Simple Summary
In Central Europe, lizards are frequent hosts for immature stages of the Ixodes ricinus tick, the principal vector of Lyme borreliosis (LB). This is the most common tick-borne disease and is caused by several species of bacteria from the Borrelia burgdorferi sensu lato (s.l.) complex. Of the 14 genospecies identified in I. ricinus, three account for the majority of LB cases in Europe: B. afzelii, B. garinii and B. burgdorferi sensu stricto. Each of these human-pathogenic species is associated with a particular vertebrate group acting as its natural reservoir. Borrelia afzelii depends on rodents, B. garinii depends on birds, whereas B. burgdorferi s.s. can use both birds and rodents. Certain lizard species are proven reservoir hosts of B. lusitaniae, which is implicated as a potential pathogen in humans. This study aimed to evaluate the prevalence of B. burgdorferi s.l. in sand lizards and common lizards, as well as in their I. ricinus ticks, in suburban areas of western Poland. Our results confirmed that the sand lizard but not the common lizard, can act as a competent reservoir for B. lusitaniae. Furthermore, we suggest for the first time that these two lizard species could be another group of reservoir hosts for the human pathogen B. burgdorferi s.s., alongside birds and rodents.
Abstract
This study was conducted to assess the involvement of two lizard species: the sand lizard (Lacerta agilis) and the common lizard (Zootoca vivipara), and their Ixodes ricinus ticks, in the circulation spirochetes of the Borrelia burgdorferi s.l. complex. Lizards were captured at three study sites in suburban areas of western Poland. Common lizards were less abundant and occurred only at one site. A total of 1129 ticks were collected from 167 sand lizards and 164 individuals from 42 common lizards. Biopsies of the distal part of the lizard tail were taken from 172 animals. All samples that tested positive by real-time PCR underwent subsequent nested PCR targeting the flaB gene, followed by sequencing. At least 6.3% of I. ricinus ticks (MIR) from L. agilis, and 6.1% from Z. vivipara, were infected. Borrelia lusitaniae was the most prevalent genospecies in L. agilis-derived ticks, accounting for 73.2% of all infected samples, followed by B. burgdorferi s.s. (23.0%). Conversely, this latter species prevailed (90%) over B. lusitaniae (10%) in tick samples from Z. vivipara. Therefore, we believe that sand lizards are competent reservoir hosts for B. lusitaniae, while the role of Z. vivipara for this species is unclear. The high prevalence of B. burgdorferi s.s. was also found in infected larval samples (40.7%) and biopsies (60%) of L. agilis. Thus, in our opinion, these two lizard species could be another group of reservoir hosts for this human pathogen, along with birds and rodents.
1. Introduction
The sand lizard, Lacerta agilis Linnaeus, 1758 is widespread throughout Eurasia and has one of the largest ranges of any reptile species, ranging from the western border of France and eastern United Kingdom to Mongolia and north-western China [1]. It is represented by at least ten distinct geographical subspecies belonging to the L. agilis complex [2,3]. Sand lizards inhabit a wide range of open or semi-open habitats and occur both in natural and anthropogenic ecosystems characterized by high sunlight exposure, low vegetation, and access to hiding places [4]. They prefer low-dense cover of grass and bushes interspersed with patches of bare ground, and avoid the over-shaded wooded areas. This species is most commonly found in dry grasslands on sandy soils, heathlands, along forest edges, in sparse low pine stands, on grassy or bushy edges of trenches, roadsides, slopes, wastelands, rock rubble, railway and flood embankments [5,6,7].
The common (viviparous) lizard, Zootoca vivipara (Lichtenstein, 1823) formerly known as Lacerta vivipara, is smaller than L. agilis, and its distribution covers nearly the whole of Europe, northern and central Asia and as far as Japan. In consequence, it has the largest and most northerly range of any lizard in the Lacertidae family, including the subarctic regions of Eurasia. Within this distribution, the common lizard has adapted to a cooler, more humid climate [8,9]. It prefers moist, less sunny places, such as peat bogs, wet meadows and forest edges, and avoids dry areas. Zootoca vivipara often co-exists simpartically with L. agilis lizards [10,11,12]. In Europe, both lizard species are common hosts of Ixodes ricinus larvae and nymphs transmitting a wide variety of blood-borne bacterial agents including spirochetes of the Borrelia burgdorferi sensu lato (s.l.) complex [13,14]. Currently, this complex comprises at least 23 genospecies (hereafter referred to as species), of which 14 have been reported in European I. ricinus ticks [15,16]. At least five of them, B. afzelii, B. garinii, B. burgdorferi sensu stricto (s.s.), B. spielmanii, and B. bavariensis are considered the causative agents of Lyme borreliosis (LB). The first three predominate in European patients, while the latter two are rarely reported [17,18]. Furthermore, three other spirochaete species, B. bissettiae, B. valaisiana and B. lusitaniae, have been occasionally detected or isolated from human samples and are thought to have pathogenic potential [19].
At least four lizard species: Lacerta viridis (Laurenti, 1768), L. agilis, Z. vivipara and Podarcis muralis (Laurenti, 1768) have been implicated in the maintenance of local cycles of B. lusitaniae in Central Europe acting as reservoir hosts for the bacterium [20,21,22,23,24]. This spirochete, which was first detected in Portugal in 1993, occurs in Central and Southeastern Europe, but its prevalence appears to be low and focal. By contrast, in Mediterranean countries such as Portugal, Morocco, Tunisia and Italy, B. lusitaniae appears to infect I. ricinus ticks more frequently than other spirochaete species [25]. Apart from lizards, this bacterium has sporadically been reported in immature I. ricinus ticks collected from birds [26,27] and Apodemus sylvaticus mice [28]. Furthermore, de Carvalho et al. [29] isolated B. lusitaniae from the same species of mouse. However, these reports did not provide evidence that birds or mice may act as reservoirs for the bacterium. In suitable habitats, lizards can be more important hosts for ticks than rodents or birds. Consequently, their local dominance may negatively impact the spread of spirochaete species other than B. lusitaniae [21,30]. Nevertheless, the role of lizards in the circulation of tick-borne pathogens, especially in urban and peri-urban areas, appears to be still underestimated compared to that of mammals and birds [31]. Such areas are highly fragmented environments, composed of a mosaic of patches of different sizes and types of vegetation and land use. However, they might provide suitable conditions for lizards.
