The Influence of Different Light Spectra on Broiler Chicken Endocrine Systems and Productivity
Simple Summary
Abstract
1. Introduction
2. Light Detection Pathways: Visual and Non-Visual Mechanisms in Physiological Regulation
3. Interactions Between the Endocrine System and the Circadian Clock in Avian Physiology
4. Pineal Gland
5. Hypothalamus
6. Implications of Hormone Signaling in Muscle Tissue Development
7. The Somatotropic Axis
7.1. Hormonal Influence on Tissues
7.2. Hormone-Mediated Regulatory Signaling
7.3. The Effect of Light on the Somatotropic Axis
8. Hypothalamic–Pituitary–Gonadal Axis
8.1. Hormonal Influence on Tissues
8.2. Hormone-Mediated Regulatory Signaling
8.3. The Effect of Light on the Hypothalamic-Pituitary-Gonadal (HPG) Axis
9. Hypothalamic-Pituitary-Thyroid Axis
9.1. Anatomy and Histology of the Thyroid Gland
9.2. Hormonal Influence on Tissues
9.3. Hormone-Mediated Regulatory Signaling
9.4. The Effect of Light on the Hypothalamic-Pituitary-Thyroid (HPT) Axis
10. Hypothalamus-Pituitary-Adrenal Axis
10.1. Anatomy and Histology of the Adrenal Glands
10.2. Hormonal Influence on Tissues
10.3. Hormone-Mediated Regulatory Signaling
10.4. The Effect of Light on the Hypothalamic–Pituitary–Adrenal (HPA) Axis
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LED | Light-emitting diode |
| GHRH | Growth hormone-releasing hormone |
| IGF-1 | Insulin-like growth factor 1 |
| GnRH | gonadotropin-releasing hormone |
| LH | luteinizing hormone |
| FSH | follicle-stimulating hormone |
| TRH | Thyrotropin-releasing hormone |
| TSH | Thyroid-stimulating hormone |
| T4 | Thyroxine |
| T3 | Triiodothyronine |
| CRH | Corticotropin-releasing hormone |
| ACTH | Adrenocorticotropic hormone |
| ALAN | Artificial light at night |
| DBPs | Deep Brain Photoreceptors |
| SCN | Suprachiasmatic nucleus |
| VA opsin | Vertebrate Ancient opsin |
| MBH | Mediobasal hypothalamus |
| Per1 | Period Circadian Regulator 1 |
| cAMP | Cyclic adenosine monophosphate |
| HPA | Hpothalamic–pituitary–adrenal |
| SCG | Superior cervical ganglia |
| Pit-1 | Pituitary-specific transcription factor 1 |
| Mel 1b | Melatonin receptor type 1b |
| Mel 1c | Melatonin receptor type 1c |
| SST | Somatostatin |
| GHRL | Ghrelin |
| Mel 1a | Melatonin receptor type 1a |
| POA | Preoptic area |
| GHR | Growth hormone receptor |
| IGFs | Insulin-like growing factors |
| IGFR1 | Insulin-like Growth Factor 1 Receptor |
| IGFBPs | Insulin-like growth factor binding proteins |
| CORT | Corticosterone |
| NR3C1 | Nuclear Receptor Subfamily 3, Group C, Member 1 |
| CBG | Corticosteroid-Binding Globulin |
| FCR | Feed conversion ratio |
| DIOs | Deiodinases |
| ECM | Extracellular matrix |
| HGF | Hepatocyte growth factor |
| FGF2 | Fibroblast growth factor 2 |
| IGF | Insulin-like growth factor |
| TGF-β | Transforming growth factor-beta |
| MyoD1 | Myoblast determination protein 1 |
| MYF5 | Myogenic factor 5 |
| MRFs | Myogenic regulatory factors |
| IGF2 | Insulin-like Growth Factor 2 |
| THs | Thyroid hormones |
| SSTR2 | Somatostatin receptor 2 |
| IGFBP1 | Insulin-like Growth Factor Binding Protein 1 |
| IGFBP7 | Insulin-like Growth Factor Binding Protein 7 |
| IGFBP6 | Insulin-like Growth Factor Binding Protein 6 |
| IGFALS | Insulin-like Growth Factor Acid-Labile Subunit |
| IGFBP3 | Insulin-like Growth Factor Binding Protein 3 |
| IGFBP2 | Insulin-like Growth Factor Binding Protein 2 |
| IGFBP4 | Insulin-like Growth Factor Binding Protein 4 |
| IGFBP5 | Insulin-like Growth Factor Binding Protein 5 |
| PVN | Hypothalamic paraventricular nucleus |
| GPCR | G Protein-Coupled Receptor |
| ATP | Adenosine Triphosphate |
| PKA | Protein kinase A |
| CREB | cAMP Response Element-Binding protein |
| SSTR2A | Somatostatin Receptor 2A |
| SSTR2B | Somatostatin Receptor 2B |
| RL | Red light |
| GL | Green light |
| SSTR1–5 | Somatostatin Receptors 1 to 5 |
| JAK2/STAT5 | Janus Kinase 2/Signal Transducer and Activator of Transcription 5 |
| Src/ERK | Src kinase/Extracellular signal-Regulated Kinase |
| Akt | Protein Kinase B |
| mTOR | Mechanistic Target of Rapamycin |
| TSC2 | Tuberous sclerosis complex 2 |
| RPTOR | Regulatory Associated Protein of mTOR |
| mTORC1 | Mechanistic Target of Rapamycin Complex 1 |
| SREBPs | Sterol regulatory element-binding proteins |
| S6Ks | Ribosomal Protein S6 kinases |
| BL | Blue light |
| PCNA | Proliferating Cell Nuclear Antigen |
| WL | White light |
| BDNF | Brain-Derived Neurotrophic Factor |
| TrkB | Ropomyosin receptor kinase B |
| ERK | Extracellular signal-regulated kinase |
| GnIH | Gonadotropin-Inhibitory Hormone |
| cGnRH-I | chicken Gonadotropin-Releasing Hormone I |
| HPG | hypothalamo–pituitary–gonadal axis |
| VIP | Vasoactive intestinal peptide |
| NPY | neuropeptide Y |
| cGnRH-R | chicken Gonadotropin-Releasing Hormone Receptor |
| cGnRH-II | chicken Gonadotropin-Releasing Hormone II |
| PLC | phospholipase C |
| PIP2 | phosphatidylinositol-4,5-bisphosphate |
| IP3 | inositol-1,4,5-trisphosphate |
| DAG | diacylglycerol |
| Gi | Guanine nucleotide-binding protein, inhibitory |
| Gs | Guanine nucleotide-binding protein, stimulatory |
| TG | Thyroglobulin |
| TH | thyroid hormone |
| HPT | Hypothalamic-pituitary-thyroid axis |
| CRHR2 | Corticotropin-Releasing Hormone Receptor 2 |
| TTR | Transthyretin |
| TBG | Thyroxine-binding globulin |
| DIO1 | Type 1 Deiodinase |
| DIO2 | Type 2 Deiodinase |
| THRA | Thyroid Hormone Receptor Alpha |
| THRB | Thyroid Hormone Receptor Beta |
| DIO3 | Type 3 Deiodinase |
| T2 | 3,5-diiodo-L-thyronine |
| rT3 | Reverse Triiodothyronine |
| THRs | Thyroid hormone receptors |
| α-MSH | α-melanocyte-stimulating hormone |
| NA | Noradrenaline |
| TRHR-1 | Thyrotropin-Releasing Hormone Receptor 1 |
| TSHR | Thyroid-Stimulating Hormone Receptor |
| MIT | Monoiodotyrosine |
| DIT | Diiodotyrosine |
| TREs | Thyroid hormone response elements |
| FT3 | Free Triiodothyronine |
| MHC | Myosin Heavy Chain |
| AD | Adrenaline |
| CRHRs | Corticotropin-releasing hormone receptors |
| POMC | Precursor proopiomelanocortin |
| PC1/3 | Prohormone convertase 1/3 |
| MC2R | Melanocortin-2 receptor |
| MRAPs | Melanocortin receptor accessory proteins |
| HDAC6 | Histone deacetylase 6 |
| GRs | Glucocorticoid receptors |
| GCs | Circulating glucocorticoids |
References
- Parvin, R.; Mushtaq, M.; Kim, M.; Choi, H. Light Emitting Diode (LED) as a Source of Monochromatic Light: A Novel Lighting Approach for Behaviour, Physiology and Welfare of Poultry. World Poult. Sci. J. 2014, 70, 543–556. [Google Scholar] [CrossRef]
- Pap, T.I.; Szabó, R.T.; Bodnár, Á.; Pajor, F.; Egerszegi, I.; Podmaniczky, B.; Pacz, M.; Mezőszentgyörgyi, D.; Kovács-Weber, M. Effect of Lighting Methods on the Production, Behavior and Meat Quality Parameters of Broiler Chickens. Animals 2024, 14, 1827. [Google Scholar] [CrossRef]
- Luo, G.; Cheng, Y.; Xu, Y.; Liu, J.; Yang, W.; Liu, J.; Guo, B.; Zhu, H. Monochromatic Light Impacts the Growth Performance, Intestinal Morphology, Barrier Function, Antioxidant Status, and Microflora of Yangzhou Geese. Animals 2025, 15, 1815. [Google Scholar] [CrossRef]
- Wathes, C.; Spechter, H.; Bray, T. The Effects of Light Illuminance and Wavelength on the Growth of Broiler Chickens. J. Agric. Sci. 1982, 98, 195–201. [Google Scholar] [CrossRef]
- Prayitno, D.; Phillips, C.; Omed, H. The Effects of Color of Lighting on the Behavior and Production of Meat Chickens. Poult. Sci. 1997, 76, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Rozenboim, I.; Biran, I.; Uni, Z.; Robinzon, B.; Halevy, O. The Effect of Monochromatic Light on Broiler Growth and Development. Poult. Sci. 1999, 78, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Liu, W.; Wang, Z.; Xie, D.; Jia, L.; Chen, Y. Green and Blue Monochromatic Lights Promote Growth and Development of Broilers via Stimulating Testosterone Secretion and Myofiber Growth. J. Appl. Poult. Res. 2008, 17, 211–218. [Google Scholar] [CrossRef]
- Ke, Y.; Liu, W.; Wang, Z.; Chen, Y. Effects of Monochromatic Light on Quality Properties and Antioxidation of Meat in Broilers. Poult. Sci. 2011, 90, 2632–2637. [Google Scholar] [CrossRef]
- Mohamed, R.; Eltholth, M.; El-Saidy, N. Rearing Broiler Chickens under Monochromatic Blue Light Improve Performance and Reduce Fear and Stress during Pre-Slaughter Handling and Transportation. Biotechnol. Anim. Husb. 2014, 30, 457–471. [Google Scholar] [CrossRef]
- Soliman, F.N.; El-Sabrout, K. Light Wavelengths/Colors: Future Prospects for Broiler Behavior and Production. J. Vet. Behav. 2020, 36, 34–39. [Google Scholar] [CrossRef]
- Abdel-Moneim, E.A.-M.; Siddiqui, S.A.; Shehata, A.M.; Biswas, A.; Abougabal, M.S.; Kamal, A.M.; Mesalam, N.M.; Elsayed, M.A.; Yang, B.; Ebeid, T.A. Impact of lIght Wavelength on Growth and Welfare of broIler chIckens–overvIew and Future perspectIve. Ann. Anim. Sci. 2024, 24, 731–748. [Google Scholar] [CrossRef]
- Rozenboim, I.; El Halawani, M.; Kashash, Y.; Piestun, Y.; Halevy, O. The Effect of Monochromatic Photostimulation on Growth and Development of Broiler Birds. Gen. Comp. Endocrinol. 2013, 190, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Gharahveysi, S.; Irani, M.; Kenari, T.A.; Mahmud, K.I. Effects of Colour and Intensity of Artificial Light Produced by Incandescent Bulbs on the Performance Traits, Thyroid Hormones, and Blood Metabolites of Broiler Chickens. Ital. J. Anim. Sci. 2020, 19, 1–7. [Google Scholar] [CrossRef]
- Jin, E.; Jia, L.; Li, J.; Yang, G.; Wang, Z.; Cao, J.; Chen, Y. Effect of Monochromatic Light on Melatonin Secretion and Arylalkylamine N-acetyltransferase mRNA Expression in the Retina and Pineal Gland of Broilers. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2011, 294, 1233–1241. [Google Scholar] [CrossRef]
- Hayat, K.; Zheng, R.; Zeng, L.; Ye, Z.; Pan, J. Impact of Full-Spectrum and Infrared Lighting on Growth, Oxidative Stress, and Cecal Microbiota in Broilers. Antioxidants 2024, 13, 1442. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, Y.; Pan, J.; Ying, Y.; Zhou, H. A New Method to Manipulate Broiler Chicken Growth and Metabolism: Response to Mixed LED Light System. Sci. Rep. 2016, 6, 25972. [Google Scholar] [CrossRef]
- Halevy, O.; Biran, I.; Rozenboim, I. Various Light Source Treatments Affect Body and Skeletal Muscle Growth by Affecting Skeletal Muscle Satellite Cell Proliferation in Broilers. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 120, 317–323. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.; Chen, Y. Effects of Monochromatic Light on Developmental Changes in Satellite Cell Population of Pectoral Muscle in Broilers during Early Posthatch Period. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2010, 293, 1315–1324. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, J.; Wang, Z.; Dong, Y.; Chen, Y. Melatonin Modulates Monochromatic Light-Induced GHRH Expression in the Hypothalamus and GH Secretion in Chicks. Acta Histochem. 2016, 118, 286–292. [Google Scholar] [CrossRef]
- Reyad, A.R.N.; Mohamed, O.A. A Comparative Study of The Effect of White And Blue-Green LED Light Wavelengths On Growth Performance, Feed Efficiency, And Behavioural Traits In Cobb Broiler Chickens. Egypt. Poult. Sci. J. 2024, 44, 393–402. [Google Scholar] [CrossRef]
- Olanrewaju, H.; Miller, W.; Maslin, W.; Collier, S.; Purswell, J.; Branton, S. Effects of Light Sources and Intensity on Broilers Grown to Heavy Weights. Part 1: Growth Performance, Carcass Characteristics, and Welfare Indices. Poult. Sci. 2016, 95, 727–735. [Google Scholar] [CrossRef]
- Nelson, J.R.; Bray, J.L.; Delabbio, J.; Archer, G.S. Light Emitting Diode (LED) Color and Broiler Growth: Effect of Supplementing Blue/Green LED to White LED Light on Broiler Growth, Stress, and Welfare. Poult. Sci. 2020, 99, 3519–3524. [Google Scholar] [CrossRef] [PubMed]
- Russart, K.L.; Nelson, R.J. Light at Night as an Environmental Endocrine Disruptor. Physiol. Behav. 2018, 190, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.Q.; Davies, S.; Dominoni, D. Hormonally Mediated Effects of Artificial Light at Night on Behavior and Fitness: Linking Endocrine Mechanisms with Function. J. Exp. Biol. 2018, 221, jeb156893. [Google Scholar] [CrossRef] [PubMed]
