of a New Strain of Guizhou Recessive White-Feather Chicken and Study on Its Hybridization Matching Effect with Local Chickens. Master’s Thesis, Guizhou University, Guiyang, China, 2025. (In Chinese). [Google Scholar]
- Li, Y. Research on the Hybridization Effect and Molecular Marker Associated with Feather/Shank Color between Taihang and Recessive White Feather Chicken. Master’s Thesis, Hebei Agricultural University, Hebei, China, 2023. (In Chinese). [Google Scholar]
- Shen, J.; Wu, Y.; Pi, J.; Pan, A.; Huang, T.; Niang, Z.; Zhang, H.; Fu, M.; Chen, Y. The breeding and hybridizing maternal line of a new line of recessive white feather chicken. Hubei Agric. Sci. 2024, 63, 143–146. (In Chinese) [Google Scholar]
- NY/T 823-2020; People’s Republic of China Agricultural Industry Standard: Terminology and Statistical Methods for Poultry Production Performance. China Poultry Industry Guide. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2020.
- Xiao, L.; Qi, L.; Fu, R.; Nie, Q.; Zhang, X.; Luo, W. A large-scale comparison of the meat quality characteristics of different chicken breeds in South China. Poult. Sci. 2024, 103, 103740. [Google Scholar] [CrossRef] [PubMed]
- Hagan, B.A.; Asumah, C.; Yeboah, E.D.; Lamptey, V.K. Modeling the growth of four commercial broiler genotypes reared in the tropics. Trop. Anim. Health Prod. 2022, 54, 75. [Google Scholar] [CrossRef]
- Dekkers, J.C.; Chakraborty, R. Optimizing purebred selection for crossbred performance using QTL with different degrees of dominance. Genet. Sel. Evol. 2004, 36, 297–324. [Google Scholar] [CrossRef]
- Yang, H.; Ni, A.; Wu, Y.; Li, Y.; Yuan, J.; Ma, H.; Zong, Y.; Han, X.; Chen, J.; Sun, Y. Research note: Differential heterosis of spent laying hens’ carcass characteristics and meat quality in reciprocal crosses between White Leghorn and Beijing-You chickens. Poult. Sci. 2024, 103, 104–198. [Google Scholar] [CrossRef] [PubMed]
- Sungkhapreecha, P.; Chankitisakul, V.; Duangjinda, M.; Boonkum, W. Combining abilities, heterosis, growth performance, and carcass characteristics in a diallel cross from black-bone chickens and Thai native chickens. Animals 2022, 12, 1602. [Google Scholar] [CrossRef]
- Li, Z.; Mushtaq, M.; Khan, M.; Fu, J.; Rahman, A.; Long, Y.; Liu, Y.; Zi, X.; Sun, D.; Ge, C.; et al. Evaluation of the growth performance and meat quality of different F1 crosses of Tengchong Snow and Xichou Black Bone chicken breeds. Animals 2024, 14, 3099. [Google Scholar] [CrossRef]
- García-González, F.; Simmons, L.W. Paternal indirect genetic effects on offspring viability and the benefits of polyandry. Curr. Biol. 2007, 17, 32–36. [Google Scholar] [CrossRef]
- Feil, R.; Khosla, S.; Cappai, P.; Loi, P. Genomic imprinting in ruminants: Allele-specific gene expression in parthenogenetic sheep. Mamm. Genome 1998, 9, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Giannoukakis, N.; Deal, C.; Paquette, J.; Goodyer, C.G.; Polychronakos, C. Parental genomic imprinting of the human IGF2 gene. Nat. Genet. 1993, 4, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, R.; Nyström, A.; Pfeifer-Ohlsson, S.; Töhönen, V.; Hedborg, F.; Schofield, P.; Flam, F.; Ekström, T.J. IGF2 is parentally imprinted during human embryogenesis and in the Beckwith-Wiedemann syndrome. Nat. Genet. 1993, 4, 94–97. [Google Scholar] [CrossRef]