The aim of this study was to assess the association between two lizard species (L. agilis and Z. vivipara) and their larval and nymphal I. ricinus ticks in the spreading spirochetes of the Borrelia burgdorferi s.l. group in anthropogenically transformed suburban areas of western Poland.
2. Material and Methods
2.1. Study Sites
The research was conducted from April to September 2017 in three separate study sites (I, II and III) in the Lubusz Province in western Poland (Figure 1). They depict transformed suburban or former agricultural areas that vary in terms of their habitats. Site I: a small village called Olcha that is now part of the city of Zielona Góra (51°52.188′ N, 15°27.122′ E). It has been partially excluded from agricultural use and developed for a new low-rise housing estate. The largest proportion (70%) of the land cover comprised grasslands, arable lands, and mosaics of fresh meadows, with a small area covered by riparian forest. Site II: a post-gravel pit area is located within the boundaries of the town of Żary (51°37.690′ N, 15°05.387′ E). Most of the site is covered by young Scots pines, birch and aspen trees, as well as humid meadows. Wetlands with grass and several small seasonal water bodies covered about 30% of the area. Site III: a converted post-sand extraction area is located within the boundaries of the town of Nowa Sól (51°43.850′ N, 15°43.816′ E). It is an exceptionally dry and sunny place dominated by sandy grasslands, which cover over 80% of the slopes. Only 10% of the area was covered by scattered shrubs and trees, and about 5% was a small water body.
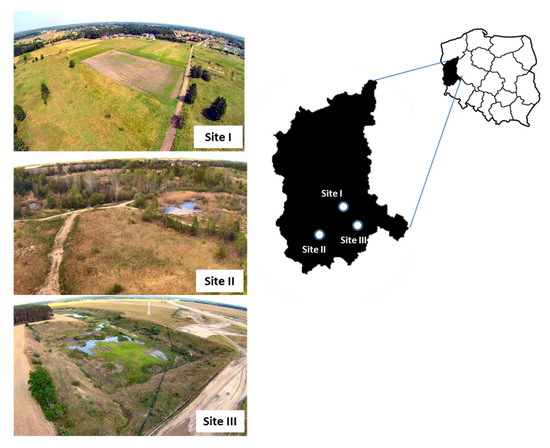
Figure 1.
Geographical location of the three collection sites where tick-infested lizards were captured in the Lubusz Province in western Poland. The common lizard was less abundant, being recorded only at site I.
2.2. Lizard Capture and Tick Collection
In the present study, only lizards infested with ixodid ticks (n = 209) were analyzed. Two species of lizards: the sand lizard L. agilis (n = 167) and the common lizard Z. vivipara (n = 42) were captured alive by hand. Both species are depicted in Figure 2. The sex (male or female) and age status (adult or juvenile) of the lizards were determined. Each individual was checked for the presence of feeding ticks, which were removed using forceps and stored in 75% ethanol for further analyses. Ticks were identified to the species level using a stereoscope microscope based on morphological criteria according to Siuda [32]. Most of the ticks that fed upon both lizard species were recorded in May and June. Furthermore, a biopsy of up to 1 cm long from the distal part of the lizard’s tail was successfully taken with sterile scissors from 172 animals (131 from L. agilis and 41 from Z. vivipara). Biopsies were placed in separate vials with 75% ethanol. After examination each animal was released at the site of capture.


Figure 2.
The top photo shows a male of the sand lizard (Lacerta agilis), and the bottom photo shows a male of the common lizard (Zootoca vivipara). Both lizards are infested with immature Ixodes ricinus ticks (photo by B. Najbar).
2.3. DNA Extraction
Genomic DNA from the tails of lizards and ticks was isolated by alkaline hydrolysis, according to previous reference [33]. Ticks were processed individually (60 larvae and 79 nymphs) and in pools (184 larval pools and 89 nymphal pools). A larval pool contained between two and ten individuals, whereas a nymphal pool yielded between two and five. Each pool originated from the same animal. A tick or pooled sample was placed in a 1.5 mL Eppendorf tube and mechanically homogenized using a sterile micropestle. A total of 412 DNA samples (244 from larvae and 168 from nymphs) were obtained from ticks. The obtained lysates were kept at −20 °C.
2.4. Screening for Borrelia burgdorferi s.l. DNA
In order to detect Borrelia DNA, Real-Time PCR was performed with the primers Bb23Sf and Bb23Sr complementary to a 75 bp fragment of the 23S rRNA gene of B. burgdorferi s.l. and with a TaqMan Bb23Sp-FAM probe following the methodology described by Courtney et al. [34]. The reaction was conducted using Real-Time HS-PCR Mastermix Probe (A&A Biotechnology, Gdynia, Poland) following the manufacturer’s instructions. Reaction conditions were as follows: denaturation/polymerase activation at 95 °C for 10 min and 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s, extension at 60 °C for 30 s. Plasmids pJET1 (A&A Biotechnology, Poland), into which the amplified fragments of the target genes were cloned, were used as positive controls. The negative control consisted of double-distilled water. All real-time DNA amplification reactions were performed using the Mx3005P Real-Time QPCR System (Stratagene, La Jolla, CA, USA) in Department of Tropical Parasitology at the Medical University of Gdańsk.
Positive samples were retested by amplification of the flaB gene fragment using two primer sets 132f/905r and 220f/823r [35]. DNA extracted from a tick infected by B. afzelii was used to control unspecific detection of Borrelia DNA by flagellin gene amplification. Amplification products were separated on 1% agarose gel stained with Midori Green DNA Stain (ABO, Gdańsk, Poland).