- Falcón, J.; Torriglia, A.; Attia, D.; Viénot, F.; Gronfier, C.; Behar-Cohen, F.; Martinsons, C.; Hicks, D. Exposure to Artificial Light at Night and the Consequences for Flora, Fauna, and Ecosystems. Front. Neurosci. 2020, 14, 602796. [Google Scholar] [CrossRef]
- Grunst, M.L.; Grunst, A.S. Endocrine Effects of Exposure to Artificial Light at Night: A Review and Synthesis of Knowledge Gaps. Mol. Cell. Endocrinol. 2023, 568, 111927. [Google Scholar] [CrossRef]
- Atkinson, H.C.; Waddell, B.J. Circadian Variation in Basal Plasma Corticosterone and Adrenocorticotropin in the Rat: Sexual Dimorphism and Changes across the Estrous Cycle. Endocrinology 1997, 138, 3842–3848. [Google Scholar] [CrossRef]
- Guchhait, P.; Haldar, C. Circadian Rhythms of Melatonin and Sex Steroids in a Nocturnal Bird, Indian Spotted Owlet Athene Brama during Reproductively Active and Inactive Phases. Biol. Rhythm Res. 1999, 30, 508–516. [Google Scholar] [CrossRef]
- Li, S.; Cao, J.; Wang, Z.; Dong, Y.; Wang, W.; Chen, Y. Melatonin Mediates Monochromatic Light-induced Insulin-like Growth Factor 1 Secretion of Chick Liver: Involvement of Membrane Receptors. Photochem. Photobiol. 2016, 92, 595–603. [Google Scholar] [CrossRef]
- Çalişlar, S.; Yeter, B.; Şahin, A. Importance of Melatonin on Poultry. Tarim Doga Derg. 2018, 21, 987. [Google Scholar] [CrossRef]
- Chmura, H.E.; Wingfield, J.C.; Hahn, T.P. Non-photic Environmental Cues and Avian Reproduction in an Era of Global Change. J. Avian Biol. 2020, 51, e02243. [Google Scholar] [CrossRef]
- Hussein, A.A.; Bloem, E.; Fodor, I.; Baz, E.-S.; Tadros, M.M.; Soliman, M.F.; El-Shenawy, N.S.; Koene, J.M. Slowly Seeing the Light: An Integrative Review on Ecological Light Pollution as a Potential Threat for Mollusks. Environ. Sci. Pollut. Res. 2021, 28, 5036–5048. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.; Cunningham, F. Effect of Increasing Day Length and Intermittent Lighting Schedules in the Domestic Hen on Plasma Concentrations of Luteinizing Hormone (LH) and the LH Response to Exogenous Progesterone. Gen. Comp. Endocrinol. 1980, 41, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Manser, C.E.; Elliott, H.; Morris, T.; Broom, D. The Use of a Novel Operant Test to Determine the Strength of Preference for Flooring in Laboratory Rats. Lab. Anim. 1996, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Deep, A.; Schwean-Lardner, K.; Crowe, T.; Fancher, B.; Classen, H. Effect of Light Intensity on Broiler Production, Processing Characteristics, and Welfare. Poult. Sci. 2010, 89, 2326–2333. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Fero, K.; Driever, W.; Burgess, H.A. Enlightening the Brain: Linking Deep Brain Photoreception with Behavior and Physiology. Bioessays 2013, 35, 775–779. [Google Scholar] [CrossRef]
- Aulsebrook, A.E.; Johnsson, R.D.; Lesku, J.A. Light, Sleep and Performance in Diurnal Birds. Clocks Sleep 2021, 3, 115–131. [Google Scholar] [CrossRef]
- ViviD, D.; Bentley, G.E. Seasonal Reproduction in Vertebrates: Melatonin Synthesis, Binding, and Functionality Using Tinbergen’s Four Questions. Molecules 2018, 23, 652. [Google Scholar] [CrossRef]
- Collins, S.; Forkman, B.; Kristensen, H.H.; Sandøe, P.; Hocking, P.M. Investigating the Importance of Vision in Poultry: Comparing the Behaviour of Blind and Sighted Chickens. Appl. Anim. Behav. Sci. 2011, 133, 60–69. [Google Scholar] [CrossRef]
- Dawson, A.; King, V.M.; Bentley, G.E.; Ball, G.F. Photoperiodic Control of Seasonality in Birds. J. Biol. Rhythm. 2001, 16, 365–380. [Google Scholar] [CrossRef]
- Baxter, M.; Joseph, N.; Osborne, V.; Bédécarrats, G. Red Light Is Necessary to Activate the Reproductive Axis in Chickens Independently of the Retina of the Eye. Poult. Sci. 2014, 93, 1289–1297. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, J.; Quan, S.; Yang, Y. Light Regimen on Health and Growth of Broilers: An Update Review. Poult. Sci. 2022, 101, 101545. [Google Scholar] [CrossRef]
- Bowmaker, J.K.; Knowles, A. The Visual Pigments and Oil Droplets of the Chicken Retina. Vis. Res. 1977, 17, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Kram, Y.A.; Mantey, S.; Corbo, J.C. Avian Cone Photoreceptors Tile the Retina as Five Independent, Self-Organizing Mosaics. PLoS ONE 2010, 5, e8992. [Google Scholar] [CrossRef] [PubMed]
- Golombek, D.A.; Rosenstein, R.E. Physiology of Circadian Entrainment. Physiol. Rev. 2010, 90, 1063–1102. [Google Scholar] [CrossRef] [PubMed]
- Halford, S.; Pires, S.S.; Turton, M.; Zheng, L.; González-Menéndez, I.; Davies, W.L.; Peirson, S.N.; García-Fernández, J.M.; Hankins, M.W.; Foster, R.G. VA Opsin-Based Photoreceptors in the Hypothalamus of Birds. Curr. Biol. 2009, 19, 1396–1402. [Google Scholar] [CrossRef]
- Kuenzel, W.J.; Kang, S.W.; Zhou, Z.J. Exploring Avian Deep-Brain Photoreceptors and Their Role in Activating the Neuroendocrine Regulation of Gonadal Development. Poult. Sci. 2015, 94, 786–798. [Google Scholar] [CrossRef]
- Wang, G.; Wingfield, J.C. Immunocytochemical Study of Rhodopsin-Containing Putative Encephalic Photoreceptors in House Sparrow, Passer domesticus. Gen. Comp. Endocrinol. 2011, 170, 589–596. [Google Scholar] [CrossRef]
- Nakane, Y.; Yoshimura, T. Deep Brain Photoreceptors and a Seasonal Signal Transduction Cascade in Birds. Cell Tissue Res. 2010, 342, 341–344. [Google Scholar] [CrossRef]
- Kang, S.W. Central Nervous System Associated with Light Perception and Physiological Responses of Birds. Front. Physiol. 2021, 12, 723454. [Google Scholar] [CrossRef]
- Davies, W.I.; Turton, M.; Peirson, S.N.; Follett, B.K.; Halford, S.; Garcia-Fernandez, J.M.; Sharp, P.J.; Hankins, M.W.; Foster, R.G. Vertebrate Ancient Opsin Photopigment Spectra and the Avian Photoperiodic Response. Biol. Lett. 2012, 8, 291–294. [Google Scholar] [CrossRef]
- Menaker, M.; Keatts, H. Extraretinal Light Perception in the Sparrow. II. Photoperiodic Stimulation of Testis Growth. Proc. Natl. Acad. Sci. USA 1968, 60, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Nakane, Y.; Ikegami, K.; Ono, H.; Yamamoto, N.; Yoshida, S.; Hirunagi, K.; Ebihara, S.; Kubo, Y.; Yoshimura, T. A Mammalian Neural Tissue Opsin (Opsin 5) Is a Deep Brain Photoreceptor in Birds. Proc. Natl. Acad. Sci. USA 2010, 107, 15264–15268. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Wang, Z.; Chen, Y.; Cao, J. Melanopsin-Mediated Optical Entrainment Regulates Circadian Rhythms in Vertebrates. Commun. Biol. 2023, 6, 1054. [Google Scholar] [CrossRef] [PubMed]
- Hankins, M.W.; Davies, W.I.; Foster, R.G. The Evolution of Non-Visual Photopigments in the Central Nervous System of Vertebrates. In Evolution of Visual and Non-Visual Pigments; Springer: Boston, MA, USA, 2014; pp. 65–103. [Google Scholar]
- Foster, R.; Follett, B.; Lythgoe, J. Rhodopsin-like Sensitivity of Extra-Retinal Photoreceptors Mediating the Photoperiodic Response in Quail. Nature 1985, 313, 50–52. [Google Scholar] [CrossRef]
- Hartwig, H.; Van Veen, T. Spectral Characteristics of Visible Radiation Penetrating into the Brain and Stimulating Extraretinal Photoreceptors: Transmission Recordings in Vertebrates. J. Comp. Physiol. 1979, 130, 277–282. [Google Scholar] [CrossRef]
- Yasuo, S.; Watanabe, M.; Okabayashi, N.; Ebihara, S.; Yoshimura, T. Circadian Clock Genes and Photoperiodism: Comprehensive Analysis of Clock Gene Expression in the Mediobasal Hypothalamus, the Suprachiasmatic Nucleus, and the Pineal Gland of Japanese Quail under Various Light Schedules. Endocrinology 2003, 144, 3742–3748. [Google Scholar] [CrossRef]
- Follett, B.; Nicholls, T. Influences of Thyroidectomy and Thyroxine Replacement on Photoperiodically Controlled Reproduction in Quail. J. Endocrinol. 1985, 107, 211–221. [Google Scholar] [CrossRef]
- Yoshimura, T.; Yasuo, S.; Watanabe, M.; Iigo, M.; Yamamura, T.; Hirunagi, K.; Ebihara, S. Light-Induced Hormone Conversion of T4 to T3 Regulates Photoperiodic Response of Gonads in Birds. Nature 2003, 426, 178–181. [Google Scholar] [CrossRef]
- Cassone, V.M.; Yoshimura, T. Circannual Cycles and Photoperiodism. In Sturkie’s Avian Physiology; Elsevier: Amsterdam, The Netherland, 2022; pp. 1183–1201. [Google Scholar]
- Xu, J.; Jarocha, L.E.; Zollitsch, T.; Konowalczyk, M.; Henbest, K.B.; Richert, S.; Golesworthy, M.J.; Schmidt, J.; Déjean, V.; Sowood, D.J. Magnetic Sensitivity of Cryptochrome 4 from a Migratory Songbird. Nature 2021, 594, 535–540. [Google Scholar] [CrossRef]
- Nader, N.; Chrousos, G.P.; Kino, T. Interactions of the Circadian CLOCK System and the HPA Axis. Trends Endocrinol. Metab. 2010, 21, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Cermakian, N.; Lange, T.; Golombek, D.; Sarkar, D.; Nakao, A.; Shibata, S.; Mazzoccoli, G. Crosstalk between the Circadian Clock Circuitry and the Immune System. Chronobiol. Int. 2013, 30, 870–888. [Google Scholar] [CrossRef] [PubMed]
- Markowska, M.; Majewski, P.M.; Skwarło-Sońta, K. Avian Biological Clock–Immune System Relationship. Dev. Comp. Immunol. 2017, 66, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Jerigova, V.; Zeman, M.; Okuliarova, M. Circadian Disruption and Consequences on Innate Immunity and Inflammatory Response. Int. J. Mol. Sci. 2022, 23, 13722. [Google Scholar] [CrossRef]
- Mishra, I.; Singh, D.; Kumar, V. Temporal Expression of C-Fos and Genes Coding for Neuropeptides and Enzymes of Amino Acid and Amine Neurotransmitter Biosynthesis in Retina, Pineal and Hypothalamus of a Migratory Songbird: Evidence for Circadian Rhythm-Dependent Seasonal Responses. Neuroscience 2018, 371, 309–324. [Google Scholar] [CrossRef]
- Natesan, A.; Geetha, L.; Zatz, M. Rhythm and Soul in the Avian Pineal. Cell Tissue Res. 2002, 309, 35–45. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. BMAL1 but Not CLOCK Is Associated with Monochromatic Green Light-Induced Circadian Rhythm of Melatonin in Chick Pinealocytes. Endocr. Connect. 2019, 8, 57–68. [Google Scholar] [CrossRef]
- Beker, M.C.; Caglayan, B.; Caglayan, A.B.; Kelestemur, T.; Yalcin, E.; Caglayan, A.; Kilic, U.; Baykal, A.T.; Reiter, R.J.; Kilic, E. Interaction of Melatonin and Bmal1 in the Regulation of PI3K/AKT Pathway Components and Cellular Survival. Sci. Rep. 2019, 9, 19082. [Google Scholar] [CrossRef]
- Gotlieb, N.; Moeller, J.; Kriegsfeld, L.J. Circadian Control of Neuroendocrine Function: Implications for Health and Disease. Curr. Opin. Physiol. 2018, 5, 133–140. [Google Scholar] [CrossRef]
- Rich, E.; Romero, L. Daily and Photoperiod Variations of Basal and Stress-Induced Corticosterone Concentrations in House Sparrows (Passer domesticus). J. Comp. Physiol. B 2001, 171, 543–547. [Google Scholar]
- Schwabl, P.; Bonaccorso, E.; Goymann, W. Diurnal Variation in Corticosterone Release among Wild Tropical Forest Birds. Front. Zool. 2016, 13, 19. [Google Scholar] [CrossRef]
- Huffeldt, N.P.; Merkel, F.R.; Jenni-Eiermann, S.; Goymann, W.; Helm, B. Melatonin and Corticosterone Profiles under Polar Day in a Seabird with Sexually Opposite Activity-Rhythms. Gen. Comp. Endocrinol. 2020, 285, 113296. [Google Scholar] [CrossRef]
- Greives, T.; Eshleman, M.; Galante, H.; Elderbrock, E.; Deimel, C.; Hau, M. Early Nighttime Testosterone Peaks Are Correlated with GnRH-Induced Testosterone in a Diurnal Songbird. Gen. Comp. Endocrinol. 2021, 312, 113861. [Google Scholar] [CrossRef]
- Graham, J.L.; Needham, K.B.; Bertucci, E.M.; Pearson, A.A.; Bauer, C.M.; Greives, T.J. Onset of Daily Activity in a Female Songbird Is Related to Peak-Induced Estradiol Levels. Integr. Comp. Biol. 2019, 59, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Elderbrock, E.K.; Hau, M.; Greives, T.J. Sex Steroids Modulate Circadian Behavioral Rhythms in Captive Animals, but Does This Matter in the Wild? Horm. Behav. 2021, 128, 104900. [Google Scholar] [CrossRef]
- Gwinner, F. Testosterone Induces” Splitting” of Circadian Locomotor Activity Rhythms in Birds. Science 1974, 185, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Quay, W. Histological Structure and Cytology of the Pineal Organ in Birds and Mammals. Prog. Brain Res. 1965, 10, 49–86. [Google Scholar] [PubMed]
- Fejér, Z.; Röhlich, P.; Szél, Á.; Dávid, C.; Zádori, A.; João Manzano, M.; Vígh, B. Comparative Ultrastructure and Cytochemistry of the Avian Pineal Organ. Microsc. Res. Tech. 2001, 53, 12–24. [Google Scholar] [CrossRef]
- Haldar, C.; Bishnupuri, K. Comparative View of Pineal Gland Morphology of Nocturnal and Diurnal Birds of Tropical Origin. Microsc. Res. Tech. 2001, 53, 25–32. [Google Scholar] [CrossRef]