- Van Kaam, J.B.C.H.M.; Van Arendonk, J.A.M.; Groenen, M.A.M.; Bovenhuis, H.; Vereijken, A.L.J.; Crooijmans, R.P.M.A.; Van der Poel, J.J.; Veenendaal, A. Whole genome scan for quantitative trait loci affecting body weight in chickens using a three-generation design. Livest. Prod. Sci. 1998, 54, 133–150. [Google Scholar] [CrossRef]
- Jacobsson, L.; Park, H.B.; Wahlberg, P.; Fredriksson, R.; Perez-Enciso, M.; Siegel, P.B.; Andersson, L. Many QTLs with minor additive effects are associated with a large difference in growth between two selection lines in chickens. Genet. Res. 2005, 86, 115–125. [Google Scholar] [CrossRef]
- Wahlberg, P.; Carlborg, O.; Foglio, M.; Tordoir, X.; Syvänen, A.C.; Lathrop, M.; Gut, I.G.; Siegel, P.B.; Andersson, L. Genetic analysis of an F2 intercross between two chicken lines divergently selected for body-weight. BMC Genom. 2009, 10, 248. [Google Scholar] [CrossRef]
- Van Laere, A.S.; Nguyen, M.; Braunschweig, M.; Nezer, C.; Collette, C.; Moreau, L.; Archibald, A.L.; Haley, C.S.; Buys, N.; Tally, M.; et al. A regulatory mutation in IGF2 causes a major QTL effect on muscle growth in the pig. Nature 2003, 425, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Tortereau, F.; Gilbert, H.; Heuven, H.C.; Bidanel, J.P.; Groenen, M.A.; Riquet, J. Number and mode of inheritance of QTL influencing backfat thickness on SSC2p in Sino-European pig pedigrees. Genet. Sel. Evol. 2011, 43, 11. [Google Scholar] [CrossRef]
- Imumorin, I.G.; Kim, E.H.; Lee, Y.M.; De Koning, D.J.; van Arendonk, J.A.; De Donato, M.; Taylor, J.F.; Kim, J.J. Genome scan for parent-of-origin QTL effects on bovine growth and carcass traits. Front. Genet. 2011, 2, 44. [Google Scholar] [CrossRef]
- Li, L.L.; Keverne, E.B.; Aparicio, S.A.; Ishino, F.; Barton, S.C.; Surani, M.A. Regulation of maternal behavior and offspring growth by paternally expressed Peg3. Science 1999, 284, 330–334. [Google Scholar] [CrossRef]
- Nie, Q.; Sun, B.; Zhang, D.; Luo, C.; Ishag, N.A.; Lei, M.; Yang, G.; Zhang, X. High diversity of the chicken growth hormone gene and effects on growth and carcass traits. J. Hered. 2005, 96, 698–703. [Google Scholar] [CrossRef]
- Vasilatos-Younken, R.; Wang, X.H.; Zhou, Y.; Day, J.R.; McMurtry, J.P.; Rosebrough, R.W.; Decuypere, E.; Buys, N.; Darras, V.; Beard, J.L.; et al. New insights into the mechanism and actions of growth hormone (GH) in poultry. Domest. Anim. Endocrinol. 1999, 17, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.G.; Clayton, P.E. Disorders of growth hormone in childhood. In Endotext; Feingold, K.R., Boyce, A.M., Koch, C., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2022. [Google Scholar]
- Martinez, M.E.; Duarte, C.W.; Stohn, J.P.; Karaczyn, A.; Wu, Z.; DeMambro, V.E.; Hernandez, A. Thyroid hormone influences brain gene expression programs and behaviors in later generations by altering germ line epigenetic information. Mol. Psychiatry 2020, 25, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.E.; Wu, Z.; Hernandez, A. Paternal developmental thyrotoxicosis disrupts neonatal leptin leading to increased adiposity and altered physiology of the melanocortin system. Front. Endocrinol. 2023, 14, 1210414. [Google Scholar] [CrossRef]