2.5. Identification of Borrelia Species by Sequencing
PCR-positive products were purified with the clean-up purification (A&A Biotechnology, Gdynia, Poland) and sequenced in both directions by using the same primer pairs (220f and 823r) by firm Macrogen Europe B.V. (Amsterdam, The Netherlands). The obtained sequences (604 bp) were compared with those available in the GenBank databases using BLAST program (US National Institutes of Health, Bethesda, MD, USA) (http://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 21 July 2025). Aligned sequences representing flaB gene fragments of Borrelia strains were examined with MEGA X version 10.0.1 [36]. A total of 96 partial sequences of the flaB gene were deposited in GenBank under the following accession numbers: ON086818-ON086836, ON086838-ON086843, ON086845-ON086848; ON086850, ON086852-ON086854, ON086858-ON086860, ON086863-ON086865, ON086881-ON086882-ON086885, ON086887, ON086889, ON086900, ON086902, ON086903, ON086895-ON086909 (B. lusitaniae), ON086837, ON086849, ON086851, ON086861, ON086862, ON086866-ON086872-ON086880, ON086888, N086890-N086893, ON086896-ON086899, ON086901, ON086904, ON086910-ON086912 (B. burgdorferi s.s.); ON086894, ON086905 (B. afzelii), OM970780, OM970781 (B. miyamotoi).
2.6. Data Analysis
The prevalence of B. burgdorferi s.l. infection in ticks was estimated using the Minimum Infection Rate (MIR), i.e., the minimum infected proportion expressed as a percentage: MIR = (p/N) × 100%, where p = the number of positive pools and N = the total number of ticks tested. This method assumes that at least one infected tick is present in each positive pool [37]. Differences in the prevalence of Borrelia infection in tick samples were evaluated statistically using the 2-tailed chi square test (χ2).
3. Results
3.1. Lizards and Their Ticks
A total of 167 tick-infested sand lizards (L. agilis) were captured during our study. They were caught at each of the three different study sites in comparable numbers. In total, 1129 feeding ticks identified as I. ricinus (837 larvae and 292 nymphs) were removed from these animals (Table 1). Larvae predominated, accounting for 74% of all ticks, with a ratio of 2.9 larvae to nymphs. Sixty-three animals (37.7%) hosted both larvae and nymphs. Mono-infestation with either larvae or nymphs was recorded in 58 (34.7%) and 46 (27.5%) of the lizards, respectively. On average, one infested animal hosted 6.8 ticks. The number of ticks attached to lizards differed greatly between the study sites. The highest mean intensity of infestation (average number of ticks per tick-infested host) was found at site II, and the lowest at site III (10.8 and 2.8 ticks per animal, respectively).

Table 1.
Prevalence of Borrelia burgdorferi s.l. genospecies identified in Ixodes ricinus ticks collected from two lizard species: Lacerta agilis (LA) and Zootoca vivipara (ZV) examined in three separate study sites (I, II and III) in the Lubusz Province in western Poland.
A total of 42 common lizards (Z. vivipara) were captured, all of which were found only at site I. From these individuals, 164 feeding ticks (116 larvae and 48 nymphs) representing I. ricinus were collected. A 70% predominance of larvae was recorded, with a ratio of 2.4 larvae to nymphs (Table 1). Ten of the animals (24.4%) were co-infested by both larvae and nymphs. Mono-infestation with either larvae or nymphs was observed in 21 (51.1%) and 10 (24.4%) of the lizards, respectively. The mean intensity reached 3.9 ticks per host, which was comparable to the tick number (5.5 ticks per host) recorded on sand lizards coexisting at the same site.
3.2. Borrelia burgdorferi s.l. DNA in Ticks and Lizards
In total, 71 out of 358 tick samples obtained from the sand lizards yielded Borrelia DNA. The overall infection prevalence, evaluated as the minimum infection rate (MIR) and calculated for combined tick samples, reached 6.3% (71 of 1129 ticks) (Table 1). The prevalence of B. burgdorferi s.l. differed between study sites, ranging from 5.7% to 9.7%. However, these differences were not statistically significant (χ2 test, p = 0.38). Combined data from all study sites, showed that nymphs were almost five-fold more frequently infected than larvae (15.1% vs. 3.2%, respectively; χ2 test, p < 0.0001). However, this trend was only recorded at sites II and III (24.2% vs. 1.7%, and 13.6% vs. 7.1%, respectively). In total, 55 (33.0%) out of the 167 lizards hosted at least one Borrelia positive tick.
Ten out of 54 tick samples obtained from common lizards were found to be infected with Borrelia spp. Thus, at least 6.1% of 164 I. ricinus ticks carried spirochetes (Table 1). Infection rates calculated for nymphs and larvae were similar (6.3% and 6.0%, respectively). Eight (9.0%) of the 42 Z. vivipara lizards were found to be infested with B. burgdorferi s.l. infected ticks. Analysis of biopsies taken from the tails of 131 sand lizards and 41 common lizards revealed that 15 (11.5%) and two (4.9%) of the samples, respectively, yielded B. burgdorferi s.l. DNA (Table 2).

Table 2.
Prevalence of Borrelia burgdorferi s.l. species identified in biopsies taken from the distal part of two lizard species’ tails: Lacerta agilis (LA) and Zootoca vivipara (ZV) examined in three separate study sites (I, II and III) in the Lubusz Province in western Poland.