- Przybylska-Gornowicz, B.; Lewczuk, B.; Prusik, M.; Nowicki, M. Post-Hatching Development of the Turkey Pineal Organ: Histological and Immunohistochemical Studies. Neuroendocrinol. Lett. 2005, 26, 383–392. [Google Scholar]
- Prusik, M.; Lewczuk, B.; Nowicki, M.; Przybylska-Gornowicz, B. Histology and Ultrastructure of the Pineal Organ in the Domestic Goose. Histol. Histopathol. 2006, 21, 1075–1090. [Google Scholar]
- Sato, T.; Wake, K. Innervation of the Avian Pineal Organ: A Comparative Study. Cell Tissue Res. 1983, 233, 237–264. [Google Scholar] [CrossRef]
- Korf, H.-W.; Zimmerman, N.H.; Oksche, A. Intrinsic Neurons and Neural Connections of the Pineal Organ of the House Sparrow, Passer domesticus, as Revealed by Anterograde and Retrograde Transport of Horseradish Peroxidase. Cell Tissue Res. 1982, 222, 243–260. [Google Scholar] [CrossRef]
- Csernus, V.J. The Avian Pineal Gland. Chronobiol. Int. 2006, 23, 329–339. [Google Scholar] [CrossRef]
- Okano, T.; Yoshizawa, T.; Fukada, Y. Pinopsin Is a Chicken Pineal Photoreceptive Molecule. Nature 1994, 372, 94–97. [Google Scholar] [CrossRef]
- Robertson, L.M.; Takahashi, J.S. Circadian Clock in Cell Culture: II. In Vitro Photic Entrainment of Melatonin Oscillation from Dissociated Chick Pineal Cells. J. Neurosci. 1988, 8, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S.; Murakami, N.; Nikaido, S.S.; Pratt, B.L.; Robertson, L.M. The Avian Pineal, a Vertebrate Model System of the Circadian Oscillator: Cellular Regulation of Circadian Rhythms by Light, Second Messengers, and Macromolecular Synthesis. In Proceedings of the 1988 Laurentian Hormone Conference; Elsevier: Amsterdam, The Netherlands, 1989; Volume 45, pp. 279–352. [Google Scholar]
- Prusik, M. Developmental Morphology of the Turkey Pineal Gland in Histological Images and 3D Models. Micron 2022, 153, 103196. [Google Scholar] [CrossRef] [PubMed]
- Olah, I.; Glick, B. Lymphopineal Tissue in the Chicken. Dev. Comp. Immunol. 1984, 8, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Doi, O.; Ohno, A.; Kaneda, T.; Iwasawa, A.; Nakamura, T. Immunohistochemical Demonstration and Daily Rhythms of Melatonin in the Chicken Pineal Gland. Jpn. Poult. Sci. 1995, 32, 90–98. [Google Scholar] [CrossRef]
- Timothy, C.; Birdsall, N. The Biological Effects and Clinical Uses of the Pineal Hormone Melatonin. Altern. Med. Rev. 1996, 1, 94–102. [Google Scholar]
- Barrenetxe, J.; Delagrange, P.; Martinez, J. Physiological and Metabolic Functions of Melatonin. J. Physiol. Biochem. 2004, 60, 61–72. [Google Scholar] [CrossRef]
- Ziółkowska, N.; Prusik, M.; Lewczuk, B.; Przybylska-Gornowicz, B. Regulation of Melatonin Secretion in the Pineal Organ of the Domestic Duck–an in Vitro Study. Pol. J. Vet. Sci. 2015, 18, 635–644. [Google Scholar]
- Sinkalu, V.O.; Ayo, J.O.; Abimbola, A.A.; Ibrahim, J.E. Effects of Melatonin on Cloacal Temperature and Erythrocyte Osmotic Fragility in Layer Hens during the Hot-Dry Season. J. Appl. Anim. Res. 2015, 43, 52–60. [Google Scholar] [CrossRef]
- Şener, G. Karanlığın Hormonu: Melatonin. Marmara Pharm. J. 2010, 14, 112–120. [Google Scholar] [CrossRef]
- Bubenik, G.A. Gastrointestinal Melatonin: Localization, Function, and Clinical Relevance. Dig. Dis. Sci. 2002, 47, 2336–2348. [Google Scholar] [CrossRef]
- Cassone, V.M.; Brooks, D.S.; Kelm, T.A. Comparative Distribution of 2 [125I] Iodomelatonin Binding in the Brains of Diurnal Birds: Outgroup Analysis with Turtles. Brain Behav. Evol. 1995, 45, 241–256. [Google Scholar] [CrossRef]
- Lee, P.P.; Pang, S.F. Identification and Characterization of Melatonin Binding Sites in the Gastrointestinal Tract of Ducks. Life Sci. 1992, 50, 117–125. [Google Scholar] [CrossRef]
- Dishon, L.; Avital-Cohen, N.; Malamud, D.; Heiblum, R.; Druyan, S.; Porter, T.; Gumułka, M.; Rozenboim, I. In-Ovo Monochromatic Green Light Photostimulation Enhances Embryonic Somatotropic Axis Activity. Poult. Sci. 2017, 96, 1884–1890. [Google Scholar] [CrossRef]
- Xu, Y.; Tang, Y.; Cheng, Y.; Yang, W.; Liu, J.; Guo, B.; Luo, G.; Zhu, H. Effects of Different Monochromatic Light on Growth Performance and Liver Circadian Rhythm of Yangzhou Geese. Poult. Sci. 2025, 104, 104496. [Google Scholar] [CrossRef]
- Yue, L.; Qin, X.; Liu, X.; Wang, Z.; Dong, Y.; Chen, Y.; Cao, J. Melatonin Receptor Mel1b-and Mel1c-mediated Green Light Induced the Secretion of Growth Hormone in Anterior Pituitary of Chicks. Photochem. Photobiol. 2019, 95, 1387–1394. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Wang, Z.; Dong, Y.; Chen, Y.; Cao, J. Mel1b and Mel1c Melatonin Receptors Mediate Green Light-Induced Secretion of Growth Hormone in Chick Adenohypophysis Cells via the AC/PKA and ERK1/2 Signalling Pathways. J. Photochem. Photobiol. B Biol. 2021, 225, 112322. [Google Scholar] [CrossRef]
- Lazăr, R.; Solcan, C.; Creţu, C.; Lazăr, M.; Muntean, C.; Boişteanu, P. Characterization of the Relations between Morphology and Physiological Status of the Pineal Gland in Connection with the Somatic Development Level in Turkeys Reared in Romania. Arq. Bras. Med. Veterinária Zootec. 2015, 67, 763–770. [Google Scholar] [CrossRef]
- Clark, W.; Classen, H. The Effects of Continuously or Diurnally Fed Melatonin on Broiler Performance and Health. Poult. Sci. 1995, 74, 1900–1904. [Google Scholar] [CrossRef]
- Zeman, M.; Výboh, P.; Jurani, M.; Lamosova, D.; Kostal, L.; Bilcik, B.; Blazicek, P.; Juraniova, E. Effects of Exogenous Melatonin on Some Endocrine, Behavioural and Metabolic Parameters in Japanese Quail Coturnix coturnix japonica. Comp. Biochem. Physiol. Part A Physiol. 1993, 105, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Zeman, M.; Buyse, J.; Lamosova, D.; Herichova, I.; Decuypere, E. Role of Melatonin in the Control of Growth and Growth Hormone Secretion in Poultry. Domest. Anim. Endocrinol. 1999, 17, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.; Decuypere, E.; Darras, V.; Berghman, L. Differential Effects of T4 and T3 on TRH and GRF-Induced GH Secretion in the Domestic Fowl. Reprod. Nutr. Dev. 1991, 31, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Horodincu, L.; Solcan, C. Influence of Different Light Spectra on Melatonin Synthesis by the Pineal Gland and Influence on the Immune System in Chickens. Animals 2023, 13, 2095. [Google Scholar] [CrossRef]
- Zheng, L.; Ma, Y.; Gu, L.; Yuan, D.; Shi, M.; Guo, X.; Zhan, X. Growth Performance, Antioxidant Status, and Nonspecific Immunity in Broilers under Different Lighting Regimens. J. Appl. Poult. Res. 2013, 22, 798–807. [Google Scholar] [CrossRef]
- Bubenik, G.A. Localization, Physiological Significance and Possible Clinical Implication of Gastrointestinal Melatonin. Neurosignals 2001, 10, 350–366. [Google Scholar] [CrossRef]
- Ahmed, H.; Essawy, G.; Salem, H.; Abdel Daim, M. Melatonin Has a Strong Antioxidant Activity and Improves Liver and Kidney Functions in Broiler Chicks. Egypt. J. Basic Appl. Physiol. 2005, 4, 77–92. [Google Scholar]
- Sahin, N.; Onderci, M.; Sahin, K.; Gursu, M.; Smith, M. Ascorbic Acid and Melatonin Reduce Heat-Induced Performance Inhibition and Oxidative Stress in Japanese Quails. Br. Poult. Sci. 2004, 45, 116–122. [Google Scholar] [CrossRef]
- Ohta, Y.; Kongo, M.; Sasaki, E.; Nishida, K.; Ishiguro, I. Therapeutic Effect of Melatonin on Carbon Tetrachloride-induced Acute Liver Injury in Rats. J. Pineal Res. 2000, 28, 119–126. [Google Scholar] [CrossRef]
- Kankova, Z.; Drozdova, A.; Hodova, V.; Zeman, M. Effect of Blue and Red Monochromatic Light during Incubation on the Early Post-Embryonic Development of Immune Responses in Broiler Chicken. Br. Poult. Sci. 2022, 63, 541–547. [Google Scholar] [CrossRef]
- Brandstätter, R.; Abraham, U. Hypothalamic Circadian Organization in Birds. I. Anatomy, Functional Morphology, and Terminology of the Suprachiasmatic Region. Chronobiol. Int. 2003, 20, 637–655. [Google Scholar] [CrossRef]
- Pearson, C.A.; Placzek, M. Development of the Medial Hypothalamus: Forming a Functional Hypothalamic-Neurohypophyseal Interface. Curr. Top. Dev. Biol. 2013, 106, 49–88. [Google Scholar]
- Machluf, Y.; Gutnick, A.; Levkowitz, G. Development of the Zebrafish Hypothalamus. Ann. N. Y. Acad. Sci. 2011, 1220, 93–105. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, F.; Cao, J.; Dong, Y.; Wang, Z.; Chen, Y. Melatonin Modulates Monochromatic Light-Induced Melatonin Receptor Expression in the Hypothalamus of Chicks. Acta Histochem. 2017, 119, 733–739. [Google Scholar] [CrossRef]
- Levine, J.E. An Introduction to Neuroendocrine Systems. In Handbook of Neuroendocrinology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 3–19. [Google Scholar]
- Schulkin, J. Evolutionary Conservation of Glucocorticoids and Corticotropin Releasing Hormone: Behavioral and Physiological Adaptations. Brain Res. 2011, 1392, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, K.; Yoshimura, T. The Hypothalamic–Pituitary–Thyroid Axis and Biological Rhythms: The Discovery of TSH’s Unexpected Role Using Animal Models. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.G.; Robinson, L.C. Up and down the Growth Hormone Cascade. Cytokine Growth Factor Rev. 1996, 7, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Gahete, M.D.; Luque, R.M.; Castaño, J.P. Models of GH Deficiency in Animal Studies. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 693–704. [Google Scholar] [CrossRef]
- Vance, M.L. Growth-Hormone-Releasing Hormone. Clin. Chem. 1990, 36, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, G.S.; Painson, J.-C.; Lapointe, M.; Gurd, W.; McCarthy, G.F. Interplay of Somatostatin and Growth Hormone-Releasing Hormone in Genesis of Episodic Growth Hormone Secretion. Metabolism 1990, 39, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Tuggle, C.; Trenkle, A. Control of Growth Hormone Synthesis. Domest. Anim. Endocrinol. 1996, 13, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Toogood, A.A.; Harvey, S.; Thorner, M.O.; Gaylinn, B.D. Cloning of the Chicken Pituitary Receptor for Growth Hormone-Releasing Hormone. Endocrinology 2006, 147, 1838–1846. [Google Scholar] [CrossRef]
- Harvey, S.; Gineste, C.; Gaylinn, B. Growth Hormone (GH)-Releasing Activity of Chicken GH-Releasing Hormone (GHRH) in Chickens. Gen. Comp. Endocrinol. 2014, 204, 261–266. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Wang, C.Y.; Kwok, A.H.Y.; Leung, F.C. Identification of the Endogenous Ligands for Chicken Growth Hormone-Releasing Hormone (GHRH) Receptor: Evidence for a Separate Gene Encoding GHRH in Submammalian Vertebrates. Endocrinology 2007, 148, 2405–2416. [Google Scholar] [CrossRef]
- Woelfle, J.; Chia, D.J.; Massart-Schlesinger, M.B.; Moyano, P.; Rotwein, P. Molecular Physiology, Pathology, and Regulation of the Growth Hormone/Insulin-like Growth Factor-I System. Pediatr. Nephrol. 2005, 20, 295–302. [Google Scholar] [CrossRef]
- Dewil, E.; Darras, V.M.; Spencer, G.S.G.; Lauterio, T.J.; Decuypere, E. The Regulation of GH-Dependent Hormones and Enzymes after Feed Restriction in Dwarf and Control Chickens. Life Sci. 1999, 64, 1359–1371. [Google Scholar] [CrossRef]
- Stewart, C.E.; Rotwein, P. Growth, Differentiation, and Survival: Multiple Physiological Functions for Insulin-like Growth Factors. Physiol. Rev. 1996, 76, 1005–1026. [Google Scholar] [CrossRef]
- Armstrong, D.; McKay, C.; Morrell, D.; Goddard, C. Insulin-like Growth Factor-I Binding Proteins in Serum from the Domestic Fowl. J. Endocrinol. 1989, 120, 373–378. [Google Scholar] [CrossRef]
- Lu, B.; Liang, W.; Liang, C.; Yu, Z.; Xie, X.; Chen, Z. Effect of Heat Stress on Expression of Main Reproductive Hormone in Hypothalamic-Pituitary-Gonadal Axis of Wenchang Chicks. Braz. J. Poult. Sci. 2021, 23, eRBCA-2019. [Google Scholar] [CrossRef]
- Calogero, A.E.; Bernardini, R.; Margioris, A.N.; Bagdy, G.; Gallucci, W.T.; Munson, P.J.; Tamarkin, L.; Tomai, T.P.; Brady, L.; Gold, P.W. Effects of Serotonergic Agonists and Antagonists on Corticotropin-Releasing Hormone Secretion by Explanted Rat Hypothalami. Peptides 1989, 10, 189–200. [Google Scholar] [CrossRef]