- Manjula, P.; Park, H.B.; Seo, D.; Choi, N.; Jin, S.; Ahn, S.J.; Heo, K.N.; Kang, B.S.; Lee, J.H. Estimation of heritability and genetic correlation of body weight gain and growth curve parameters in Korean native chicken. Asian-Australas. J. Anim. Sci. 2017, 31, 26–34. [Google Scholar] [CrossRef]
- Tutkun, M. Comparison of Nonlinear Growth Models of Native Chickens (Gallus gallus domesticus L.; Atak-S) Reared in Organic Production. Appl. Ecol. Environ. Res. 2025, 23, 7123–7135. [Google Scholar] [CrossRef]
- Xie, W.; Pan, N.; Zeng, H.; Yan, H.; Wang, X.; Gao, C. Comparison of nonlinear models to describe the feather growth and development curve in yellow-feathered chickens. Animal 2020, 14, 1005–1013. [Google Scholar] [CrossRef]
- Setiaji, A.; Lestari, D.A.; Pandupuspitasari, N.S.; Agusetyaningsih, I.; Sutopo, S.; Tamaningrum, T.; Philco, S. Growth curve evaluation for Indonesian indigenous Red Kedu chicken by using non-linear models. J. Livest. Sci. Technol. 2026, 14, 1–7. [Google Scholar]
- Galeano-Vasco, L.F.; Cerón-Muñoz, M.F.; Narváez-Solarte, W. Ability of non-linear mixed models to predict growth in laying hens. R. Bras. Zootec. 2014, 43, 573–578. [Google Scholar] [CrossRef]
- Ercanli, I.; Gunlu, A.; Başkent, E.Z. Mixed effect models for predicting breast height diameter from stump diameter of Oriental beech in Göldağ. Sci. Agric. 2015, 72, 245–251. [Google Scholar] [CrossRef]
- Stamps, J.A. Sexual size dimorphism in species with asymptotic growth after maturity. Biol. J. Linn. Soc. 1993, 50, 123–145. [Google Scholar] [CrossRef]
- Sinha-Hikim, I.; Roth, S.M.; Lee, M.I.; Bhasin, S. Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am. J. Physiol.-Endocrinol. Metab. 2003, 285, E197–E205. [Google Scholar] [CrossRef]
- Cao, J.; Liu, W.; Wang, Z.; Xie, D.; Jia, L.; Chen, Y. Green and blue monochromatic lights promote growth and development of broilers via stimulating testosterone secretion and myofiber growth. J. Appl. Poult. Res. 2008, 17, 211–218. [Google Scholar] [CrossRef]
- Ali Decuypere, E.; Buyse, J. Endocrine control of postnatal growth in poultry. J. Poult. Sci. 2005, 42, 1–13. [Google Scholar] [CrossRef]
- Isaac, U.C.; Ezejesi, H.C. Genotype impact on body weight and linear body measurements of main and reciprocal crosses of Isa Brown and native chickens. Niger. J. Anim. Prod. 2023, 50, 1–9. [Google Scholar]
- Haunshi, S.; Ullengala, R.; Prince, L.L.; Ramasamy, K.; Gurunathan, K.; Devatkal, S.; Chatterjee, R.N. Genetic parameters of growth traits, trend of production and reproduction traits, and meat quality status of Ghagus, an indigenous chicken of India. Trop. Anim. Health Prod. 2022, 54, 170. [Google Scholar] [CrossRef]
- Moto, E.; Rubanza, C.D. Phenotypic characterisation of indigenous chicken in the central zone of Tanzania. Open Agric. 2023, 8, 20220218. [Google Scholar] [CrossRef]
- Wahlberg Johnson, P.A.; Giles, J.R. The hen as a model of ovarian cancer. Nat. Rev. Cancer 2013, 13, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Hocking, P.M. Relationships between egg size, body weight and pelvic dimensions in turkeys. Anim. Sci. 1993, 56, 145–150. [Google Scholar] [CrossRef]
- Baéza, E.; Arnould, C.; Jlali, M.; Chartrin, P.; Gigaud, V.; Mercerand, F.; Durand, C.; Méteau, K.; Le Bihan-Duval, E.; Berri, C. Influence of increasing slaughter age of chickens on meat quality, welfare, and technical and economic results. J. Anim. Sci. 2012, 90, 2003–2013. [Google Scholar] [CrossRef]