3.3. Prevalence of B. burgdorferi Sensu Lato Species
Borrelia lusitaniae was found to be the most prevalent species in L. agilis-derived ticks, accounting for 73.2% (52 out of 71) of all infected samples, followed by B. burgdorferi s.s. (23.0%; n = 17), and B. afzelii (2.8%; n = 2). The first two species were recorded at all study sites, whereas B. afzelii was only detected in two pools, one of which was obtained from larvae and the other from nymphs collected at sites I and II. In PCR-positive nymphal samples B. lusitaniae was found to be more common than B. burgdorferi s.s. (84.1% vs. 13.6%, respectively). Among the infected larval samples, the two dominant species reached relatively similar prevalences (55.6% and 40.7%, respectively). Furthermore, the DNA of B. miyamotoi, a spirochaete belonging to the Borrelia relapsing fever group, was found in two nymphs collected from two sand lizards at sites I and III.
In the infected tick samples (n = 10) derived from common lizards, two spirochaete species were identified, with the predominance of B. burgdorferi s.s. (90%; n = 9) over B. lusitaniae (10%; n = 1). The first species was identified in seven larval and two nymphal samples, whereas the latter was only found in a single pool of nymphs (Table 1).
Of the 15 PCR-positive tail biopsies collected from sand lizards, B. burgdorferi s.s. was the most prevalent (60.0%; n = 9), followed by B. lusitaniae (33.3%; n = 5) and B. afzelii (6.7%; n = 1). Borrelia lusitaniae was the only species found in two PCR-positive common lizards (Table 2).
4. Discussion
In this study, we provide evidence that two lizard species, L. agilis and Z. vivipara, along with immature I. ricinus ticks, are involved in the circulation of spirochetes from the B. burgdorferi s.l. complex in disturbed suburban habitats of western Poland. The sand lizard is the most prevalent and abundant species of lizard in Poland. The presence of the species was identified at all three of the selected locations, while the common lizard was recorded only at one. Both species confirmed their importance as hosts for immature I. ricinus ticks, particularly for larvae, which clearly dominated over nymphs, accounting for nearly 74% of the 1293 ticks that were collected. This finding is consistent with the results of our previous two-year study of L. agilis in the same area [38]. A similar trend of larval predominance on L. agilis and/or on Z. vivipara was also reported in southwestern Poland [39,40], Hungary [22] and in the Netherlands [41]. On the other hand, a nymphal dominance over larvae was observed on L. agilis in Poland [12,42] and on L. viridis in Slovakia [20]. Moreover, in our two-year radiotelemetry study at site I, we removed three times more nymphs (n = 1899) than larvae (n = 628) from 16 monitored sand lizards [43]. According to Dyczko et al. [40], L. agilis is a more significant host in maintaining I. ricinus populations than Z. vivipara. However, in our study, the number of ticks collected from both lizard species which co-occurred only at site I was comparable (5.5 and 3.9 ticks per animal, respectively).
At least 6.3% of I. ricinus ticks (calculated as MIR) collected from L. agilis, and 6.1% from Z. vivipara, were found to be infected with B. burgdorferi s.l. It should be emphasized that the overall prevalence of B. burgdorferi s.l. found in ticks from the latter species is the highest reported to date. Only 1% of 103 ticks obtained from common lizards in southwestern Poland yielded the bacterium [40]. Apart from the cited Polish report [40], there is no published data on the role of the common lizard in the transmission cycle of B. burgdorferi s.l. Studies on sand lizards conducted in Poland showed that the mean infection rates of B. burgdorferi s.l. detected in ticks parasitizing these hosts ranged from 4.1% (12/290) [23] to 12% (41/342) [40]. To date, the highest prevalence of the bacterium, 21% (32/152), has been documented by Majláthová et al. [44] in Slovakia. On the other hand, only 0.2% of 1355 ticks from sand lizards examined in a coastal dune ecosystem in the Netherlands carried the bacterium [41].
Despite the finding of comparable prevalences of B. burgdorferi s.l. in tick samples obtained from L. agilis and Z. vivipara, the prevalence of the predominant spirochete species was different. Among PCR-positive tick samples from sand lizards, B. lusitaniae proved to be the most prevalent species (73.2%), followed by B. burgdorferi s.s. (23.9%). This species prevailed in both infected nymphs (84.1%) and larvae (55.6%), and was present at all study sites. Furthermore, it was detected in 33.3% (5/15) of PCR-positive tail biopsies taken from L. agilis. Larval samples obtained from three of these animals also yielded B. lusitaniae, implying that they acquired the spirochetes while feeding on them. Therefore, we believe that sand lizards act as competent reservoir hosts for B. lusitaniae in suburban habitats where I. ricinus populations are present. The involvement of L. agilis in the maintenance of B. lusitanie was for the first time demonstrated by Richter and Matuschka [21] in Germany. The authors found that all subadult I. ricinus ticks that acquired spirochetes from L. agilis harbored B. lusitaniae. This species distinctly dominated in Borrelia-infected ticks collected from L. agilis in Slovakia (94%) and Romania (100%) [44]. Studies conducted in Poland also showed that B. lusitaniae prevailed in ticks removed from sand lizards, accounting for 66.7% [23] and 88% of all infections [40]. Unexpectedly, B. lusitaniae was the dominant species among questing ticks (50%; 21/42) sampled in green areas of Zielona Góra (our site I) [45]. This is the highest infection rate of this species ever recorded in Poland. According to the authors, the high proportion of B. lusitaniae indicates a significant expansion of lizards in the city. Therefore, lizards could shape the diversity of Borrelia species in ticks inhabiting urban areas. Furthermore, Musilová et al. [24] found that the pathogen was present in 67% of Borrelia-infected questing nymphs in the Czech Republic. Of note is that these nymphs were only collected at a site where high numbers of ticks were found on green lizards. Thus, the strong association of this species with lizards may determine its focal distribution in tick populations. The above reports are consistent with the data published by Cirkovic et al. [25], which shows that B. lusitaniae is becoming more prevalent in Central Europe and not just in Mediterranean countries.
To date, two strains of B. lusitaniae have been isolated from human patients with suspected LB [46] and vasculitis syndrome [47] in Portugal. Furthermore, this spirochete was recently isolated from the blood of a Serbian patient with multiple erythema migrans [48]. These reports implicate the pathogenic potential of B. lusitaniae in humans. However, its pathogenicity appears to be limited to some genetic variants [49]. To date, no human cases associated with this spirochete species have been reported in Central European countries.