- Hayashi, H.; Imai, K.; Imai, K. Characterization of Chicken ACTH and α-MSH: The Primary Sequence of Chicken ACTH Is More Similar to Xenopus ACTH than to Other Avian ACTH. Gen. Comp. Endocrinol. 1991, 82, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Whitnall, M.H. Regulation of the Hypothalamic Corticotropin-Releasing Hormone Neurosecretory System. Prog. Neurobiol. 1993, 40, 573–629. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xie, L.; Abou-Samra, A. Molecular Cloning of a Type A Chicken Corticotropin-Releasing Factor Receptor with High Affinity for Urotensin I. Endocrinology 1996, 137, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Webster, E.; Lewis, D.; Torpy, D.; Zachman, E.; Rice, K.; Chrousos, G. In Vivo and in Vitro Characterization of Antalarmin, a Nonpeptide Corticotropin-Releasing Hormone (CRH) Receptor Antagonist: Suppression of Pituitary ACTH Release and Peripheral Inflammation. Endocrinology 1996, 137, 5747–5750. [Google Scholar] [CrossRef]
- Latour, M.A.; Laiche, S.; Thompson, J.; Pond, A.; Peebles, E.D. Continuous Infusion of Adrenocorticotropin Elevates Circulating Lipoprotein Cholesterol and Corticosterone Concentrations in Chickens. Poult. Sci. 1996, 75, 1428–1432. [Google Scholar] [CrossRef]
- De Groef, B.; Geris, K.; Manzano, J.; Bernal, J.; Millar, R.; Abou-Samra, A.-B.; Porter, T.; Iwasawa, A.; Kühn, E.; Darras, V. Involvement of Thyrotropin-Releasing Hormone Receptor, Somatostatin Receptor Subtype 2 and Corticotropin-Releasing Hormone Receptor Type 1 in the Control of Chicken Thyrotropin Secretion. Mol. Cell. Endocrinol. 2003, 203, 33–39. [Google Scholar] [CrossRef]
- De Groef, B.; Grommen, S.V.; Mertens, I.; Schoofs, L.; Kühn, E.R.; Darras, V.M. Cloning and Tissue Distribution of the Chicken Type 2 Corticotropin-Releasing Hormone Receptor. Gen. Comp. Endocrinol. 2004, 138, 89–95. [Google Scholar] [CrossRef]
- Taylor, T.; Wondisford, F.; Blaine, T.; Weintraub, B. The Paraventricular Nucleus of the Hypothalamus Has a Major Role in Thyroid Hormone Feedback Regulation of Thyrotropin Synthesis and Secretion. Endocrinology 1990, 126, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Szkudlinski, M.W.; Fremont, V.; Ronin, C.; Weintraub, B.D. Thyroid-Stimulating Hormone and Thyroid-Stimulating Hormone Receptor Structure-Function Relationships. Physiol. Rev. 2002, 82, 473–502. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lu, X.; Gershengorn, M. G-Protein-Coupled Receptor Signaling in Neuroendocrine Systems. Thyrotropin-Releasing Hormone Receptors—Similarities and Differences. J. Mol. Endocrinol. 2003, 30, 87–97. [Google Scholar] [CrossRef]
- Sam, S.; Frohman, L.A. Normal Physiology of Hypothalamic Pituitary Regulation. Endocrinol. Metab. Clin. N. Am. 2008, 37, 1–22. [Google Scholar] [CrossRef]
- De Groef, B.; Grommen, S.; Darras, V. The Chicken Embryo as a Model for Developmental Endocrinology: Development of the Thyrotropic, Corticotropic, and Somatotropic Axes. Mol. Cell. Endocrinol. 2008, 293, 17–24. [Google Scholar] [CrossRef]
- Nillni, E.A. Regulation of the Hypothalamic Thyrotropin Releasing Hormone (TRH) Neuron by Neuronal and Peripheral Inputs. Front. Neuroendocrinol. 2010, 31, 134–156. [Google Scholar] [CrossRef]
- Grossmann, M.; Weintraub, B.D.; Szkudlinski, M.W. Novel Insights into the Molecular Mechanisms of Human Thyrotropin Action: Structural, Physiological, and Therapeutic Implications for the Glycoprotein Hormone Family. Endocr. Rev. 1997, 18, 476–501. [Google Scholar] [CrossRef]
- Decuypere, E.; Van As, P.; Van der Geyten, S.; Darras, V. Thyroid Hormone Availability and Activity in Avian Species: A Review. Domest. Anim. Endocrinol. 2005, 29, 63–77. [Google Scholar] [CrossRef]
- Decuypere, E.; Buyse, J. Endocrine Control of Postnatal Growth in Poultry. J. Poult. Sci. 2005, 42, 1–13. [Google Scholar] [CrossRef]
- Larsen, P.R.; Zavacki, A.M. Role of the Iodothyronine Deiodinases in the Physiology and Pathophysiology of Thyroid Hormone Action. Eur. Thyroid J. 2013, 1, 232–242. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid Hormone Regulation of Metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Velleman, S. Muscle Development in the Embryo and Hatchling. Poult. Sci. 2007, 86, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Bangsbo, J.; Gollnick, P.; Graham, T.; Saltin, B. Substrates for Muscle Glycogen Synthesis in Recovery from Intense Exercise in Man. J. Physiol. 1991, 434, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Oksbjerg, N.; Gondret, F.; Vestergaard, M. Basic Principles of Muscle Development and Growth in Meat-Producing Mammals as Affected by the Insulin-like Growth Factor (IGF) System. Domest. Anim. Endocrinol. 2004, 27, 219–240. [Google Scholar] [CrossRef]
- Hansen-Smith, F.M.; Picou, D.; Golden, M. Muscle Satellite Cells in Malnourished and Nutritionally Rehabilitated Children. J. Neurol. Sci. 1979, 41, 207–221. [Google Scholar] [CrossRef]
- Jirmanová, I.; Thesleff, S. Ultrastructural Study of Experimental Muscle Degeneration and Regeneration in the Adult Rat. Z. Zellforsch. Mikrosk. Anat. 1972, 131, 77–97. [Google Scholar] [CrossRef]
- McGeachie, J.K.; Grounds, M.D. Initiation and Duration of Muscle Precursor Replication after Mild and Severe Injury to Skeletal Muscle of Mice: An Autoradiographic Study. Cell Tissue Res. 1987, 248, 125–130. [Google Scholar] [CrossRef]
- Phillips, W.D.; Bennett, M.R. Elimination of Distributed Synaptic Acetylcholine Receptor Clusters on Developing Avian Fast-Twitch Muscle Fibres Accompanies Loss of Polyneuronal Innervation. J. Neurocytol. 1987, 16, 785–797. [Google Scholar] [CrossRef]
- d’Albis, A.; Lenfant-Guyot, M.; Janmot, C.; Chanoine, C.; Weinman, J.; Gallien, C.L. Regulation by Thyroid Hormones of Terminal Differentiation in the Skeletal Dorsal Muscle: I. Neonate Mouse. Dev. Biol. 1987, 123, 25–32. [Google Scholar] [CrossRef]
- Bischoff, R. Regeneration of Single Skeletal Muscle Fibers in Vitro. Anat. Rec. 1975, 182, 215–235. [Google Scholar] [CrossRef]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite Cells and the Muscle Stem Cell Niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef]
- Kuang, S.; Kuroda, K.; Le Grand, F.; Rudnicki, M.A. Asymmetric Self-Renewal and Commitment of Satellite Stem Cells in Muscle. Cell 2007, 129, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, N.; Asakura, A. Muscle Satellite Cell Heterogeneity and Self-Renewal. Front. Cell Dev. Biol. 2014, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Cornelison, D.; Wold, B.J. Single-Cell Analysis of Regulatory Gene Expression in Quiescent and Activated Mouse Skeletal Muscle Satellite Cells. Dev. Biol. 1997, 191, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.; Tajbakhsh, S.; Mouly, V.; Cossu, G.; Buckingham, M.; Butler-Browne, G. In Vivo Satellite Cell Activation via Myf5 and MyoD in Regenerating Mouse Skeletal Muscle. J. Cell Sci. 1999, 112, 2895–2901. [Google Scholar] [CrossRef]
- Mak, K.-L.; To, R.Q.; Kong, Y.; Konieczny, S.F. The MRF4 Activation Domain Is Required to Induce Muscle-Specific Gene Expression. Mol. Cell. Biol. 1992, 12, 4334–4346. [Google Scholar]
- Sabourin, L.A.; Rudnicki, M.A. The Molecular Regulation of Myogenesis. Clin. Genet. 2000, 57, 16–25. [Google Scholar] [CrossRef]
- Perry, R.; Rudnick, M.A. Molecular Mechanisms Regulating Myogenic Determination and Differentiation. Front. Biosci. 2000, 5, D750–D767. [Google Scholar] [CrossRef]
- Lehka, L.; Rędowicz, M.J. Mechanisms Regulating Myoblast Fusion: A Multilevel Interplay. Semin. Cell Dev. Biol. 2020, 104, 81–92. [Google Scholar] [CrossRef]
- Glass, D.J. Skeletal Muscle Hypertrophy and Atrophy Signaling Pathways. Int. J. Biochem. Cell Biol. 2005, 37, 1974–1984. [Google Scholar] [CrossRef]
- Smith, J.H. Relation of Body Size to Muscle Cell Size and Number in the Chicken. Poult. Sci. 1963, 42, 283–290. [Google Scholar] [CrossRef]
- Allen, R.E.; Merkel, R.A.; Young, R.B. Cellular Aspect of Muscle Growth: Myogenic Cell Proliferation. J. Anim. Sci. 1979, 49, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Halevy, O.; Geyra, A.; Barak, M.; Uni, Z.; Sklan, D. Early Posthatch Starvation Decreases Satellite Cell Proliferation and Skeletal Muscle Growth in Chicks. J. Nutr. 2000, 130, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.; Velleman, S.; Cowieson, A.; Singh, M.; Muir, W. Influence of Chick Hatch Time and Access to Feed on Broiler Muscle Development. Poult. Sci. 2016, 95, 1433–1448. [Google Scholar] [CrossRef]
- Torrey, S.; Mohammadigheisar, M.; Dos Santos, M.N.; Rothschild, D.; Dawson, L.C.; Liu, Z.; Kiarie, E.G.; Edwards, A.M.; Mandell, I.; Karrow, N. In Pursuit of a Better Broiler: Growth, Efficiency, and Mortality of 16 Strains of Broiler Chickens. Poult. Sci. 2021, 100, 100955. [Google Scholar] [CrossRef]
- Weintraub, H. The MyoD Family and Myogenesis: Redundancy, Networks, and Thresholds. Cell 1993, 75, 1241–1244. [Google Scholar] [CrossRef]
- Yablonka-Reuveni, Z.; Rudnicki, M.A.; Rivera, A.J.; Primig, M.; Anderson, J.E.; Natanson, P. The Transition from Proliferation to Differentiation Is Delayed in Satellite Cells from Mice Lacking MyoD. Dev. Biol. 1999, 210, 440–455. [Google Scholar] [CrossRef]
- Yablonka–Reuveni, Z.; Paterson, B.M. MyoD and Myogenin Expression Patterns in Cultures of Fetal and Adult Chicken Myoblasts. J. Histochem. Cytochem. 2001, 49, 455–462. [Google Scholar] [CrossRef]
- Velleman, S.; Nestor, K.; Coy, C.; Harford, I.; Anthony, N. Effect of Posthatch Feed Restriction on Broiler Breast Muscle Development and Muscle Transcriptional Regulatory Factor Gene and Heparan Sulfate Proteoglycan Expression. Int. J. Poult. Sci. 2010, 9, 417–425. [Google Scholar] [CrossRef]
- Velleman, S.; Coy, C.; Emmerson, D. Effect of the Timing of Posthatch Feed Restrictions on Broiler Breast Muscle Development and Muscle Transcriptional Regulatory Factor Gene Expression. Poult. Sci. 2014, 93, 1484–1494. [Google Scholar] [CrossRef]
- Averous, J.; Gabillard, J.-C.; Seiliez, I.; Dardevet, D. Leucine Limitation Regulates Myf5 and myoD Expression and Inhibits Myoblast Differentiation. Exp. Cell Res. 2012, 318, 217–227. [Google Scholar] [CrossRef]
- Powell, D.; McFarland, D.; Cowieson, A.; Muir, W.; Velleman, S. The Effect of Nutritional Status on Myogenic Gene Expression of Satellite Cells Derived from Different Muscle Types. Poult. Sci. 2014, 93, 2278–2288. [Google Scholar] [CrossRef] [PubMed]
- Rehfeldt, C.; Te Pas, M.; Wimmers, K.; Brameld, J.; Nissen, P.; Berri, C.; Valente, L.; Power, D.; Picard, B.; Stickland, N. Advances in Research on the Prenatal Development of Skeletal Muscle in Animals in Relation to the Quality of Muscle-Based Food. I. Regulation of Myogenesis and Environmental Impact. Animal 2011, 5, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Duclos, M.J. Regulation of Chicken Muscle Growth by Insulin-like Growth Factors a. Ann. N. Y. Acad. Sci. 1998, 839, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Duclos, M.J.; Chevalier, B.; Rémignon, H.; Ricard, F.; Goddard, C.; Simon, J. Divergent Selection for High or Low Growth Rate Modifies the Response of Muscle Cells to Serum or Insulin-like Growth Factor-I in Vitro. Growth Regul. 1996, 6, 176–184. [Google Scholar]
- Kumegawa, M.; Ikeda, E.; Hosoda, S.; Takuma, T. In Vitro Effects of Thyroxine and Insulin on Myoblasts from Chick Embryo Skeletal Muscle. Dev. Biol. 1980, 79, 493–499. [Google Scholar] [CrossRef]
- Suthama, N.; Hayashi, K.; Toyomizu, M.; Tomita, Y. Effect of Dietary Thyroxine on Growth and Muscle Protein Metabolism in Broiler Chickens. Poult. Sci. 1989, 68, 1396–1401. [Google Scholar] [CrossRef]
- He, J.; Cao, M.; Gao, F.; Wang, J.; Hayashi, K. Dietary Thyroid Hormone Improves Growth and Muscle Protein Accumulation of Black-Boned Chickens. Br. Poult. Sci. 2006, 47, 567–571. [Google Scholar] [CrossRef]
- El-Husseiny, O.; Hashish, S.; Arafa, S.; Madian, A. Response of Poultry Performance to Environmental Light Colour. Egypt. Poult. Sci. J. 2000, 20, 385–402. [Google Scholar]
- Rozenboim, I.; Biran, I.; Chaiseha, Y.; Yahav, S.; Rosenstrauch, A.; Sklan, D.; Halevy, O. The Effect of a Green and Blue Monochromatic Light Combination on Broiler Growth and Development. Poult. Sci. 2004, 83, 842–845. [Google Scholar] [CrossRef]
- Abdelazeem, A.F. Productive and Physiological Response of Broiler Chickens Exposed to Different Colored Light-Emitting Diode and Reared under Different Stocking Densities. Egypt. Poult. Sci. J. 2019, 38, 1243–1264. [Google Scholar] [CrossRef]