- Mebratie, W.S. The Genetics of Body Weight and Feed Efficiency in Broiler Chickens. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 2019. [Google Scholar]
- Wolf, J.B.; Wade, M.J. What are maternal effects (and what are they not)? Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1107–1115. [Google Scholar] [CrossRef]
- Katemala, S.; Molee, A.; Thumanu, K.; Yongsawatdigul, J. A comparative study of meat quality and vibrational spectroscopic properties of different chicken breeds. Poult. Sci. 2022, 101, 101829. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.S.; England, E.M. Postmortem glycolysis and glycogenolysis: Insights from species comparisons. Meat Sci. 2018, 144, 118–126. [Google Scholar] [CrossRef]
- Wolcott, M.L.; Johnston, D.J.; Barwick, S.A.; Iker, C.L.; Thompson, J.M.; Burrow, H.M. Genetics of meat quality and carcass traits and the impact of tenderstretching in two tropical beef genotypes. Anim. Prod. Sci. 2009, 49, 383–398. [Google Scholar] [CrossRef]
- Zielbauer, B.I.; Franz, J.; Viezens, B.; Vilgis, T.A. Physical aspects of meat cooking: Time dependent thermal protein denaturation and water loss. Food Biophys. 2016, 11, 34–42. [Google Scholar] [CrossRef]

| Measurement Index | Measurement Method |
|---|---|
| Body Slope Length (BSL)/cm | Measured as the distance from the shoulder joint to the sciatic tuberosity using a tape measure. |
| Keel Length (KL)/cm | Measured as the distance from the anterior tip to the posterior end of the keel using a tape measure. |
| Chest Depth (CD)/mm | Measured as the vertical distance from the first thoracic vertebra to the anterior edge of the keel using calipers. |
| Chest Width (CW)/mm | Measured as the distance between the left and right shoulder joints using calipers. |
| Pelvis Width (PW)/mm | Measured as the distance between the left and right iliac tuberosities using calipers. |
| Shank Length (SL)/mm | Measured as the distance from the hock joint to the tip of the third toe using calipers. |
| Shin Girth (SG)/cm | Measured as the circumference at the midpoint of the shank using a tape measure. |
| Live Weight Before Slaughter (LW)/g | Live weight measured after a 12 h fast prior to slaughter. |
| Dressed Weight (DW)/g | Weight after exsanguination, defeathering, and removal of claw sheaths and beak sheaths. |
| Half-eviscerated Weight with Giblet (HEW)/g | Carcass weight after removal of the trachea, esophagus, crop, gastrointestinal tract, spleen, pancreas, gallbladder, reproductive organs, and stomach contents. |
| Eviscerated Weight (EW)/g | Calculated as half-eviscerated weight with giblets minus the heart, liver, proventriculus, gizzard, lungs, abdominal fat, head, and feet. |
| Breast Muscle Weight (BMW)/g | Weight of the pectoral muscles completely removed from both sides of the keel using a scalpel. |
| Leg Muscle Weight (LMW)/g | Weight of thigh and drumstick muscles after removal of skin, bones, and fat. |
| Dressed Percentage (DP) | (DW/LW) × 100% |
| Percentage Half-eviscerated Yield with Giblet (PHE) | (HEW/LW) × 100% |
| Percentage of Eviscerated Yield (PE) | (EW/LW) × 100% |
| Percentage of Breast Muscle Yield (PBM) | (BMW/EW) × 100% |
| Percentage of Leg Muscle Yield (PLM) | (LMW/EW) × 100% |
| Models | Formula | Inflection Point Weight | Inflection Point Weekly Age |
|---|---|---|---|