Our analysis of infected tick samples collected from Z. vivipara revealed a clear predominance of B. burgdorferi s.s. (90%) over B. lusitaniae (10%). To the best of our knowledge, this is the highest rate of the bacterium observed in ticks originating from lizards in Central Europe. Apart from two nymphal samples, the species was identified in larval samples, which were collected from seven animals that were not concurrently infested by nymphs. Therefore, it cannot be excluded that the larvae most likely contracted the infection from the host animals. In this case, it is highly likely that Z. vivipara could act as a reservoir host for B. burgdorferi s.s., but not for B. lusitaniae, which was identified only in one nymphal sample and in two biopsies. This means that Z. vivipara exhibits a very low infectivity for the lizard-associated B. lusitaniae compared to L. agilis, which is its competent reservoir host. This is consistent with the Polish report, in which only one of the 103 ticks parasitizing Z. vivipara carried the pathogen [40].
It should be emphasized that we also found very high prevalences of B. burgdorferi s.s. in biopsies of sand lizards (60%) and in infected larval samples (40.7%) collected from these hosts. In a study conducted in Hungary, 13% of 31 Borrelia-infected ticks obtained from lizards yielded this spirochete species [22]. Similar prevalences of B. burgdorferi s.s. in ticks from sand lizards have been documented in Poland with a range from 14.3% [42] to 16.7% [23]. However, Dyczko et al. [40] failed to detect the bacterium in ticks collected from both Z. vivipara and L. agilis in urban areas of the city of Wrocław in south-western Poland. A low infection rate of 5% (3/60) in ticks from L. viridis was reported in the Czech Republic [24].
The human-pathogenic B. burgdorferi s.s. is considered a generalist species because it is capable of infecting many different groups of vertebrate species including birds and mammalian hosts, especially rodents [50,51]. Interestingly, this spirochete was recently identified in two chiropterophilic Ixodes tick species, which were collected from cave bats captured in Poland and Romania [52]. Therefore, given that various groups of vertebrates and tick vectors can be infected by this generalist species, both Z. vivipara and L. agilis could locally act as its reservoir hosts. This is important from an epidemiological point of view because B. burgdorferi s.s. is responsible for Lyme arthritis (LA) and neurological complications [53]. The data available in Poland show that, in many regions, this is a dominant species among Borrelia-infected questing ticks [54,55,56], in contrast to most other European countries [17]. Furthermore, a retrospective study based on data submitted to the Polish National Institute of Public Health revealed that erythema migrans and LA were the most prevalent symptoms of LB between 2015 and 2019 [57]. In our opinion, the high incidence of LA cases could be attributed to the predominance of B. burgdorferi s.s. in questing ticks, which acquire the pathogen by feeding on competent reservoir host species.
5. Conclusions
Our research provides evidence that two lizard species, L. agilis and Z. vivipara, along with their immature I. ricinus ticks are involved in the circulation of two species from the B. burgdorferi s.l. complex, but in different ways. Lacerta agilis showed a distinctly higher infectivity for B. lusitaniae, as 73% of infected tick samples carried this spirochete, compared to only 10% derived from Z. vivipara. Therefore, at our study sites, only the sand lizard served as a competent reservoir host for this pathogen. Further research is needed to determine the reservoir competence of Z. vivipara for the lizard-associated B. lusitaniae. On the other hand, the unexpectedly high prevalence of B. burgdorferi s.s. found in infected ticks collected from Z. vivipara (90%) and additionally in biopsies (60%) and larval samples (40.7%) obtained from sand lizards, suggests that these two species could preferentially maintain the circulation of this human-pathogenic spirochete. Of note is that lizard-derived ticks infected with B. lusitaniae and B. burgdorferi s.s. were found at all study sites, indicating that both spirochete species may be widespread among lizard populations inhabiting suburban areas of western Poland. In such disturbed habitats, lizards could be another group of reservoir hosts for the less specialized B. burgdorferi s.s., along with birds and rodents. Based on our data, it can be assumed that this species could potentially be more prevalent in areas inhabited by lizards, but further research is needed to prove this hypothesis. Consequently, the two lizards may influence the diversity of Borrelia species in tick populations in urban and suburban areas. In conclusion, our findings highlight the importance of the host element in the ecology of European spirochete species belonging to the B. burgdorferi s.l. group.
Author Contributions
Conceptualization, M.W., R.G., B.N., B.S. and J.M.; methodology, M.W., R.G., B.N. and J.M.; validation, R.G. and J.M.; formal analysis, M.W., R.G., B.N., B.S. and J.M.; investigation, M.W., R.G., B.S. and J.M.; material collection, M.W. and B.N.; data curation, M.W. and R.G.; writing—original draft preparation, M.W., R.G., B.N., B.S. and J.M.; visualization, R.G., B.N. and J.M.; supervision, R.G., B.N. and J.M.; project administration, R.G. and B.N.; funding acquisition, R.G. and B.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The authors declare that no experimentation on animals has been conducted to obtain the data presented in this paper. The trapping and handling procedures of lizards were approved by the Regional Directorate for Environmental Protection (permit No. WPNI.6401.206.2-15.JK) and the Local Ethics Commission for Animal Experiments (Resolution No. 70/2016).
Informed Consent Statement
Not applicable.
Data Availability Statement
All necessary data are available in the text.