- Soliman, E.S.; Hassan, R.A. Impact of Lighting Color and Duration on Productive Performance and Newcastle Disease Vaccination Efficiency in Broiler Chickens. Vet. World 2019, 12, 1052. [Google Scholar] [CrossRef]
- Senaratna, D.; Samarakone, T.; Gunawardena, W. Red Color Light at Different Intensities Affects the Performance, Behavioral Activities and Welfare of Broilers. Asian-Australas. J. Anim. Sci. 2015, 29, 1052. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Role of Monochromatic Light on Daily Variation of Clock Gene Expression in the Pineal Gland of Chick. J. Photochem. Photobiol. B Biol. 2016, 164, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, N.M.; Krueckl, S.L.; McRory, J.E. The Origin and Function of the Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP)/Glucagon Superfamily. Endocr. Rev. 2000, 21, 619–670. [Google Scholar] [PubMed]
- Bossis, I.; Porter, T.E. Identification of the Somatostatin Receptor Subtypes Involved in Regulation of Growth Hormone Secretion in Chickens. Mol. Cell. Endocrinol. 2001, 182, 203–213. [Google Scholar] [CrossRef]
- Meng, F.; Huang, G.; Gao, S.; Li, J.; Yan, Z.; Wang, Y. Identification of the Receptors for Somatostatin (SST) and Cortistatin (CST) in Chickens and Investigation of the Roles of cSST28, cSST14, and cCST14 in Inhibiting cGHRH1–27NH2-Induced Growth Hormone Secretion in Cultured Chicken Pituitary Cells. Mol. Cell. Endocrinol. 2014, 384, 83–95. [Google Scholar] [CrossRef]
- Florini, J.R.; Ewton, D.Z.; Coolican, S.A. Growth Hormone and the Insulin-like Growth Factor System in Myogenesis. Endocr. Rev. 1996, 17, 481–517. [Google Scholar]
- Kajimoto, Y.; Rotwein, P. Structure and Expression of a Chicken Insulin-like Growth Factor I Precursor. Mol. Endocrinol. 1989, 3, 1907–1913. [Google Scholar] [CrossRef]
- D’Costa, A.P.; Prevette, D.M.; Houenou, L.J.; Wang, S.; Zackenfels, K.; Rohrer, H.; Zapf, J.; Caroni, P.; Oppenheim, R.W. Mechanisms of Insulin-like Growth Factor Regulation of Programmed Cell Death of Developing Avian Motoneurons. J. Neurobiol. 1998, 36, 379–394. [Google Scholar] [CrossRef]
- Duclos, M.J.; Goddard, C. Insulin-like Growth Factor Receptors in Chicken Liver Membranes: Binding Properties, Specificity, Developmental Pattern and Evidence for a Single Receptor Type. J. Endocrinol. 1990, 125, 199–206. [Google Scholar] [CrossRef]
- Baxter, R.C. Insulin-like Growth Factor (IGF)-Binding Proteins: Interactions with IGFs and Intrinsic Bioactivities. Am. J. Physiol.-Endocrinol. Metab. 2000, 278, E967–E976. [Google Scholar] [CrossRef]
- Allander, S.V.; Ehrenborg, E.; Luthman, H.; Powell, D.R. Conservation of IGFBP Structure during Evolution: Cloning of Chicken Insulin-like Growth Factor Binding Protein-5. Prog. Growth Factor Res. 1995, 6, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Allander, S.V.; Coleman, M.; Luthman, H.; Powell, D.R. Chicken Insulin-like Growth Factor Binding Protein (IGFBP)-5: Conservation of IGFBP-5 Structure and Expression during Evolution. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1997, 116, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Schoen, T.J.; Bondy, C.A.; Zhou, J.; Dhawan, R.; Mazuruk, K.; Arnold, D.R.; Rodriguez, I.R.; Waldbillig, R.J.; Beebe, D.C.; Chader, G.J. Differential Temporal and Spatial Expression of Insulin-like Growth Factor Binding Protein-2 in Developing Chick Ocular Tissues. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2652–2662. [Google Scholar]
- Kelley, K.; Schmidt, K.; Berg, L.; Sak, K.; Galima, M.; Gillespie, C.; Balogh, L.; Hawayek, A.; Reyes, J.; Jamison, M. Beyond Carrier Proteins Comparative Endocrinology of the Insulin-like Growth Factor-Binding Protein. J. Endocrinol. 2002, 175, 3–18. [Google Scholar] [CrossRef]
- Daza, D.O.; Sundström, G.; Bergqvist, C.A.; Duan, C.; Larhammar, D. Evolution of the Insulin-like Growth Factor Binding Protein (IGFBP) Family. Endocrinology 2011, 152, 2278–2289. [Google Scholar] [CrossRef]
- Wang, H.-B.; Li, H.; Wang, Q.-G.; Zhang, X.-Y.; Wang, S.-Z.; Wang, Y.-X.; Wang, X.-P. Profiling of Chicken Adipose Tissue Gene Expression by Genome Array. BMC Genom. 2007, 8, 193. [Google Scholar] [CrossRef]
- Yamanaka, Y.; Wilson, E.M.; Rosenfeld, R.G.; Oh, Y. Inhibition of Insulin Receptor Activation by Insulin-like Growth Factor Binding Proteins. J. Biol. Chem. 1997, 272, 30729–30734. [Google Scholar] [CrossRef]
- Kim, J.W. The Endocrine Regulation of Chicken Growth. Asian-Australas. J. Anim. Sci. 2010, 23, 1668–1676. [Google Scholar] [CrossRef]
- Baxter, R. Insulin-like Growth Factor (IGF) Binding Proteins: The Role of Serum IGFBPs in Regulating IGF Availability. Acta Paediatr. 1991, 80, 107–114. [Google Scholar] [CrossRef]
- Frommer, K.W.; Reichenmiller, K.; Schutt, B.S.; Hoeflich, A.; Ranke, M.B.; Dodt, G.; Elmlinger, M.W. IGF-Independent Effects of IGFBP-2 on the Human Breast Cancer Cell Line Hs578T. J. Mol. Endocrinol. 2006, 37, 13–23. [Google Scholar] [CrossRef]
- Schutt, B.; Langkamp, M.; Rauschnabel, U.; Ranke, M.; Elmlinger, M. Integrin-Mediated Action of Insulin-like Growth Factor Binding Protein-2 in Tumor Cells. J. Mol. Endocrinol. 2004, 32, 859–868. [Google Scholar] [CrossRef]
- Mohan, S.; Nakao, Y.; Honda, Y.; Landale, E.; Leser, U.; Dony, C.; Lang, K.; Baylink, D.J. Studies on the Mechanisms by Which Insulin-like Growth Factor (IGF) Binding Protein-4 (IGFBP-4) and IGFBP-5 Modulate IGF Actions in Bone Cells (∗). J. Biol. Chem. 1995, 270, 20424–20431. [Google Scholar] [CrossRef]
- Hashimoto, R.; Ono, M.; Fujiwara, H.; Higashihashi, N.; Yoshida, M.; Enjoh-Kimura, T.; Sakano, K. Binding Sites and Binding Properties of Binary and Ternary Complexes of Insulin-like Growth Factor-II (IGF-II), IGF-Binding Protein-3, and Acid-Labile Subunit. J. Biol. Chem. 1997, 272, 27936–27942. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Boisclair, Y.R. Growth Hormone Signaling in the Regulation of Acid Labile Subunit. Asian-Australas. J. Anim. Sci. 2008, 21, 754–768. [Google Scholar] [CrossRef]
- Kim, H.; Fu, Y.; Hong, H.J.; Lee, S.-G.; Lee, D.S.; Kim, H.M. Structural Basis for Assembly and Disassembly of the IGF/IGFBP/ALS Ternary Complex. Nat. Commun. 2022, 13, 4434. [Google Scholar] [CrossRef] [PubMed]
- Suwanichkul, A.; Boisclair, Y.R.; Olney, R.C.; Durham, S.K.; Powell, D.R. Conservation of a Growth Hormone-Responsive Promoter Element in the Human and Mouse Acid-Labile Subunit Genes. Endocrinology 2000, 141, 833–838. [Google Scholar] [CrossRef]
- Baxter, R.C.; Dai, J. Purification and Characterization of the Acid-Labile Subunit of Rat Serum Insulin-like Growth Factor Binding Protein Complex. Endocrinology 1994, 134, 848–852. [Google Scholar] [CrossRef]
- Ooi, G.T.; Cohen, F.J.; Tseng, L.Y.-H.; Rechler, M.M.; Boisclair, Y.R. Growth Hormone Stimulates Transcription of the Gene Encoding the Acid-Labile Subunit (ALS) of the Circulating Insulin-like Growth Factor-Binding Protein Complex and ALS Promoter Activity in Rat Liver. Mol. Endocrinol. 1997, 11, 997–1007. [Google Scholar] [CrossRef]
- Bunn, R.C.; Fowlkes, J.L. Insulin-like Growth Factor Binding Protein Proteolysis. Trends Endocrinol. Metab. 2003, 14, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Durham, S.K.; Riggs, B.L.; Harris, S.A.; Conover, C.A. Alterations in Insulin-like Growth Factor (IGF)-Dependent IGF-Binding Protein-4 Proteolysis in Transformed Osteoblastic Cells. Endocrinology 1995, 136, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.A.; Lang, C.H. Differential Effects of Insulin-like Growth Factor I (IGF-I) and IGF-Binding Protein-1 on Protein Metabolism in Human Skeletal Muscle Cells. Endocrinology 1999, 140, 3962–3970. [Google Scholar] [CrossRef]
- Fisher, M.C.; Meyer, C.; Garber, G.; Dealy, C.N. Role of IGFBP2, IGF-I and IGF-II in Regulating Long Bone Growth. Bone 2005, 37, 741–750. [Google Scholar] [CrossRef]
- Ewton, D.Z.; Coolican, S.A.; Mohan, S.; Chernausek, S.D.; Florini, J.R. Modulation of Insulin-like Growth Factor Actions in L6A1 Myoblasts by Insulin-like Growth Factor Binding Protein (IGFBP)-4 and IGFBP-5: A Dual Role for IGFBP-5. J. Cell. Physiol. 1998, 177, 47–57. [Google Scholar] [CrossRef]
- Ballard, F.J.; Johnson, R.J.; Owens, P.C.; Francis, G.L.; Upton, F.M.; McMurtry, J.P.; Wallace, J.C. Chicken Insulin-like Growth Factor-I: Amino Acid Sequence, Radioimmunoassay, and Plasma Levels between Strains and during Growth. Gen. Comp. Endocrinol. 1990, 79, 459–468. [Google Scholar] [CrossRef]
- Porter, T.E.; Ellestad, L.E.; Fay, A.; Stewart, J.L.; Bossis, I. Identification of the Chicken Growth Hormone-Releasing Hormone Receptor (GHRH-R) mRNA and Gene: Regulation of Anterior Pituitary GHRH-R mRNA Levels by Homologous and Heterologous Hormones. Endocrinology 2006, 147, 2535–2543. [Google Scholar] [CrossRef]
- Kajkowski, E.M.; Price, L.A.; Pausch, M.H.; Young, K.H.; Ozenberger, B.A. Investigation of Growth Hormone Releasing Hormone Receptor Structure and Activity Using Yeast Expression Technologies. J. Recept. Signal Transduct. 1997, 17, 293–303. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Li, J.; Leung, F. Expression Profiles of Growth Hormone-Releasing Hormone and Growth Hormone-Releasing Hormone Receptor during Chicken Embryonic Pituitary Development. Poult. Sci. 2006, 85, 569–576. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Wang, C.Y.; Kwok, A.Y.; Zhang, X.; Leung, F. Characterization of the Receptors for Chicken GHRH and GHRH-Related Peptides: Identification of a Novel Receptor for GHRH and the Receptor for GHRH-LP (PRP). Domest. Anim. Endocrinol. 2010, 38, 13–31. [Google Scholar] [CrossRef]
- Mayo, K.E.; Miller, T.L.; DeAlmeida, V.; Zheng, J.; Godfrey, P.A. The Growth-Hormone-Releasing Hormone Receptor: Signal Transduction, Gene Expression, and Physiological Function in Growth Regulation. Ann. N. Y. Acad. Sci. 1996, 805, 184–203. [Google Scholar] [CrossRef]
- Struthers, R.S.; Vale, W.W.; Arias, C.; Sawchenko, P.E.; Montminy, M.R. Somatotroph Hypoplasia and Dwarfism in Transgenic Mice Expressing a Non-Phosphorylatable CREB Mutant. Nature 1991, 350, 622–624. [Google Scholar] [CrossRef]
- Bilezikjian, L.M.; Vale, W.W. Stimulation of Adenosine 3′, 5′-Monophosphate Production by Growth Hormone-Releasing Factor and Its Inhibition by Somatostatin in Anterior Pituitary Cells in Vitro. Endocrinology 1983, 113, 1726–1731. [Google Scholar] [CrossRef]
- Møller, L.N.; Stidsen, C.E.; Hartmann, B.; Holst, J.J. Somatostatin Receptors. Biochim. Biophys. Acta (BBA)-Biomembr. 2003, 1616, 1–84. [Google Scholar] [CrossRef]
- Reisine, T.; Kong, H.; Raynor, K.; Yano, H.; Takeda, J.; Yasuda, K.; Bell, G.I. Splice Variant of the Somatostatin Receptor 2 Subtype, Somatostatin Receptor 2B, Couples to Adenylyl Cyclase. Mol. Pharmacol. 1993, 44, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Cakir, M.; Dworakowska, D.; Grossman, A. Somatostatin Receptor Biology in Neuroendocrine and Pituitary Tumours: Part 1—Molecular Pathways. J. Cell. Mol. Med. 2010, 14, 2570–2584. [Google Scholar] [CrossRef]
- Qin, X.; Liu, X.; Yan, X.; Long, M.; Wang, Z.; Dong, Y.; Chen, Y.; Cao, J. Melatonin Mediates Monochromatic Light-Induced Expression of Somatostatin in the Hypothalamus and Pituitary of Chicks. Poult. Sci. 2021, 100, 101285. [Google Scholar] [CrossRef] [PubMed]
- Dehkhoda, F.; Lee, C.M.; Medina, J.; Brooks, A.J. The Growth Hormone Receptor: Mechanism of Receptor Activation, Cell Signaling, and Physiological Aspects. Front. Endocrinol. 2018, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.J.; Wooh, J.W.; Tunny, K.A.; Waters, M.J. Growth Hormone Receptor; Mechanism of Action. Int. J. Biochem. Cell Biol. 2008, 40, 1984–1989. [Google Scholar] [CrossRef]
- Lan, H.; Li, W.; Fu, Z.; Yang, Y.; Wu, T.; Liu, Y.; Zhang, H.; Cui, H.; Li, Y.; Hong, P. Differential Intracellular Signalling Properties of the Growth Hormone Receptor Induced by the Activation of an Anti-GHR Antibody. Mol. Cell. Endocrinol. 2014, 390, 54–64. [Google Scholar] [CrossRef]
- Hull, K.; Thiagarajah, A.; Harvey, S. Cellular Localization of Growth Hormone Receptors/Binding Proteins in Immune Tissues. Cell Tissue Res. 1996, 286, 69–80. [Google Scholar] [CrossRef]
- Lawlor, M.A.; Alessi, D.R. PKB/Akt: A Key Mediator of Cell Proliferation, Survival and Insulin Responses? J. Cell Sci. 2001, 114, 2903–2910. [Google Scholar] [CrossRef] [PubMed]
- Fingar, D.C.; Blenis, J. Target of Rapamycin (TOR): An Integrator of Nutrient and Growth Factor Signals and Coordinator of Cell Growth and Cell Cycle Progression. Oncogene 2004, 23, 3151–3171. [Google Scholar] [CrossRef] [PubMed]