| Logistic | Y = A/(1 + Be−kt) | A/2 | (ln B)/k |
| Gompertz | Y = Ae −Bexp(−kt) | A/e | (ln B)/k |
| VonBertalanffy | Y = A (1 − Be−kt)3 | 8A/27 | (ln 3B)/k |
| Week | Gender | Body Weight/g | H%(WQ)/95%CI | H%(QW)/95%CI | |||
|---|---|---|---|---|---|---|---|
| WW | WQ | QW | |||||
| 0 | Male | 33.68 ± 2.47 a | 30.47 ± 2.8 b | 31.68 ± 3.37 b | 31.77 ± 2.22 b | 1.23 (−2.92%, 0.46%) | −0.95 (−2.34%, 0.44%) |
| Female | 33.26 ± 2.33 a | 30.19 ± 2.65 c | 30.39 ± 3.19 bc | 32.11 ± 3.12 ab | 4.21 (−6.21%, −2.21%) | 1.21 (−0.71%, 3.13%) | |
| 2 | Male | 112.6 ± 18.53 b | 116.88 ± 12.64 ab | 122.42 ± 14.64 a | 98.29 ± 13.36 c | 6.69 (4.49%, 8.89%) | −14.34 (−16.60%, −12.08%) |
| Female | 104.22 ± 15.34 b | 111.41 ± 14.36 a | 106.52 ± 15.8 ab | 95.06 ± 15.69 c | −1.20 (−4.65%, 2.25%) | −11.83 (−15.26%, −8.40%) | |
| 4 | Male | 257.48 ± 43.81 a | 239.6 ± 24.17 b | 266.81 ± 33.94 a | 217.36 ± 38.77 b | 7.35 (3.65%, 11.05%) | −12.55 (−16.43%, −8.67%) |
| Female | 225.3 ± 36.12 a | 211.48 ± 28.8 ab | 220.24 ± 34.39 a | 197.89 ± 23.31 b | 0.85 (−2.42%, 4.12%) | −9.39 (−12.29%, −6.49%) | |
| 6 | Male | 541.15 ± 69.73 a | 383.64 ± 45.18 c | 500.23 ± 62.32 b | 402.44 ± 82.83 c | 8.18 (4.70%, 11.66%) | −12.97 (−17.28%, −8.66%) |
| Female | 472.56 ± 64.67 a | 326.07 ± 51.71 d | 405.28 ± 52.09 b | 375.48 ± 32.03 c | 1.49 (−1.21%, 4.19%) | −5.97 (−8.40%, −3.54%) | |
| 8 | Male | 862.23 ± 80.78 a | 557.55 ± 54.54 d | 744.7 ± 97.7 b | 673.08 ± 91.8 c | 4.90 (1.72%, 8.08%) | −5.19 (−8.26%, −2.10%) |
| Female | 693.56 ± 76.18 a | 463.07 ± 60.96 c | 574.78 ± 85.56 b | 569.08 ± 68.46 b | −0.61 (−3.71%, 2.49%) | −1.60 (−4.40%, 1.20%) | |
| 10 | Male | 1036.01 ± 107.29 a | 720.15 ± 81.78 c | 965.17 ± 132.84 b | 930.07 ± 110.53 b | 9.92 (6.33%, 13.51%) | 5.92 (2.71%, 9.13%) |
| Female | 845.84 ± 76.33 a | 600.49 ± 74.63 c | 717.08 ± 90.17 b | 752.1 ± 94.88 b | −0.84 (−3.82%, 2.14%) | 4.00 (0.89%, 7.09%) | |
| 12 | Male | 1298.66 ± 130.69 a | 864.16 ± 97.49 c | 1209.83 ± 143.61 b | 1157.09 ± 126.81 b | 11.88 (8.26%, 15.50%) | 7.00 (3.63%, 10.37%) |
| Female | 1037.2 ± 93.15 a | 693.29 ± 75.27 c | 916.87 ± 109.57 b | 898.49 ± 108.21 b | 5.97 (2.96%, 8.98%) | 3.84 (0.84%, 6.84%) | |
| 14 | Male | 1499.21 ± 165.7 a | 990.84 ± 110.45 c | 1376.42 ± 135.55 b | 1427.75 ± 154.47 ab | 10.55 (7.92%, 13.20%) | 14.68 (11.78%, 17.58%) |
| Female | 1182.21 ± 122.85 a | 782.95 ± 84.68 c | 1062.63 ± 124.77 b | 1036.98 ± 134.77 b | 8.15 (5.35%, 10.95%) | 5.54 (2.58%, 8.50%) | |
| 16 | Male | 1689.1 ± 203.12 a | 1179.8 ± 120.44 c | 1582.6 ± 181.64 b | 1536.85 ± 169.58 b | 10.33 (6.99%, 13.67%) | 7.14 (3.94%, 10.34%) |
| Female | 1322.24 ± 149.78 a | 897.27 ± 93.76 c | 1181.68 ± 141.11 b | 1138.41 ± 151.64 b | 6.48 (3.54%, 9.42%) | 2.58 (−0.52%, 5.68%) | |
| 18 | Male | 1884.89 ± 219.67 a | 1290.33 ± 128.98 d | 1784.1 ± 182.07 b | 1627.94 ± 178.41 c | 12.38 (9.15%, 15.61%) | 2.54 (−0.66%, 5.74%) |
| Female | 1455.96 ± 147.47 a | 1003.24 ± 101.76 c | 1328.57 ± 154.9 b | 1279.41 ± 183.01 b | 8.05 (5.04%, 11.04%) | 4.05 (0.67%, 7.43%) | |
| Gender | Model | Parameter Estimates | Inflection Point Weight/g | Inflection Point Weekly Age | |||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | K | R2 | AIC | BIC | ||||
| WW Male | Logistic | 1950.874 | 18.255 | 0.306 | 0.991 | 117.9 | 119.1 | 975.44 | 9.49 |