Acknowledgments
We sincerely thank Joanna Stańczak from the Department of Tropical Parasitology, Medical University of Gdańsk, for her assistance with the laboratory research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bischoff, W. Zur Verbreitung und Systematik der Zauneidechse, Lacerta agilis LINNAEUS, 1758. Mertensiella 1988, 1, 11–30. [Google Scholar]
- Tuniyev, S.B.; Tuniyev, B.S. Interspecific variation of the sand lizard (Lacerta agilis) from the Western Caucasus and description of a new subspecies Lacerta agilis mzymtensis ssp. nov. (Reptilia: Sauria). Russ. J. Herpetol. 2008, 15, 55–66. [Google Scholar]
- Andres, C.; Franke, F.; Bleidorn, C.; Bernhard, D.; Schlegel, M. Phylogenetic analysis of the Lacerta agilis subspecies complex. Syst. Biodivers. 2014, 12, 43–54. [Google Scholar] [CrossRef]
- Gill, I.; McGeorge, I.; Jameson, T.J.M.; Moulton, N.; Wilkie, M.; Försäter, K.; Gardner, R.; Bockreiß, L.; Simpson, S.; Garcia, G. EAZA Best Practice Guidelines for Sand Lizard (Lacerta agilis), 1st ed.; European Association of Zoos and Aquariums: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Nemes, S.; Vogrin, M.; Hartel, T.; Öllerer, K. Habitat selection at the sand lizard (Lacerta agilis): Ontogenetic shifts. North-West. J. Zool. 2006, 2, 17–26. [Google Scholar]
- Čeirāns, A. Microhabitat Characteristics For Reptiles Lacerta agilis, Zootoca vivipara, Anguis fragilis, Natrix natrix, and Vipera berus in Latvia. Russ. J. Herpetol. 2007, 14, 172–176. [Google Scholar]
- Heltai, B.; Sály, P.; Kovács, D.; Kiss, I. Niche segregation of sand lizard (Lacerta agilis) and green lizard (Lacerta viridis) in an urban semi-natural habitat. Amphib. Reptil. 2015, 36, 389–399. [Google Scholar] [CrossRef]
- Berman, D.I.; Bulakhova, N.A.; Alfimov, A.V.; Meshcheryakova, E.N. How the most northern lizard, Zootoca vivipara, overwinters in Siberia. Polar Biol. 2016, 39, 2411–2425. [Google Scholar] [CrossRef]
- Horreo, J.L.; Pelaez, M.L.; Suárez, T.; Breedveld, M.C.; Heulin, B.; Surget-Groba, Y.; Oksanen, T.A.; Fitze, P.S. Phylogeography, evolutionary history and effects of glaciations in a species (Zootoca vivipara) inhabiting multiple biogeographic regions. J. Biogeogr. 2018, 45, 1616–1627. [Google Scholar] [CrossRef]
- Bauwens, D.; Strijbosch, H.; Stumpel, A.H.P. The Lizards Lacerta agilis and L. vivipara as Hosts to Larvae and Nymphs of the Tick Ixodes ricinus. Holarct. Ecography 1983, 6, 32–40. [Google Scholar] [CrossRef]
- Ekner, A.; Majlath, I.; Majlathova, V.; Hromada, M.; Bona, M.; Antczak, M.; Bogaczyk, M.; Tryjanowski, P. Densities and Morphology of Two Co-existing Lizard Species (Lacerta agilis and Zootoca vivipara) in Extensively Used Farmland in Poland. Folia Biol. 2008, 56, 165–167. [Google Scholar] [CrossRef]
- Gwiazdowicz, D.J.; Gdula, A.K.; Kurczewski, R.; Zawieja, B. Factors influencing the level of infestation of Ixodes ricinus (Acari: Ixodidae) on Lacerta Agilis and Zootoca vivipara (Squamata: Lacertidae). Acarologia 2020, 60, 390–397. [Google Scholar] [CrossRef]
- Mendoza-Roldan, J.A.; Mendoza-Roldan, M.A.; Otranto, D. Reptile vector-borne diseases of zoonotic concern. Int. J. Parasitol. Parasites Wildl. 2021, 15, 32–42. [Google Scholar] [CrossRef]
- Nowak, T.A.; Burke, R.L.; Diuk-Wasser, M.A.; Lin, Y.P. Lizards and the enzootic cycle of Borrelia burgdorferi sensu lato. Mol. Microbiol. 2024, 121, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, K.A.; Margos, G.; Fingerle, V.; Becker, N. Host association of Borrelia burgdorferi sensu lato: A review. Ticks Tick-Borne Dis. 2021, 12, 101766. [Google Scholar] [CrossRef]
- Wodecka, B.; Kolomiiets, V. Genetic Diversity of Borreliaceae Species Detected in Natural Populations of Ixodes ricinus Ticks in Northern Poland. Life 2023, 13, 972. [Google Scholar] [CrossRef] [PubMed]
- Strnad, M.; Hönig, V.; Růžek, D.; Grubhoffer, L.; Rego, R.O.M. Europe-wide meta-analysis of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus ticks. Appl. Environ. Microbiol. 2017, 83, e00609-17. [Google Scholar] [CrossRef]
- Stanek, G.; Strle, F. Lyme borreliosis—From tick bite to diagnosis and treatment. FEMS Microbiol. Rev. 2018, 42, 233–258. [Google Scholar] [CrossRef] [PubMed]
- Steinbrink, A.; Brugger, K.; Margos, G.; Kraiczy, P.; Klimpel, S. The evolving story of Borrelia burgdorferi sensu lato transmission in Europe. Parasitol. Res. 2022, 121, 781–803. [Google Scholar] [CrossRef]
- Majláthová, V.; Majláth, I.; Derdáková, M.; Víchová, B.; Pet’ko, B. Borrelia lusitaniae and green lizards (Lacerta viridis), Karst Region, Slovakia. Emerg. Infect. Dis. 2006, 12, 1895–1901. [Google Scholar] [CrossRef]