- Potter, C.J.; Pedraza, L.G.; Xu, T. Akt Regulates Growth by Directly Phosphorylating Tsc2. Nat. Cell Biol. 2002, 4, 658–665. [Google Scholar] [CrossRef]
- Inoki, K.; Li, Y.; Zhu, T.; Wu, J.; Guan, K.-L. TSC2 Is Phosphorylated and Inhibited by Akt and Suppresses mTOR Signalling. Nat. Cell Biol. 2002, 4, 648–657. [Google Scholar] [CrossRef]
- Kim, D.-H.; Sarbassov, D.D.; Ali, S.M.; King, J.E.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. mTOR Interacts with Raptor to Form a Nutrient-Sensitive Complex That Signals to the Cell Growth Machinery. Cell 2002, 110, 163–175. [Google Scholar] [CrossRef]
- Schieke, S.M.; Phillips, D.; McCoy, J.P.; Aponte, A.M.; Shen, R.-F.; Balaban, R.S.; Finkel, T. The Mammalian Target of Rapamycin (mTOR) Pathway Regulates Mitochondrial Oxygen Consumption and Oxidative Capacity. J. Biol. Chem. 2006, 281, 27643–27652. [Google Scholar] [CrossRef]
- Lipton, J.O.; Sahin, M. The Neurology of mTOR. Neuron 2014, 84, 275–291. [Google Scholar] [CrossRef]
- Peterson, T.R.; Sengupta, S.S.; Harris, T.E.; Carmack, A.E.; Kang, S.A.; Balderas, E.; Guertin, D.A.; Madden, K.L.; Carpenter, A.E.; Finck, B.N. mTOR Complex 1 Regulates Lipin 1 Localization to Control the SREBP Pathway. Cell 2011, 146, 408–420. [Google Scholar] [CrossRef]
- Chauvin, C.; Koka, V.; Nouschi, A.; Mieulet, V.; Hoareau-Aveilla, C.; Dreazen, A.; Cagnard, N.; Carpentier, W.; Kiss, T.; Meyuhas, O. Ribosomal Protein S6 Kinase Activity Controls the Ribosome Biogenesis Transcriptional Program. Oncogene 2014, 33, 474–483. [Google Scholar] [CrossRef]
- Sultana, S.; Hassan, M.R.; Choe, H.S.; Ryu, K.S. The Effect of Monochromatic and Mixed LED Light Colour on the Behaviour and Fear Responses of Broiler Chicken. Avian Biol. Res. 2013, 6, 207–214. [Google Scholar] [CrossRef]
- Halevy, O.; Piestun, Y.; Rozenboim, I.; Yablonka-Reuveni, Z. In Ovo Exposure to Monochromatic Green Light Promotes Skeletal Muscle Cell Proliferation and Affects Myofiber Growth in Posthatch Chicks. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2006, 290, R1062–R1070. [Google Scholar] [CrossRef]
- Bai, X.; Wang, Y.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. In Ovo Exposure to Monochromatic Lights Affect Posthatch Muscle Growth and Satellite Cell Proliferation of Chicks: Role of IGF-1. Growth Factors 2016, 34, 107–118. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Wang, J.; Wu, S.; Qiao, X.; Yue, H.; Yao, J.; Qi, G. Stimulation with Monochromatic Green Light during Incubation Alters Satellite Cell Mitotic Activity and Gene Expression in Relation to Embryonic and Posthatch Muscle Growth of Broiler Chickens. Animal 2014, 8, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Helva, I.; Aksit, M.; Yalcin, S. Effects of Monochromatic Light on Growth Performance, Welfare and Hormone Levels in Broiler Chickens. Eur. Poult. Sci. 2019, 83, 1–12. [Google Scholar] [CrossRef]
- Arowolo, M.; He, J.; He, S.; Adebowale, T. The Implication of Lighting Programmes in Intensive Broiler Production System. World’s Poult. Sci. J. 2019, 75, 17–28. [Google Scholar] [CrossRef]
- Schwean-Lardner, K.; Fancher, B.I.; Classen, H.L. Impact of Daylength on Behavioural Output in Commercial Broilers. Appl. Anim. Behav. Sci. 2012, 137, 43–52. [Google Scholar] [CrossRef]
- Olanrewaju, H.; Thaxton, J.; Dozier, W.; Purswell, J.; Roush, W.; Branton, S. A Review of Lighting Programs for Broiler Production. Int. J. Poult. Sci. 2006, 5, 301–308. [Google Scholar] [CrossRef]
- Magee, C.; Olanrewaju, H.; Campbell, J.; Purswell, J. Effect of Photoperiod on Live Performance in Broiler Chicks from Placement to 14-Days-of-Age. J. Appl. Poult. Res. 2022, 31, 100295. [Google Scholar] [CrossRef]
- Yang, Y.; Cong, W.; Liu, J.; Zhao, M.; Xu, P.; Han, W.; Wang, D.; Zhao, R. Constant Light in Early Life Induces Fear-Related Behavior in Chickens with Suppressed Melatonin Secretion and Disrupted Hippocampal Expression of Clock-and BDNF-Associated Genes. J. Anim. Sci. Biotechnol. 2022, 13, 67. [Google Scholar] [CrossRef]
- Ashabranner, G.G.; Czarick, M., III; Fairchild, B.D. Evaluating the Effect of Daylength (24, 20, and 18 Hours) during Brooding on Broiler Performance and Physiological Responses to Light Environment. J. Appl. Poult. Res. 2025, 34, 100558. [Google Scholar] [CrossRef]
- Jiang, S.; Fu, Y.; Cheng, H. Daylight Exposure and Circadian Clocks in Broilers: Part I—Photoperiod Effect on Broiler Behavior, Skeletal Health, and Fear Response. Poult. Sci. 2023, 102, 103162. [Google Scholar] [CrossRef] [PubMed]
- Fidan, D.E.; Nazligü, A.; Türkyilmaz, M.K.; Aypak, S.U.; Kilimci, F.S.; Karaarslan, S.; Kaya, M. Effect of photoperiod length and light intensity on some welfare criteria, carcass, and meat quality characteristics in broilers. R. Bras. Zootec 2017, 46, 202–210. [Google Scholar] [CrossRef]
- Dunn, I.; Ciccone, N.; Joseph, N. Endocrinology and Genetics of the Hypothalamic-Pituitary-Gonadal Axis. In Biology of Breeding Poultry; CABI: Wallingford, UK, 2009; pp. 61–88. [Google Scholar]
- Robinson, F.; Etches, R. Ovarian Steroidogenesis during Foillicular Maturation in the Domestic Fowl (Gallus domesticus). Biol. Reprod. 1986, 35, 1096–1105. [Google Scholar] [CrossRef]
- Ubuka, T.; Bentley, G.E.; Tsutsui, K. Neuroendocrine Regulation of Gonadotropin Secretion in Seasonally Breeding Birds. Front. Neurosci. 2013, 7, 38. [Google Scholar] [CrossRef]
- Rangel, P.; Gutierrez, C. Reproduction in Hens: Is Testosterone Necessary for the Ovulatory Process? Gen. Comp. Endocrinol. 2014, 203, 250–261. [Google Scholar] [CrossRef]
- Terada, O.; Shimada, K.; Saito, N. Effect of Oestradiol Replacement in Ovariectomized Chickens on Pituitary LH Concentrations and Concentrations of mRNAs Encoding LH β and α Subunits. Reproduction 1997, 111, 59–64. [Google Scholar] [CrossRef]
- Bain, M.M.; Nys, Y.; Dunn, I.C. Increasing Persistency in Lay and Stabilising Egg Quality in Longer Laying Cycles. What Are the Challenges? Br. Poult. Sci. 2016, 57, 330–338. [Google Scholar] [CrossRef]
- Ottinger, M.A.; Bakst, M.R. Endocrinology of the Avian Reproductive System. J. Avian Med. Surg. 1995, 9, 242–250. [Google Scholar]
- Ubuka, T.; Ukena, K.; Sharp, P.J.; Bentley, G.E.; Tsutsui, K. Gonadotropin-Inhibitory Hormone Inhibits Gonadal Development and Maintenance by Decreasing Gonadotropin Synthesis and Release in Male Quail. Endocrinology 2006, 147, 1187–1194. [Google Scholar] [CrossRef]
- Chowdhury, V.S.; Yamamoto, K.; Ubuka, T.; Bentley, G.E.; Hattori, A.; Tsutsui, K. Melatonin Stimulates the Release of Gonadotropin-Inhibitory Hormone by the Avian Hypothalamus. Endocrinology 2010, 151, 271–280. [Google Scholar] [CrossRef]
- Ubuka, T.; Bentley, G.E.; Ukena, K.; Wingfield, J.C.; Tsutsui, K. Melatonin Induces the Expression of Gonadotropin-Inhibitory Hormone in the Avian Brain. Proc. Natl. Acad. Sci. USA 2005, 102, 3052–3057. [Google Scholar] [CrossRef]
- Jozsa, R.; Mess, A.; Gladwell, R.; Cunningham, F.; Sharp, P. The Stimulatory Action of the Glutamate Agonist, N-Methyl-Aspartate, on Luteinizing Hormone Release in the Cockerel with Immunocytochemical Observations on Its Mode of Action. In Neuroendocrinology: Retrospect and Perspectives; Springer: Berlin/Heidelberg, Germany, 1997; pp. 151–162. [Google Scholar]
- Teruyama, R.; Beck, M. Double Immunocytochemistry of Vasoactive Intestinal Peptide and cGnRH-I in Male Quail: Photoperiodic Effects. Cell Tissue Res. 2001, 303, 403–414. [Google Scholar] [CrossRef]
- Contijoch, A.M.; Malamed, S.; Sarkar, D.K.; Advis, J.-P. β-Endorphin Regulation of LHRH Release at the Median Eminence Level: Immunocytochemical and Physiological Evidence in Hens. Neuroendocrinology 1993, 57, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Boswell, T.; Dunn, I.; Corr, S. Hypothalamic Neuropeptide Y mRNA Is Increased after Feed Restriction in Growing Broilers. Poult. Sci. 1999, 78, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, N.; Dunn, I.; Sharp, P. Increased Food Intake Stimulates GnRH-I, Glycoprotein Hormone α-Subunit and Follistatin mRNAs, and Ovarian Follicular Numbers in Laying Broiler Breeder Hens. Domest. Anim. Endocrinol. 2007, 33, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Advis, J.; Contijoch, A. The Median Eminence as a Site for Neuroendocrine Control of Reproduction in Hens. Poult. Sci. 1993, 72, 932–939. [Google Scholar] [CrossRef]
- Bhatt, R.; Youngren, O.; Kang, S.; El Halawani, M. Dopamine Infusion into the Third Ventricle Increases Gene Expression of Hypothalamic Vasoactive Intestinal Peptide and Pituitary Prolactin and Luteinizing Hormone β Subunit in the Turkey. Gen. Comp. Endocrinol. 2003, 130, 41–47. [Google Scholar] [CrossRef]
- Thayananuphat, A.; Kang, S.; Bakken, T.; Millam, J.; El Halawani, M. Rhythmic Dependent Light Induction of Gonadotrophin-releasing hormone-I Expression and Activation of Dopaminergic Neurones within the Premammillary Nucleus of the Turkey Hypothalamus. J. Neuroendocrinol. 2007, 19, 399–406. [Google Scholar] [CrossRef]
- Fraley, G.S.; Kuenzel, W.J. Immunocytochemical and Histochemical Analyses of Gonadotrophin Releasing Hormone, Tyrosine Hydroxylase, and Cytochrome Oxidase Reactivity within the Hypothalamus of Chicks Showing Early Sexual Maturation. Histochemistry 1993, 99, 221–229. [Google Scholar] [CrossRef]
- Contijoch, A.M.; Gonzalez, C.; Singh, H.N.; Malamed, S.; Troncoso, S.; Advis, J.-P. Dopaminergic Regulation of Luteinizing Hormone-Releasing Hormone Release at the Median Eminence Level: Immunocytochemical and Physiological Evidence in Hens. Neuroendocrinology 1992, 55, 290–300. [Google Scholar] [CrossRef]
- Meddle, S.L.; Follett, B.K. Photoperiodically Driven Changes in Fos Expression within the Basal Tuberal Hypothalamus and Median Eminence of Japanese Quail. J. Neurosci. 1997, 17, 8909–8918. [Google Scholar] [CrossRef]
- Yamamura, T.; Yasuo, S.; Hirunagi, K.; Ebihara, S.; Yoshimura, T. T3 Implantation Mimics Photoperiodically Reduced Encasement of Nerve Terminals by Glial Processes in the Median Eminence of Japanese Quail. Cell Tissue Res. 2006, 324, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Proudman, J.; Vandesande, F.; Berghman, L. Immunohistochemical Evidence That Follicle-Stimulating Hormone and Luteinizing Hormone Reside in Separate Cells in the Chicken Pituitary. Biol. Reprod. 1999, 60, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Dunn, I.C.; Millam, J.R. Gonadotropin Releasing Hormone: Forms and Functions in Birds. Avian Poult. Biol. Rev. 1998, 9, 61–85. [Google Scholar]
- Proudman, J.A.; Scanes, C.G.; Johannsen, S.A.; Berghman, L.R.; Camp, M.J. Comparison of the Ability of the Three Endogenous GnRHs to Stimulate Release of Follicle-Stimulating Hormone and Luteinizing Hormone in Chickens. Domest. Anim. Endocrinol. 2006, 31, 141–153. [Google Scholar] [CrossRef]
- Sharp, P.; Talbot, R.; Main, G.; Dunn, I.; Fraser, H.; Huskisson, N. Physiological Roles of Chicken LHRH-I and-II in the Control of Gonadotrophin Release in the Domestic Chicken. J. Endocrinol. 1990, 124, 291–299. [Google Scholar] [CrossRef]
- Joseph, N.T.; Morgan, K.; Sellar, R.; McBride, D.; Millar, R.P.; Dunn, I.C. The Chicken Type III GnRH Receptor Homologue Is Predominantly Expressed in the Pituitary, and Exhibits Similar Ligand Selectivity to the Type I Receptor. J. Endocrinol. 2009, 202, 179. [Google Scholar] [CrossRef]
- Heding, A.; Vrecl, M.; Bogerd, J.; McGregor, A.; Sellar, R.; Taylor, P.L.; Eidne, K.A. Gonadotropin-Releasing Hormone Receptors with Intracellular Carboxyl-Terminal Tails Undergo Acute Desensitization of Total Inositol Phosphate Production and Exhibit Accelerated Internalization Kinetics. J. Biol. Chem. 1998, 273, 11472–11477. [Google Scholar] [CrossRef]
- Pawson, A.; Katz, A.; Sun, Y.; Lopes, J.; Illing, N.; Millar, R.; Davidson, J. Contrasting Internalization Kinetics of Human and Chicken Gonadotropin-Releasing Hormone Receptors Mediated by C-Terminal Tail. J. Endocrinol. 1998, 156, R9-12. [Google Scholar] [CrossRef]
- Arora, K.K.; Cheng, Z.; Catt, K.J. Dependence of Agonist Activation on an Aromatic Moiety in the DPLIY Motif of the Gonadotropin-Releasing Hormone Receptor. Mol. Endocrinol. 1996, 10, 979–986. [Google Scholar] [PubMed]