| Gompertz | 2258.256 | 3.949 | 0.165 | 0.997 | 106.4 | 107.7 | 830.85 | 8.32 | |
| Von Bertalanffy | 2547.859 | 0.817 | 0.117 | 0.998 | 102.5 | 103.7 | 754.92 | 7.66 | |
| WW Female | Logistic | 1491.06 | 16.217 | 0.309 | 0.992 | 112.0 | 113.2 | 745.53 | 9.02 |
| Gompertz | 1697.253 | 3.749 | 0.171 | 0.998 | 99.4 | 100.6 | 624.45 | 7.73 | |
| Von Bertalanffy | 1884.251 | 0.792 | 0.124 | 0.999 | 95.1 | 96.3 | 558.30 | 6.98 | |
| QQ Male | Logistic | 1417.718 | 14.355 | 0.264 | 0.993 | 107.0 | 108.2 | 708.86 | 10.09 |
| Gompertz | 1736.208 | 3.425 | 0.134 | 0.998 | 94.4 | 95.6 | 638.78 | 9.19 | |
| Von Bertalanffy | 2079.531 | 0.734 | 0.089 | 0.999 | 87.6 | 88.8 | 616.16 | 8.87 | |
| QQ Female | Logistic | 1059.915 | 12.1 | 0.268 | 0.992 | 103.3 | 104.5 | 529.96 | 9.30 |
| Gompertz | 1255.005 | 3.18 | 0.142 | 0.998 | 91.6 | 92.8 | 461.74 | 8.15 | |
| Von Bertalanffy | 1446.659 | 0.701 | 0.098 | 0.999 | 85.6 | 86.8 | 428.64 | 7.59 | |
| WQ Male | Logistic | 1893.218 | 17.486 | 0.288 | 0.994 | 113.4 | 114.7 | 946.61 | 9.94 |
| Gompertz | 2273.604 | 3.782 | 0.149 | 0.999 | 98.4 | 99.7 | 836.50 | 8.93 | |
| Von Bertalanffy | 2669.429 | 0.784 | 0.1 | 0.999 | 91.0 | 92.2 | 790.94 | 8.55 | |
| WQ Female | Logistic | 1415.121 | 15.782 | 0.283 | 0.994 | 106.2 | 107.4 | 707.56 | 9.75 |
| Gompertz | 1692.523 | 3.593 | 0.147 | 0.999 | 91.8 | 93.0 | 622.71 | 8.70 | |
| Von Bertalanffy | 1976.638 | 0.759 | 0.1 | 0.999 | 86.0 | 87.2 | 585.67 | 8.23 | |
| QW Male | Logistic | 1706.714 | 26.144 | 0.344 | 0.998 | 98.7 | 99.9 | 853.36 | 9.49 |
| Gompertz | 1951.742 | 4.678 | 0.185 | 0.999 | 97.8 | 99.0 | 718.08 | 8.34 | |
| Von Bertalanffy | 2167.389 | 0.923 | 0.133 | 0.997 | 105.3 | 106.5 | 642.19 | 7.66 | |
| QW Female | Logistic | 1308.517 | 17.465 | 0.311 | 0.995 | 105.3 | 106.5 | 654.26 | 9.20 |
| Gompertz | 1510.082 | 3.837 | 0.168 | 0.999 | 89.8 | 91.0 | 555.59 | 8.00 | |
| Von Bertalanffy | 1699.367 | 0.799 | 0.119 | 0.999 | 88.9 | 90.1 | 503.52 | 7.35 | |
| Body-Meter Index/cm | Gender | WW | WQ | QW | H%(WQ)/95%CI | H%(QW) 95%CI | |
|---|---|---|---|---|---|---|---|
| Body Slope Length | Male | 20.81 ± 0.7 a | 16.09 ± 0.87 d | 19.76 ± 0.97 b | 18.33 ± 1.23 c | 7.10 (2.35%, 11.85%) | −0.65 (−6.89%, 5.59%) |
| Female | 18.29 ± 0.86 a | 14.7 ± 0.87 d | 17.25 ± 0.82 b | 16.2 ± 0.77 c | 4.29 (0.15%, 8.43%) | −1.51 (−6.18%, 3.16%) | |
| Keel Length | Male | 10.87 ± 0.58 a | 10.01 ± 0.55 b | 10.25 ± 0.9 b | 8.84 ± 0.7 c | −1.82 (−9.97%, 6.33%) | −15.33 (−21.98%, −8.68%) |
| Female | 9.45 ± 0.46 a | 9.8 ± 0.72 a | 8.75 ± 0.61 b | 8.3 ± 0.72 b | −9.47 (−15.82%, −3.12%) | −13.71 (−20.15%, −7.27%) | |
| Chest Width | Male | 7.76 ± 0.67 a | 6.43 ± 0.41 b | 7.89 ± 0.56 a | 8.13 ± 0.67 a | 11.21 (5.87%, 16.55%) | 14.59 (8.21%, 20.97%) |
| Female | 7.18 ± 0.47 a | 6.2 ± 0.41 c | 6.58 ± 0.53 bc | 6.9 ± 0.72 ab | −1.72 (−8.05%, 4.61% | 4.11 (−2.63%, 10.85%) | |
| Chest Depth | Male | 10.59 ± 0.53 a | 8.37 ± 0.58 c | 10.4 ± 0.71 a | 9.62 ± 0.43 b | 9.70 (3.15%, 16.25%) | 1.48 (−2.41%, 5.37%) |
| Female | 9.18 ± 0.53 a | 8.4 ± 0.5 b | 9.11 ± 0.42 a | 8.6 ± 0.53 b | 3.41 (−0.98%, 7.80%) | −2.38 (−7.95%, 3.19%) | |
| Pelvis Width | Male | 10.27 ± 0.57 a | 6.97 ± 0.57 c | 9.68 ± 0.52 b | 9.82 ± 0.72 ab | 12.30 (6.98%, 17.62%) | 13.92 (7.23%, 20.61%) |
| Female | 8.72 ± 0.67 a | 6.9 ± 0.48 b | 8.82 ± 0.6 a | 8.4 ± 0.63 a | 12.43 (7.21%, 17.65%) | 7.33 (1.68%, 12.98%) | |