- Richter, D.; Matuschka, F.R. Perpetuation of the Lyme disease spirochete Borrelia lusitaniae by lizards. Appl. Environ. Microbiol. 2006, 72, 4627–4632. [Google Scholar] [CrossRef]
- Földvári, G.; Rigó, K.; Majláthová, V.; Majláth, I.; Farkas, R.; Pet’ko, B. Detection of Borrelia burgdorferi sensu lato in Lizards and Their Ticks from Hungary. Vector Borne Zoonotic Dis. 2009, 9, 331–336. [Google Scholar] [CrossRef]
- Ekner, A.; Dudek, K.; Sajkowska, Z.; Majláthová, V.; Majláth, I.; Tryjanowski, P. Anaplasmataceae and Borrelia burgdorferi Sensu Lato in the sand lizard Lacerta Agilis and co-infection of these bacteria in hosted Ixodes ricinus ticks. Parasit. Vectors 2011, 4, 182. [Google Scholar] [CrossRef]
- Musilová, L.; Kybicová, K.; Fialová, A.; Richtrová, E.; Kulma, M. First isolation of Borrelia lusitaniae DNA from green lizards (Lacerta viridis) and Ixodes ricinus ticks in the Czech Republic. Ticks Tick-Borne Dis. 2022, 13, 101887. [Google Scholar] [CrossRef]
- Cirkovic, V.; Veinovic, G.; Stankovic, D.; Mihaljica, D.; Sukara, R.; Tomanovic, S. Evolutionary dynamics and geographical dispersal of Borrelia lusitaniae. Front. Microbiol. 2024, 15, 1330914. [Google Scholar] [CrossRef] [PubMed]
- Poupon, M.; Lommano, E.; Humair, P.; Douet, V.; Rais, O.; Schaad, M.; Jenni, L.; Gern, L. Prevalence of Borrelia burgdorferi Sensu Lato in Ticks Collected from Migratory Birds in Switzerland. Appl. Environ. Microbiol. 2006, 72, 976–979. [Google Scholar] [CrossRef]
- Norte, A.C.; Margos, G.; Becker, N.S.; Ramos, J.A.; Nuncio, M.S.; Fingerle, V.; Araujo, P.M.; Adamik, P.; Alivizatos, H.; Barba, E.; et al. Host dispersal shapes the population structure of a tick-borne bacterial pathogen. Mol. Ecol. 2020, 29, 485–501. [Google Scholar] [CrossRef]
- Norte, A.C.; da Silva, A.A.; Alves, J.; da Silva, L.P.; Nuncio, M.S.; Escudero, R.; Anda, P.; Ramos, J.A.; de Carvalho, I.L. The importance of lizards and small mammals as reservoirs for Borrelia lusitaniae in Portugal. Environ. Microbiol. Rep. 2015, 7, 188–193. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, I.L.; Zeidner, N.; Ullmann, A.; Hojgaard, A.; Amaro, F.; Ze-Ze, L.; Alves, M.J.; de Sousa, R.; Piesman, J.; Nuncio, M.S. Molecular characterization of a new isolate of Borrelia lusitaniae derived from Apodemus sylvaticus in Portugal. Vector Borne Zoonotic Dis. 2010, 10, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, L.; Perazzo, S.; Venturino, E.; Giacobini, M.; Bertolotti, L.; Mannelli, A. Modeling the effects of variable feeding patterns of larval ticks on the transmission of Borrelia lusitaniae and Borrelia afzelii. Theor. Popul. Biol. 2017, 116, 27–32. [Google Scholar] [CrossRef]
- Rizzoli, A.; Silaghi, C.; Obiegala, A.; Rudolf, I.; Hubálek, Z.; Földvári, G.; Plantard, O.; Vayssier-Taussat, M.; Bonnet, S.; Spitalská, E.; et al. Ixodes ricinus and its transmitted pathogens in urban and periurban areas in Europe: New hazards and relevance for public health. Front. Public Health 2014, 2, 251. [Google Scholar] [CrossRef]
- Siuda, K. Ticks (Acari: Ixodida) of Poland. Part II: Taxonomy and Distribution; Polskie Towarzystwo Parazytologiczne: Warszawa, Poland, 1993. (In Polish) [Google Scholar]
- Rijpkema, S.; Bruinink, H. Detection of Borrelia burgdorferi sensu lato by PCR in 1070 questing Ixodes ricinus larvae from the Dutch North Sea island of Ameland. Exp. Appl. Acarol. 1996, 20, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Courtney, J.W.; Kostelnik, L.M.; Zeidner, N.S.; Massung, R.F. Multiplex real-time PCR for detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J. Clin. Microbiol. 2004, 42, 3164–3168. [Google Scholar] [CrossRef] [PubMed]
- Wodecka, B.; Leońska, A.; Skotarczak, B. A comparative analysis of molecular markers for the detection and identification of Borrelia spirochaetes in Ixodes ricinus. J. Med. Microbiol. 2010, 59, 309–314. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Fracasso, G.; Grillini, M.; Grassi, L.; Gradoni, F.; Rold, G.d.; Bertola, M. Effective Methods of Estimation of Pathogen Prevalence in Pooled Ticks. Pathogens 2023, 12, 557. [Google Scholar] [CrossRef]
- Wieczorek, M.; Najbar, B. Ectoparasitism of castor bean ticks Ixodes ricinus (Linnaeus, 1758) on sand lizards Lacerta agilis (Linnaeus, 1758) in western Poland. Stud. Biol. 2022, 16, 27–34. [Google Scholar] [CrossRef]
- Dudek, K.; Skórka, P.; Sajkowska, Z.A.; Ekner-Grzyb, A.; Dudek, M.; Tryjanowski, P. Distribution pattern and number of ticks on lizards. Ticks Tick-Borne Dis. 2016, 7, 172–179. [Google Scholar] [CrossRef]
- Dyczko, D.; Krysmann, A.; Kolanek, A.; Borczyk, B.; Kiewra, D. Bacterial pathogens in Ixodes ricinus collected from lizards Lacerta agilis and Zootoca vivipara in urban areas of Wrocław, SW Poland– preliminary study. Exp. Appl. Acarol. 2024, 93, 409–420. [Google Scholar] [CrossRef]