- Arora, K.K.; Cheng, Z.; Catt, K.J. Mutations of the Conserved DRS Motif in the Second Intracellular Loop of the Gonadotropin-Releasing Hormone Receptor Affect Expression, Activation, and Internalization. Mol. Endocrinol. 1997, 11, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Pawson, A.J.; Maudsley, S.R.; Lopes, J.; Katz, A.A.; Sun, Y.-M.; Davidson, J.S.; Millar, R.P. Multiple Determinants for Rapid Agonist-Induced Internalization of a Nonmammalian Gonadotropin-Releasing Hormone Receptor: A Putative Palmitoylation Site and Threonine Doublet within the Carboxyl-Terminal Tail Are Critical. Endocrinology 2003, 144, 3860–3871. [Google Scholar] [CrossRef] [PubMed]
- McArdle, C.A.; Franklin, J.; Green, L.; Hislop, J. Signalling, Cycling and Desensitisation of Gonadotrophin-Releasing Hormone Receptors. J. Endocrinol. 2002, 173, 1–11. [Google Scholar] [CrossRef]
- McArdle, C.A.; Davidson, J.S.; Willars, G.B. The Tail of the Gonadotrophin-Releasing Hormone Receptor: Desensitization at, and Distal to, G Protein-Coupled Receptors. Mol. Cell. Endocrinol. 1999, 151, 129–136. [Google Scholar] [CrossRef]
- Ruf, F.; Fink, M.Y.; Sealfon, S.C. Structure of the GnRH Receptor-Stimulated Signaling Network: Insights from Genomics. Front. Neuroendocrinol. 2003, 24, 181–199. [Google Scholar] [CrossRef]
- Sun, Y.-M.; Flanagan, C.A.; Illing, N.; Ott, T.R.; Sellar, R.; Fromme, B.J.; Hapgood, J.; Sharp, P.; Sealfon, S.C.; Millar, R.P. A Chicken Gonadotropin-Releasing Hormone Receptor That Confers Agonist Activity to Mammalian Antagonists: Identification of D-Lys6 in the Ligand and Extracellular Loop Two of the Receptor as Determinants. J. Biol. Chem. 2001, 276, 7754–7761. [Google Scholar] [CrossRef]
- Shimizu, M.; Bédécarrats, G.Y. Activation of the Chicken Gonadotropin-Inhibitory Hormone Receptor Reduces Gonadotropin Releasing Hormone Receptor Signaling. Gen. Comp. Endocrinol. 2010, 167, 331–337. [Google Scholar] [CrossRef]
- You, S.; Bridgham, J.; Foster, D.; Johnson, A. Characterization of the Chicken Follicle-Stimulating Hormone Receptor (cFSH-R) Complementary Deoxyribonucleic Acid, and Expression of cFSH-R Messenger Ribonucleic Acid in the Ovary. Biol. Reprod. 1996, 55, 1055–1062. [Google Scholar] [CrossRef]
- Hernandez, A.; Bahr, J. Role of FSH and Epidermal Growth Factor (EGF) in the Initiation of Steroidogenesis in Granulosa Cells Associated with Follicular Selection in Chicken Ovaries. Reproduction 2003, 125, 683–691. [Google Scholar] [CrossRef]
- Davis, A.J.; Brooks, C.F.; Johnson, P.A. Activin A and Gonadotropin Regulation of Follicle-Stimulating Hormone and Luteinizing Hormone Receptor Messenger RNA in Avian Granulosa Cells. Biol. Reprod. 2001, 65, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Bridgham, J.; Woods, D. Cellular Mechanisms and Modulation of Activin A-and Transforming Growth Factor β-Mediated Differentiation in Cultured Hen Granulosa Cells. Biol. Reprod. 2004, 71, 1844–1851. [Google Scholar] [CrossRef]
- Johnson, A.; Bridgham, J. Regulation of Steroidogenic Acute Regulatory Protein and Luteinizing Hormone Receptor Messenger Ribonucleic Acid in Hen Granulosa Cells. Endocrinology 2001, 142, 3116–3124. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Bridgham, J.; Wagner, B. Characterization of a Chicken Luteinizing Hormone Receptor (cLH-R) Complementary Deoxyribonucleic Acid, and Expression of cLH-R Messenger Ribonucleic Acid in the Ovary. Biol. Reprod. 1996, 55, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, V.S.; Ubuka, T.; Tsutsui, K. Melatonin Stimulates the Synthesis and Release of Gonadotropin-Inhibitory Hormone in Birds. Gen. Comp. Endocrinol. 2013, 181, 175–178. [Google Scholar] [CrossRef]
- Maddineni, S.R.; Ocon-Grove, O.M.; Krzysik-Walker, S.M.; Hendricks, G.L.; Ramachandran, R. Gonadotropin-Inhibitory Hormone (GnIH) Receptor Gene Is Expressed in the Chicken Ovary: Potential Role of GnIH in Follicular Maturation. Reproduction 2008, 135, 267. [Google Scholar] [CrossRef]
- Bédécarrats, G.Y.; McFarlane, H.; Maddineni, S.R.; Ramachandran, R. Gonadotropin-Inhibitory Hormone Receptor Signaling and Its Impact on Reproduction in Chickens. Gen. Comp. Endocrinol. 2009, 163, 7–11. [Google Scholar] [CrossRef]
- Han, S.; Wang, Y.; Liu, L.; Li, D.; Liu, Z.; Shen, X.; Xu, H.; Zhao, X.; Zhu, Q.; Yin, H. Influence of Three Lighting Regimes during Ten Weeks Growth Phase on Laying Performance, Plasma Levels- and Tissue Specific Gene Expression- of Reproductive Hormones in Pengxian Yellow Pullets. PLoS ONE 2017, 12, e0177358. [Google Scholar] [CrossRef]
- Lewis, P.; Tyler, N.; Gous, R.; Dunn, I.; Sharp, P. Photoperiodic Response Curves for Plasma LH Concentrations and Age at First Egg in Female Broiler Breeders. Anim. Reprod. Sci. 2008, 109, 274–286. [Google Scholar] [CrossRef]
- Liu, M.; Tan, L.; Li, X.; Li, H.; Zhang, Y.; Zi, X.; Ge, C.; Wang, K. Analysis of Reproductive Hormones and Transcriptomics in the Hypothalamus and Pituitary under Different Photoperiods. Poult. Sci. 2025, 104, 105279. [Google Scholar] [CrossRef]
- Mobarkey, N.; Avital, N.; Heiblum, R.; Rozenboim, I. The Role of Retinal and Extra-Retinal Photostimulation in Reproductive Activity in Broiler Breeder Hens. Domest. Anim. Endocrinol. 2010, 38, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Gongruttananun, N. Influence of Red Light on Reproductive Performance, Eggshell Ultrastructure, and Eye Morphology in Thai-Native Hens. Poult. Sci. 2011, 90, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.F.; Ralph, C.; Reilly, D.P. Melatonin in the Chicken Brain: Its Origin, Diurnal Variation, and Regional Distribution. Gen. Comp. Endocrinol. 1974, 22, 499–506. [Google Scholar] [CrossRef]
- Mobarkey, N.; Avital, N.; Heiblum, R.; Rozenboim, I. The Effect of Parachlorophenylalanine and Active Immunization Against Vasoactive Intestinal Peptide on Reproductive Activities of Broiler Breeder Hens Photostimulated with Green Light. Biol. Reprod. 2013, 88, 83. [Google Scholar] [CrossRef]
- Foss, D.; Carew, L., Jr.; Arnold, E. Physiological Development of Cockerels as Influenced by Selected Wavelengths of Environmental Light. Poult. Sci. 1972, 51, 1922–1927. [Google Scholar] [CrossRef]
- Darras, V.M.; Verhoelst, C.H.; Reyns, G.E.; Kühn, E.R.; der Geyten, S.V. Thyroid Hormone Deiodination in Birds. Thyroid 2006, 16, 25–35. [Google Scholar] [CrossRef]
- Ritchie, M.; Pilny, A.A. The Anatomy and Physiology of the Avian Endocrine System. Vet. Clin. N. Am. Exot. Anim. Pract. 2008, 11, 1–14. [Google Scholar] [CrossRef]
- Abdel-Fattah, K.I.; Bobek, S.; Pietras, M.; Sechman, A.; Niezgoda, J. Hypometabolic Effect of 3, 3′, 5′-Triiodothyronine in Chickens: Interaction with Hypermetabolic Effect of 3, 5, 3′-Triiodothyronine. Gen. Comp. Endocrinol. 1990, 77, 9–14. [Google Scholar] [CrossRef]
- Kühn, E.; Herremans, M.; Dewil, E.; Vanderpooten, A.; Rudas, P.; Bartha, T.; Verheyen, G.; Berghman, L.; Decuypere, E. Thyrotropin-Releasing Hormone (TRH) Is Not Thyrotropic but Somatotropic in Fed and Starved Adult Chickens. Reprod. Nutr. Dev. 1991, 31, 431–439. [Google Scholar] [CrossRef]
- De Groef, B.; Van der Geyten, S.; Darras, V.M.; Kühn, E.R. Role of Corticotropin-Releasing Hormone as a Thyrotropin-Releasing Factor in Non-Mammalian Vertebrates. Gen. Comp. Endocrinol. 2006, 146, 62–68. [Google Scholar] [CrossRef]
- Geris, K.L.; Meeussen, G.; Kühn, E.R.; Darras, V.M. Distribution of Somatostatin in the Brain and of Somatostatin and Thyrotropin-Releasing Hormone in Peripheral Tissues of the Chicken. Brain Res. 2000, 873, 306–309. [Google Scholar] [CrossRef]
- Goel, R.; Raju, R.; Maharudraiah, J.; Kumar, G.S.S.; Ghosh, K.; Kumar, A.; Lakshmi, T.P.; Sharma, J.; Sharma, R.; Balakrishnan, L. A Signaling Network of Thyroid-Stimulating Hormone. J. Proteom. Bioinform. 2011, 4, 10–4172. [Google Scholar] [CrossRef]
- Kowalik, K.; Sechman, A. Role of Thyroid Hormones in Birds in the Light of Recent Knowledge. Rocz. Nauk. Zool. 2020, 47, 149–164. [Google Scholar]
- Richardson, S.J. Cell and Molecular Biology of Transthyretin and Thyroid Hormones. Int. Rev. Cytol. 2007, 258, 137–193. [Google Scholar] [PubMed]
- Moura Neto, A.; Zantut-Wittmann, D.E. Abnormalities of Thyroid Hormone Metabolism during Systemic Illness: The Low T3 Syndrome in Different Clinical Settings. Int. J. Endocrinol. 2016, 2016, 2157583. [Google Scholar] [CrossRef] [PubMed]
- Reyns, G.; Venken, K.; De Escobar, G.M.; Kühn, E.; Darras, V. Dynamics and Regulation of Intracellular Thyroid Hormone Concentrations in Embryonic Chicken Liver, Kidney, Brain, and Blood. Gen. Comp. Endocrinol. 2003, 134, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Van der Geyten, S.; Sanders, J.P.; Kaptein, E.; Darras, V.M.; Kühn, E.R.; Leonard, J.L.; Visser, T.J. Expression of Chicken Hepatic Type I and Type III Iodothyronine Deiodinases during Embryonic Development. Endocrinology 1997, 138, 5144–5152. [Google Scholar] [CrossRef]
- Van der Geyten, S.; Van den Eynde, I.; Segers, I.B.; Kühn, E.R.; Darras, V.M. Differential Expression of Iodothyronine Deiodinases in Chicken Tissues during the Last Week of Embryonic Development. Gen. Comp. Endocrinol. 2002, 128, 65–73. [Google Scholar] [CrossRef]
- Harvey, C.B.; Williams, G.R. Mechanism of Thyroid Hormone Action. Thyroid 2002, 12, 441–446. [Google Scholar] [CrossRef]
- Bourgeois, N.M.; Van Herck, S.L.; Vancamp, P.; Delbaere, J.; Zevenbergen, C.; Kersseboom, S.; Darras, V.M.; Visser, T.J. Characterization of Chicken Thyroid Hormone Transporters. Endocrinology 2016, 157, 2560–2574. [Google Scholar] [CrossRef]
- Newmeyer, D.D. The Nuclear Pore Complex and Nucleocytoplasmic Transport. Curr. Opin. Cell Biol. 1993, 5, 395–407. [Google Scholar] [CrossRef]
- Andersson, M.L.; Vennström, B. Chicken Thyroid Hormone Receptor α Requires the N-Terminal Amino Acids for Exclusive Nuclear Localization. FEBS Lett. 1997, 416, 291–296. [Google Scholar] [CrossRef]
- Brent, G.A. Mechanisms of Thyroid Hormone Action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef]
- Hollenberg, A.N. The Role of the Thyrotropin-Releasing Hormone (TRH) Neuron as a Metabolic Sensor. Thyroid 2008, 18, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Gorska, E.; Popko, K.; Stelmaszczyk-Emmel, A.; Ciepiela, O.; Kucharska, A.; Wasik, M. Leptin Receptors. Eur. J. Med. Res. 2010, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Joseph-Bravo, P.; Jaimes-Hoy, L.; Charli, J.-L. Advances in TRH Signaling. Rev. Endocr. Metab. Disord. 2016, 17, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Fekete, C.; Lechan, R.M. Central Regulation of Hypothalamic-Pituitary-Thyroid Axis under Physiological and Pathophysiological Conditions. Endocr. Rev. 2014, 35, 159–194. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Deng, Y.; Zhang, J.; Li, J.; Wang, Y. Characterization of a Novel Thyrotropin-Releasing Hormone Receptor, TRHR3, in Chickens. Poult. Sci. 2020, 99, 1643–1654. [Google Scholar] [CrossRef]
- Ericson, L.E.; Nilsson, M. Structural and Functional Aspects of the Thyroid Follicular Epithelium. Toxicol. Lett. 1992, 64, 365–373. [Google Scholar] [CrossRef]
- Citterio, C.E.; Targovnik, H.M.; Arvan, P. The Role of Thyroglobulin in Thyroid Hormonogenesis. Nat. Rev. Endocrinol. 2019, 15, 323–338. [Google Scholar] [CrossRef]
- Nilsson, L.-Å.; Wick, G.; Kite, J. Demonstration of Thyroglobulin in the Thyroid Glands of Obese Strain and Normal White Leghorn Chicken Embryos. Clin. Exp. Immunol. 1972, 11, 83. [Google Scholar] [PubMed]
- Jaroszewski, J.; Sundick, R.S.; Rose, N.R. Effects of Antiserum Containing Thyroglobulin Antibody on the Chicken Thyroid Gland. Clin. Immunol. Immunopathol. 1978, 10, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Avvedimento, V.E.; Tramontano, D.; Ursini, M.V.; Monticelli, A.; Di Lauro, R. The Level of Thyroglobulin mRNA Is Regulated by TSH Both in Vitro and in Vivo. Biochem. Biophys. Res. Commun. 1984, 122, 472–477. [Google Scholar] [CrossRef]
- Friedrichs, B.; Tepel, C.; Reinheckel, T.; Deussing, J.; von Figura, K.; Herzog, V.; Peters, C.; Saftig, P.; Brix, K. Thyroid Functions of Mouse Cathepsins B, K, and L. J. Clin. Investig. 2003, 111, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Rousset, B.; Dupuy, C.; Miot, F.; Dumont, J. Chapter 2 Thyroid Hormone Synthesis and Secretion. In Endotext; MDText.com, Inc.: Dartmouth, MA, USA, 2015. Available online: https://www.ncbi.nlm.nih.gov/books/NBK285550/ (accessed on 20 October 2025).