| Shank Length | Male | 11.22 ± 0.58 a | 8.76 ± 0.5 c | 10.34 ± 0.61 b | 10.29 ± 0.63 b | 3.50 (−2.13%, 9.13%) | 3.00 (−2.94%, 8.94%) |
| Female | 9.32 ± 0.69 a | 7.1 ± 0.49 c | 8.76 ± 0.7 b | 8.8 ± 0.67 b | 6.63 (1.52%, 11.74%) | 7.61 (2.13%, 13.09%) | |
| Shin Girth | Male | 4.1 ± 0.21 a | 3.63 ± 0.18 b | 3.75 ± 0.25 b | 3.02 ± 0.22 c | −2.98 (−6.37%, 0.41%) | −21.86 (−25.19%, −18.53%) |
| Female | 3.57 ± 0.21 a | 3.3 ± 0.22 b | 3.3 ± 0.24 b | 2.7 ± 0.16 c | −4.76 (−9.23%, −0.29%) | −19.77 (−23.45%, −16.09%) |
| Slaughtering Indicators | Gender | WW | WQ | QW | H%(WQ) | H%(QW) | |
|---|---|---|---|---|---|---|---|
| Live Weight Before/g Slaughter/g | Male | 1894.6 ± 211.22 a | 1162.2 ± 101.36 c | 1743.7 ± 220.48 ab | 1658.97 ± 185.36 b | 14.09 (6.45%, 21.73%) | 8.54 (1.82%, 15.26%) |
| Female | 1415.6 ± 135.1 a | 1052.55 ± 96.21 b | 1299.8 ± 151.25 a | 1279.41 ± 183.01 a | 5.33 (−1.28%, 11.94%) | 3.67 (−3.81%, 11.15%) | |
| Dressed Weight/g | Male | 1605.3 ± 202.62 a | 1027.2 ± 101.36 c | 1491.5 ± 200.73 ab | 1433.85 ± 173.54 b | 13.31 (5.83%, 20.79%) | 8.93 (2.27%, 15.59%) |
| Female | 1214.7 ± 118.1 a | 903.61 ± 105.83 b | 1120.0 ± 137.99 a | 1110.20 ± 169.66 a | 5.75 (−0.71%, 12.21%) | 4.82 (−1.53%, 11.17%) | |
| Half-eviscerated Weight with Giblet/g | Male | 1411.4 ± 190.1 a | 821.56 ± 81.09 b | 1309.45 ± 179 a | 1292.46 ± 156.29 a | 17.28 (9.51%, 25.05%) | 15.76 (7.92%, 23.60%) |
| Female | 1077.9 ± 111.41 a | 730.67 ± 86.75 c | 961.15 ± 138.98 b | 963.13 ± 146.37 b | 6.29 (−0.18%, 12.76%) | 6.50 (−0.05%, 13.05%) | |
| Eviscerated Weight/g | Male | 1159.5 ± 167.41 a | 725.47 ± 71.35 c | 1080.3 ± 152.87 ab | 1039.73 ± 136.07 b | 14.62 (7.15%, 22.09%) | 10.31 (3.02%, 17.60%) |
| Female | 887.63 ± 93.55 a | 645.48 ± 76.34 c | 790.45 ± 112.2 b | 770.74 ± 108.23 b | 3.12 (−3.35%, 9.59%) | 0.55 (−5.80%, 6.90%) | |
| Breast Muscle Weight/g | Male | 179.54 ± 38.75 a | 116.28 ± 10.87 c | 176.1 ± 26.88 a | 149.51 ± 22.1 b | 19.06 (10.82%, 27.30%) | 1.08 (−6.89%, 9.05%) |
| Female | 151.83 ± 24.77 a | 120.26 ± 6.72 c | 138.50 ± 22.07 ab | 128.70 ± 23.62 bc | 1.80 (−5.12%, 8.72%) | −5.40 (−12.09%, 1.29%) | |
| Leg Muscle Weight/g | Male | 309.20 ± 56.89 a | 154.36 ± 12.17 b | 302.95 ± 48.85 a | 282.6 ± 41.03 a | 30.71 (21.35%, 40.07%) | 21.93 (13.02%, 30.84%) |
| Female | 221.85 ± 28.89 a | 115.88 ± 11.12 c | 195.20 ± 30.79 b | 181.31 ± 28.17 b | 15.60 (7.52%, 23.68%) | 7.37 (−0.28%, 15.02%) | |
| Dressed Percentage% | Male | 84.6 ± 2.72 c | 88.3 ± 0.99 a | 85.46 ± 2.38 bc | 86.35 ± 1.59 b | −1.16 (−3.87%, 1.55%) | −0.13 (−2.81%, 2.55%) |
| Female | 85.8 ± 1.34 | 85.66 ± 2.2 | 86.1 ± 2.51 | 86.65 ± 1.61 | 0.48 (−2.01%, 2.97%) | 1.07 (−1.40%, 3.54%) | |
| Percentage Half-eviscerated Yield with Giblet% | Male | 74.3 ± 3.15 b | 70.6 ± 0.79 c | 75.02 ± 2.12 b | 77.88 ± 3.14 a | 3.49 (0.98%, 6.00%) | 7.44 (4.87%, 10.01%) |
| Female | 76.1 ± 2.94 a | 69.26 ± 1.89 b | 73.8 ± 4.5 a | 75.20 ± 2.81 a | 1.55 (−0.88%, 3.98%) | 3.44 (1.05%, 5.83%) | |
| Percentage of Eviscerated Yield% | Male | 61.0 ± 3.05 | 62.3 ± 0.68 | 61.86 ± 2.07 | 62.6 ± 3.21 | 0.25 (−2.17%, 2.67%) | 1.45 (−0.94%, 3.84%) |
| Female | 62.6 ± 1.76 a | 61.18 ± 1.64 ab | 60.7 ± 3.35 ab | 60.34 ± 3.55 b | −1.95 (−4.28%, 0.38%) | −2.55 (−4.85%, −0.25%) | |