- Köhler, C.F.; Sprong, H.; Fonville, M.; Esser, H.; De Boer, W.F.; Van der Spek, V.; Spitzen-van der Sluijs, A. Sand lizards (Lacerta agilis) decrease nymphal infection prevalence for tick-borne pathogens Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum in a coastal dune ecosystem. J. Appl. Ecol. 2023, 60, 1115–1126. [Google Scholar] [CrossRef]
- Gryczyńska-Siemiątkowska, A.; Siedlecka, A.; Stańczak, J.; Barkowska, M. Infestation of sand lizards [Lacerta Agilis] resident in the Northeastern Poland by Ixodes ricinus [L.] ticks and their infection with Borrelia burgdorferi sensu lato. Acta Parasitol. 2007, 52, 165–170. [Google Scholar] [CrossRef]
- Wieczorek, M.; Rektor, R.; Najbar, B.; Morelli, F. Tick parasitism is associated with home range area in the sand lizard, Lacerta agilis. Amphib. Reptil. 2020, 41, 479–488. [Google Scholar] [CrossRef]
- Majláthová, V.; Majláth, I.; Hromada, M.; Tryjanowski, P.; Bona, M.; Antczak, M.; Víchová, B.; Dzimko, Š.; Mihalca, A.; Peťko, B. The role of the sand lizard (Lacerta agilis) in the transmission cycle of Borrelia burgdorferi sensu lato. Int. J. Med. Microbiol. 2008, 298 (Suppl. S1), 161–167. [Google Scholar] [CrossRef]
- Ciebiera, O.; Grochowalska, R.; Łopińska, A.; Zduniak, P.; Strzała, T.; Jerzak, L. Ticks and spirochetes of the genus Borrelia in urban areas of Central-Western Poland. Exp. Appl. Acarol. 2024, 93, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Collares-Pereira, M.; Couceiro, S.; Franca, I.; Kurtenbach, K.; Schafer, S.M.; Vitorino, L.; Goncalves, L.; Baptista, S.; Vieira, M.L.; Cunha, C. First isolation of Borrelia lusitaniae from a human patient. J. Clin. Microbiol. 2004, 42, 1316–1318. [Google Scholar] [CrossRef]
- de Carvalho, I.L.; Fonseca, J.E.; Marques, J.G.; Ullmann, A.; Hojgaard, A.; Zeidner, N.; Núncio, M.S. Vasculitis-like syndrome associated with Borrelia lusitaniae infection. Clin. Rheumatol. 2008, 27, 1587–1591. [Google Scholar] [CrossRef]
- Veinović, G.; Malinić, J.; Sukara, R.; Mihaljica, D.; Katanić, N.; Poluga, J.; Tomanović, S. Isolation and cultivation of Borrelia lusitaniae from the blood of a patient with multiple erythema migrans. J. Infect. Dev. Ctries. 2025, 19, 630–635. [Google Scholar] [CrossRef]
- Vieira, J.P.; Brito, M.J.; de Carvalho, I.L. Borrelia lusitaniae Infection Mimicking Headache, Neurologic Deficits, and Cerebrospinal Fluid Lymphocytosis. J. Child Neurol. 2019, 34, 748–750. [Google Scholar] [CrossRef]
- Brisson, D.; Dykhuizen, D.E. OspC diversity in Borrelia burgdorferi: Different hosts are different niches. Genetics 2004, 168, 713–722. [Google Scholar] [CrossRef]
- Vuong, H.B.; Canham, C.D.; Fonseca, D.M.; Brisson, D.; Morin, P.J.; Smouse, P.E.; Ostfeld, R.S. Occurrence and transmission efficiencies of Borrelia burgdorferi ospC types in avian and mammalian wildlife. Infect. Genet. Evol. 2014, 27, 594–600. [Google Scholar] [CrossRef]
- Michalik, J.; Wodecka, B.; Liberska, J.; Dabert, M.; Postawa, T.; Piksa, K.; Stańczak, J. Diversity of Lyme borreliosis spirochete species in Ixodes spp. ticks (Acari: Ixodidae) associated with cave-dwelling bats from Poland and Romania. Ticks Tick-Borne Dis. 2020, 11, 101300. [Google Scholar] [CrossRef]
- Radolf, J.D.; Strle, K.; Lemieux, J.E.; Strle, F. Lyme disease in humans. Curr. Issues Mol. Biol. 2021, 42, 333–384. [Google Scholar]
- Strzelczyk, J.K.; Gaździcka, J.; Cuber, P.; Asman, M.; Trapp, G.; Gołąbek, K.; Zalewska-Ziob, M.; Nowak-Chmura, M.; Siuda, K.; Wiczkowski, A.; et al. Prevalence of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected from southern Poland. Acta Parasitol. 2015, 60, 666–674. [Google Scholar] [CrossRef]
- Wójcik-Fatla, A.; Zając, V.; Sawczyn, A.; Sroka, J.; Cisak, E.; Dutkiewicz, J. Infections and mixed infections with the selected species of Borrelia burgdorferi sensu lato complex in Ixodes ricinus ticks collected in eastern Poland: A significant increase in the course of 5 years. Exp. Appl. Acarol. 2016, 68, 197–212. [Google Scholar] [CrossRef]
- Sawczyn-Domańska, A.; Zwoliński, J.; Kloc, A.; Wójcik-Fatla, A. Prevalence of Borrelia, Neoehrlichia mikurensis and Babesia in ticks collected from vegetation in eastern Poland. Exp. Appl. Acarol. 2023, 90, 409–428. [Google Scholar] [CrossRef]
- Paradowska-Stankiewicz, I.; Zbrzeźniak, J.; Skufca, J.; Nagarajan, A.; Ochocka, P.; Pilz, A.; Vyse, A.; Begier, E.; Dzingina, M.; Blum, M.; et al. A Retrospective Database Study of Lyme Borreliosis Incidence in Poland from 2015 to 2019: A Public Health Concern. Vector Borne Zoonotic Dis. 2023, 23, 247–255. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).