| Percentage of Breast Muscle Yield% | Male | 15.3 ± 1.94 ab | 16.1 ± 2.01 a | 16.3 ± 0.91 a | 14.39 ± 1.27 b | 3.36 (0.92%, 5.80%) | −8.75 (−11.06%, −6.44%) |
| Female | 17.0 ± 1.64 b | 18.82 ± 1.93 a | 17.5 ± 1.62 ab | 16.64 ± 1.39 b | −2.15 (−4.43%, 0.13%) | −7.22 (−9.47%, −4.97%) | |
| Percentage of Leg Muscle Yield% | Male | 26.6 ± 2.53 a | 21.3 ± 0.4 b | 28 ± 1.46 a | 27.2 ± 2.18 a | 16.89 (14.02%, 19.76%) | 13.55 (10.75%, 16.35%) |
| Female | 24.9 ± 1.88 a | 18.00 ± 0.4 b | 24.7 ± 1.84 a | 23.59 ± 2.47 a | 14.96 (12.28%, 17.64%) | 9.75 (7.12%, 12.38%) |
| Meat Quality Indicators | Gender | Position | WW | WQ | QW | |
|---|---|---|---|---|---|---|
| pH (45 min) | Male | Chest | 6.29 ± 0.22 a | 5.75 ± 0.16 b | 5.82 ± 0.16 b | 5.36 ± 0.42 c |
| Leg | 6.36 ± 0.26 a | 6.11 ± 0.22 a | 6.12 ± 0.24 a | 5.59 ± 0.42 b | ||
| Female | Chest | 6.37 ± 0.27 a | 5.79 ± 0.16 c | 6.1 ± 0.27 b | 5.56 ± 0.36 c | |
| Leg | 6.39 ± 0.28 a | 6.1 ± 0.21 a | 6.15 ± 0.26 a | 5.68 ± 0.4 b | ||
| Shear force/N | Male | Chest | 18.91 ± 4.8 a | 16.56 ± 2.91 ab | 15.93 ± 6.27 ab | 12.85 ± 5.33 b |
| Leg | 33.03 ± 9.87 a | 16.12 ± 2.14 b | 32.42 ± 12.23 a | 26.45 ± 7.59 a | ||
| Female | Chest | 19.00 ± 4.57 a | 16.08 ± 2.34 ab | 14.22 ± 6.4 ab | 13.58 ± 5.4 b | |
| Leg | 27.79 ± 6.81 a | 18.19 ± 1.92 b | 21.12 ± 9.91 ab | 24.27 ± 6.21 ab | ||
| Water loss% | Male | Chest | 19.00 ± 4.17 bc | 16.18 ± 3.2 c | 23.00 ± 5.01 a | 21.22 ± 2.42 ab |
| Leg | 19.33 ± 3.87 b | 15.77 ± 3.49 b | 22.89 ± 3.89 a | 17.46 ± 3.6 b | ||
| Female | Chest | 20.41 ± 5.27 | 20.02 ± 5.84 | 24.51 ± 5.68 | 22.97 ± 2.32 | |
| Leg | 17.44 ± 3.41 | 19.68 ± 4.17 | 20.44 ± 4.18 | 17.92 ± 3.63 | ||
| Cooking loss% | Male | Chest | 37.09 ± 2.69 a | 36 ± 2.53 a | 37.06 ± 2.58 a | 28.71 ± 2.5 b |
| Leg | 41.74 ± 3.72 a | 43.41 ± 2.96 a | 43.05 ± 2.53 a | 33.06 ± 4.23 b | ||
| Female | Chest | 37.03 ± 2.05 a | 34.98 ± 4.94 a | 36.93 ± 3.17 a | 29.49 ± 3.31 b | |
| Leg | 40.15 ± 3.25 a | 40.31 ± 4.53 a | 42.58 ± 3.83 a | 33.89 ± 3.01 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Wang, X.; Yue, Y.; Arif, M.; Jiang, Y.; Liu, Q.; Du, Y.; Zhao, X.; Zhang, F. Comparative Study on Growth Performance and Meat Production Traits of Reciprocal Crosses Between Guizhou Recessive White Chickens and Qiandongnan Xiaoxiang Chickens. Animals 2025, 15, 3262. https://doi.org/10.3390/ani15223262
Tian Y, Wang X, Yue Y, Arif M, Jiang Y, Liu Q, Du Y, Zhao X, Zhang F. Comparative Study on Growth Performance and Meat Production Traits of Reciprocal Crosses Between Guizhou Recessive White Chickens and Qiandongnan Xiaoxiang Chickens. Animals. 2025; 15(22):3262. https://doi.org/10.3390/ani15223262
Chicago/Turabian StyleTian, Yingping, Xiaoya Wang, Yong Yue, Muhammad Arif, Yaozhou Jiang, Qinsong Liu, Yun Du, Xudong Zhao, and Fuping Zhang. 2025. "Comparative Study on Growth Performance and Meat Production Traits of Reciprocal Crosses Between Guizhou Recessive White Chickens and Qiandongnan Xiaoxiang Chickens" Animals 15, no. 22: 3262. https://doi.org/10.3390/ani15223262
APA StyleTian, Y., Wang, X., Yue, Y., Arif, M., Jiang, Y., Liu, Q., Du, Y., Zhao, X., & Zhang, F. (2025). Comparative Study on Growth Performance and Meat Production Traits of Reciprocal Crosses Between Guizhou Recessive White Chickens and Qiandongnan Xiaoxiang Chickens. Animals, 15(22), 3262. https://doi.org/10.3390/ani15223262