- Di Cosmo, C.; Liao, X.-H.; Dumitrescu, A.M.; Philp, N.J.; Weiss, R.E.; Refetoff, S. Mice Deficient in MCT8 Reveal a Mechanism Regulating Thyroid Hormone Secretion. J. Clin. Investig. 2010, 120, 3377–3388. [Google Scholar] [CrossRef]
- Apriletti, J.; Eberhardt, N.; Latham, K.; Baxter, J. Affinity Chromatography of Thyroid Hormone Receptors. Biospecific Elution from Support Matrices, Characterization of the Partially Purified Receptor. J. Biol. Chem. 1981, 256, 12094–12101. [Google Scholar] [CrossRef]
- Evans, R.M. The Steroid and Thyroid Hormone Receptor Superfamily. Science 1988, 240, 889–895. [Google Scholar] [CrossRef]
- Wagner, R.L.; Apriletti, J.W.; McGrath, M.E.; West, B.L.; Baxter, J.D.; Fletterick, R.J. A Structural Role for Hormone in the Thyroid Hormone Receptor. Nature 1995, 378, 690–697. [Google Scholar] [CrossRef]
- Brent, G.A.; Harney, J.W.; Chen, Y.; Warne, R.L.; Moore, D.D.; Larsen, P.R. Mutations of the Rat Growth Hormone Promoter Which Increase and Decrease Response to Thyroid Hormone Define a Consensus Thyroid Hormone Response Element. Mol. Endocrinol. 1989, 3, 1996–2004. [Google Scholar] [CrossRef]
- Hudson, L.G.; Santon, J.B.; Glass, C.K.; Gill, G.N. Ligand-Activated Thyroid Hormone and Retinoic Acid Receptors Inhibit Growth Factor Receptor Promoter Expression. Cell 1990, 62, 1165–1175. [Google Scholar] [CrossRef]
- Lazar, M.A.; Berrodin, T.J.; Harding, H.P. Differential DNA Binding by Monomeric, Homodimeric, and Potentially Heteromeric Forms of the Thyroid Hormone Receptor. Mol. Cell. Biol. 1991, 11, 5005–5015. [Google Scholar]
- Fondell, J.D.; Ge, H.; Roeder, R.G. Ligand Induction of a Transcriptionally Active Thyroid Hormone Receptor Coactivator Complex. Proc. Natl. Acad. Sci. USA 1996, 93, 8329–8333. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; Kim, B.W. Deiodinases: Implications of the Local Control of Thyroid Hormone Action. J. Clin. Investig. 2006, 116, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Mcnabb, F.A.; King, D.B. Thyroid Hormone Effects on Growth, Development, and Metabolism. In The Endocrinology of Growth, Development, and Metabolism in Vertebrates; Elsevier: Amsterdam, The Netherlands, 1993; pp. 393–417. [Google Scholar]
- May, J. Effect of Fasting on T3 and T4 Concentrations in Chicken Serum. Gen. Comp. Endocrinol. 1978, 34, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Klandorf, H.; Harvey, S. Food Intake Regulation of Circulating Thyroid Hormones in Domestic Fowl. Gen. Comp. Endocrinol. 1985, 60, 162–170. [Google Scholar] [CrossRef]
- Yahav, S. Effect of Early-Age Thermal Conditioning and Food Restriction on Performance and Thermotolerance of Male Broiler Chickens. Br. Poult. Sci. 1999, 40, 120–126. [Google Scholar] [CrossRef]
- Gancedo, B.; Alonso-Gómez, A.L.; de Pedro, N.; Delgado, M.J.; Alonso-Bedate, M. Daily Changes in Thyroid Activity in the Frog Rana Perezi: Variation with Season. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1996, 114, 79–87. [Google Scholar] [CrossRef]
- Hazlerigg, D.; Loudon, A. New Insights into Ancient Seasonal Life Timers. Curr. Biol. 2008, 18, R795–R804. [Google Scholar] [CrossRef]
- Hut, R.A. Photoperiodism: Shall EYA Compare Thee to a Summer’s Day? Curr. Biol. 2011, 21, R22–R25. [Google Scholar] [CrossRef]
- Nakao, N.; Ono, H.; Yamamura, T.; Anraku, T.; Takagi, T.; Higashi, K.; Yasuo, S.; Katou, Y.; Kageyama, S.; Uno, Y. Thyrotrophin in the Pars Tuberalis Triggers Photoperiodic Response. Nature 2008, 452, 317–322. [Google Scholar] [CrossRef]
- Osol, J.; Foss, D.; Carew, L., Jr. Effects of Light Environment and Pinealectomy on Growth and Thyroid Function in the Broiler Cockerel. Poult. Sci. 1980, 59, 647–653. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Cao, J.; Dong, Y.; Gao, T.; Chen, Y. Effects of Thyroid Hormone on Monochromatic Light Combinations Mediate Skeletal Muscle Fiber Pattern in Broilers. Poult. Sci. 2024, 103, 103999. [Google Scholar] [CrossRef]
- Yahav, S.; Straschnow, A.; Plavnik, I.; Hurwitz, S. Effects of Diurnally Cycling versus Constant Temperatures on Chicken Growth and Food Intake. Br. Poult. Sci. 1996, 37, 43–54. [Google Scholar] [CrossRef]
- Visser, W.E.; Heemstra, K.A.; Swagemakers, S.M.; Ozgur, Z.; Corssmit, E.P.; Burggraaf, J.; van Ijcken, W.F.; van der Spek, P.J.; Smit, J.W.; Visser, T.J. Physiological Thyroid Hormone Levels Regulate Numerous Skeletal Muscle Transcripts. J. Clin. Endocrinol. Metab. 2009, 94, 3487–3496. [Google Scholar] [CrossRef]
- Luongo, C.; Dentice, M.; Salvatore, D. Deiodinases and Their Intricate Role in Thyroid Hormone Homeostasis. Nat. Rev. Endocrinol. 2019, 15, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Bloise, F.F.; Cordeiro, A.; Ortiga-Carvalho, T.M. Role of Thyroid Hormone in Skeletal Muscle Physiology. J. Endocrinol. 2018, 236, R57–R68. [Google Scholar] [CrossRef] [PubMed]
- Sagliocchi, S.; Cicatiello, A.G.; Di Cicco, E.; Ambrosio, R.; Miro, C.; Di Girolamo, D.; Nappi, A.; Mancino, G.; De Stefano, M.A.; Luongo, C. The Thyroid Hormone Activating Enzyme, Type 2 Deiodinase, Induces Myogenic Differentiation by Regulating Mitochondrial Metabolism and Reducing Oxidative Stress. Redox Biol. 2019, 24, 101228. [Google Scholar] [CrossRef] [PubMed]
- Cicatiello, A.G.; Sagliocchi, S.; Nappi, A.; Di Cicco, E.; Miro, C.; Murolo, M.; Stornaiuolo, M.; Dentice, M. Thyroid Hormone Regulates Glutamine Metabolism and Anaplerotic Fluxes by Inducing Mitochondrial Glutamate Aminotransferase GPT2. Cell Rep. 2022, 38, 110409. [Google Scholar] [CrossRef]
- Villanueva, I.; Alva-Sánchez, C.; Pacheco-Rosado, J. The Role of Thyroid Hormones as Inductors of Oxidative Stress and Neurodegeneration. Oxidative Med. Cell. Longev. 2013, 2013, 218145. [Google Scholar] [CrossRef]
- Costilla, M.; Macri Delbono, R.; Klecha, A.; Cremaschi, G.A.; Barreiro Arcos, M.L. Oxidative Stress Produced by Hyperthyroidism Status Induces the Antioxidant Enzyme Transcription through the Activation of the Nrf-2 Factor in Lymphoid Tissues of Balb/c Mice. Oxidative Med. Cell. Longev. 2019, 2019, 7471890. [Google Scholar] [CrossRef]
- Dransfield, J.W. The Lymphatic System of the Domestic Fowl. Vet. J. 1945, 101, 171–174, 174.e1–174.e6, 175–179. [Google Scholar] [CrossRef]
- Freedman, S.L. The Innervation of the Suprarenal Gland of the Fowl (Gallus domesticus). Cells Tissues Organs 1968, 69, 18–25. [Google Scholar] [CrossRef]
- Unsicker, K. Fine Structure and Innervation of the Avian Adrenal Gland: II. Cholinergic Innervation of Adrenal Chromaffin Cells. Z. Zellforsch. Mikrosk. Anat. 1973, 145, 417–442. [Google Scholar] [CrossRef] [PubMed]
- Unsicker, K. Fine Structure and Innervation of the Avian Adrenal Gland: III. Non-Cholinergic Nerve Fibers. Z. Zellforsch. Mikrosk. Anat. 1973, 145, 557–575. [Google Scholar] [CrossRef]
- Unsicker, K. Fine Structure and Innervation of the Avian Adrenal Gland: V. Innervation of Interrenal Cells. Z. Zellforsch. Mikrosk. Anat. 1973, 146, 403–416. [Google Scholar] [CrossRef]
- Moawad, U.; Randa, M. Histocytological and Histochemical Features of the Adrenal Gland of Adult Egyptian Native Breeds of Chicken (Gallus gallus domesticus). Beni-Suef Univ. J. Basic Appl. Sci. 2017, 6, 199–208. [Google Scholar] [CrossRef]
- Kot, T.; Prokopenko, V. Peculiarities of the Morphology of the Adrenal Glands of Chickens. Наукoві Гoризoнти 2020, 5, 82–88. [Google Scholar]
- Kot, T.; Tkachuk, S.; Usenko, S.; Prokopenko, V. Adrenal Gland of Poultry: Anatomy, Microscopy, Morphometry, and Histochemistry. J. World’s Poult. Res. 2023, 13, 20–28. [Google Scholar] [CrossRef]
- Sarkar, S.; Islam, M.N.; Adhikary, N.; Paul, B.; Bhowmik, N. Morphological and Histological Studies on the Adrenal Gland in Male and Female Chicken (Gallus domesticus). Int. J. Biol. Pharm. Res. 2014, 5, 715–718. [Google Scholar]
- Elzoghby, I.M. Light and Electron Microscope Studies of the Adrenal Glands of the Egyptian Geese (Alopochen aegyptiacus). Lucr. Științifice Med. Vet. Univ. Științe Agric. Med. Vet. 2010, 12, 195–203. [Google Scholar]
- Ohmori, Y.; Okada, Y.; Watanabe, T. Immunohistochemical Localization of Serotonin, Galanin, Cholecystokinin, and Methionine-Enkephalin in Adrenal Medullary Cells of the Chicken. Tissue Cell 1997, 29, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Unsicker, K. Fine Structure and Innervation of the Avian Adrenal Gland: I. Fine Structure of Adrenal Chromaffin Cells and Ganglion Cells. Z. Zellforsch. Mikrosk. Anat. 1973, 145, 389–416. [Google Scholar] [CrossRef] [PubMed]
- Holmes, W.; Al-Ghawas, S.; Cronshaw, J.; Rohde, K. The Structural Organization and the Steroidogenic Responsiveness in Vitro of Adrenal Gland Tissue from the Neonatal Mallard Duck (Anas platyrhynchos). Cell Tissue Res. 1991, 263, 557–566. [Google Scholar] [CrossRef]
- Jones, I.C.; Phillips, J. The Adrenal and Interrenal Glands. In Morphological Considerations; Elsevier: Amsterdam, The Netherlands, 1986; pp. 319–350. [Google Scholar]
- Humayun, K.A.; Aoyama, M.; Sugita, S. Morphological and Histological Studies on the Adrenal Gland of the Chicken (Gallus domesticus). J. Poult. Sci. 2012, 49, 39–45. [Google Scholar] [CrossRef]
- Dadgar Moghadam, P.; Mohammadpour, A.A. Histomorphological and Stereological Study on the Adrenal Glands of Adult Female Guinea Fowl (Numida meleagris). Comp. Clin. Pathol. 2017, 26, 1227–1231. [Google Scholar] [CrossRef]
- El-Desoky, S.M.; Mustafa, F.E.A. Morphological and Histological Studies of the Adrenal Gland in the Japanese Quail (Coturnix japonica). Microsc. Res. Tech. 2021, 84, 2361–2371. [Google Scholar] [CrossRef]
- Aguilera, G. Regulation of Pituitary ACTH Secretion during Chronic Stress. Front. Neuroendocrinol. 1994, 15, 321–350. [Google Scholar] [CrossRef]
- Madison, F.; Jurkevich, A.; Kuenzel, W. Sex Differences in Plasma Corticosterone Release in Undisturbed Chickens (Gallus gallus) in Response to Arginine Vasotocin and Corticotropin Releasing Hormone. Gen. Comp. Endocrinol. 2008, 155, 566–573. [Google Scholar] [CrossRef]
- Salem, M.; Norton, H.; Nalbandov, A. A Study of ACTH and CRF in Chickens. Gen. Comp. Endocrinol. 1970, 14, 270–280. [Google Scholar] [CrossRef]
- de Matos, R. Adrenal Steroid Metabolism in Birds: Anatomy, Physiology, and Clinical Considerations. Vet. Clin. N. Am. Exot. Anim. Pract. 2008, 11, 35–57. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Song, G.; Lei, F.; Li, D.; Qu, Y. Avian Corticosteroid-Binding Globulin: Biological Function and Regulatory Mechanisms in Physiological Stress Responses. Front. Zool. 2021, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Hammond, G.L. Molecular Properties of Corticosteroid Binding Globulin and the Sex-Steroid Binding Proteins. Endocr. Rev. 1990, 11, 65–79. [Google Scholar] [CrossRef]
- Breuner, C.; Lynn, S.; Julian, G.; Cornelius, J.; Heidinger, B.; Love, O.; Sprague, R.; Wada, H.; Whitman, B. Plasma-Binding Globulins and Acute Stress Response. Horm. Metab. Res. 2006, 38, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Rosner, W. Investigation of the Binding Site of Human Corticosteroid-Binding Globulin by Affinity Labeling. Demonstration of a Cysteinyl Residue in the Binding Site. J. Biol. Chem. 1977, 252, 1895–1900. [Google Scholar] [CrossRef] [PubMed]
- Defaye, G.; Basset, M.; Monnier, N.; Chambaz, E. Electron Spin Resonance Study of Human Transcortin Thiol Groups and Binding Site Topography. Biochim. Biophys. Acta (BBA)-Protein Struct. 1980, 623, 280–294. [Google Scholar] [CrossRef]
- Mazancová, K.; Kučka, M.; Mikšík, I.; Pácha, J. Glucocorticoid Metabolism and Na+ Transport in Chicken Intestine. J. Exp. Zool. Part A Comp. Exp. Biol. 2005, 303, 113–122. [Google Scholar] [CrossRef]
- Kučka, M.; Vagnerova, K.; Klusoňová, P.; Mikšík, I.; Pacha, J. Corticosterone Metabolism in Chicken Tissues: Evidence for Tissue-Specific Distribution of Steroid Dehydrogenases. Gen. Comp. Endocrinol. 2006, 147, 377–383. [Google Scholar] [CrossRef]
- Vashchenko, G.; Das, S.; Moon, K.-M.; Rogalski, J.C.; Taves, M.D.; Soma, K.K.; Van Petegem, F.; Foster, L.J.; Hammond, G.L. Identification of Avian Corticosteroid-Binding Globulin (SerpinA6) Reveals the Molecular Basis of Evolutionary Adaptations in SerpinA6 Structure and Function as a Steroid-Binding Protein. J. Biol. Chem. 2016, 291, 11300–11312. [Google Scholar] [CrossRef]
- Reisine, T.; Rougon, G.; Barbet, J.; Affolter, H.-U. Corticotropin-Releasing Factor-Induced Adrenocorticotropin Hormone Release and Synthesis Is Blocked by Incorporation of the Inhibitor of Cyclic AMP-Dependent Protein Kinase into Anterior Pituitary Tumor Cells by Liposomes. Proc. Natl. Acad. Sci. USA 1985, 82, 8261–8265. [Google Scholar] [CrossRef]
- Coleman, M.E.; DeMayo, F.; Yin, K.C.; Lee, H.M.; Geske, R.; Montgomery, C.; Schwartz, R.J. Myogenic Vector Expression of Insulin-like Growth Factor I Stimulates Muscle Cell Differentiation and Myofiber Hypertrophy in Transgenic Mice. J. Biol. Chem. 1995, 270, 12109–12116. [Google Scholar] [CrossRef]
- Harno, E.; Gali Ramamoorthy, T.; Coll, A.P.; White, A. POMC: The Physiological Power of Hormone Processing. Physiol. Rev. 2018, 98, 2381–2430. [Google Scholar] [CrossRef]
- Mikhailova, M.V.; Mayeux, P.R.; Jurkevich, A.; Kuenzel, W.J.; Madison, F.; Periasamy, A.; Chen, Y.; Cornett, L.E. Heterooligomerization between Vasotocin and Corticotropin-Releasing Hormone (CRH) Receptors Augments CRH-Stimulated 3′, 5′-Cyclic Adenosine Monophosphate Production. Mol. Endocrinol. 2007, 21, 2178–2188. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.F.; Koritz, S.B. Action of ACTH upon Steroidogenesis in the Chicken Adrenal Gland. Endocrinology 1966, 79, 652–654. [Google Scholar] [CrossRef] [PubMed]
- De Groef, B.; Vandenborne, K.; Van As, P.; Darras, V.M.; Kühn, E.R.; Decuypere, E.; Geris, K.L. Hypothalamic Control of the Thyroidal Axis in the Chicken: Over the Boundaries of the Classical Hormonal Axes. Domest. Anim. Endocrinol. 2005, 29, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Cone, R.D. Studies on the Physiological Functions of the Melanocortin System. Endocr. Rev. 2006, 27, 736–749. [Google Scholar] [CrossRef]
- Takeuchi, S.; Kudo, T.; Takahashi, S. Molecular Cloning of the Chicken Melanocortin 2 (ACTH)-Receptor Gene. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 1998, 1403, 102–108. [Google Scholar] [CrossRef]
- Sebag, J.A.; Hinkle, P.M. Regulation of G Protein–Coupled Receptor Signaling: Specific Dominant-Negative Effects of Melanocortin 2 Receptor Accessory Protein 2. Sci. Signal. 2010, 3, ra28. [Google Scholar] [CrossRef]
- Agulleiro, M.J.; Roy, S.; Sánchez, E.; Puchol, S.; Gallo-Payet, N.; Cerdá-Reverter, J.M. Role of Melanocortin Receptor Accessory Proteins in the Function of Zebrafish Melanocortin Receptor Type 2. Mol. Cell. Endocrinol. 2010, 320, 145–152. [Google Scholar] [CrossRef]
- Barlock, T.K.; Gehr, D.T.; Dores, R.M. Analysis of the Pharmacological Properties of Chicken Melanocortin-2 Receptor (cMC2R) and Chicken Melanocortin-2 Accessory Protein 1 (cMRAP1). Gen. Comp. Endocrinol. 2014, 205, 260–267. [Google Scholar] [CrossRef]
- Metherell, L.A.; Chapple, J.P.; Cooray, S.; David, A.; Becker, C.; Rüschendorf, F.; Naville, D.; Begeot, M.; Khoo, B.; Nürnberg, P. Mutations in MRAP, Encoding a New Interacting Partner of the ACTH Receptor, Cause Familial Glucocorticoid Deficiency Type 2. Nat. Genet. 2005, 37, 166–170. [Google Scholar] [CrossRef]
- Cooray, S.N.; Almiro Do Vale, I.; Leung, K.-Y.; Webb, T.R.; Chapple, J.P.; Egertová, M.; Cheetham, M.E.; Elphick, M.R.; Clark, A.J. The Melanocortin 2 Receptor Accessory Protein Exists as a Homodimer and Is Essential for the Function of the Melanocortin 2 Receptor in the Mouse Y1 Cell Line. Endocrinology 2008, 149, 1935–1941. [Google Scholar] [CrossRef]
- Pratt, W.; Morishima, Y.; Murphy, M.; Harrell, M. Chaperoning of Glucocorticoid Receptors. Mol. Chaperones Health Dis. 2006, 172, 111–138. [Google Scholar]
- Kirschke, E.; Goswami, D.; Southworth, D.; Griffin, P.R.; Agard, D.A. Glucocorticoid Receptor Function Regulated by Coordinated Action of the Hsp90 and Hsp70 Chaperone Cycles. Cell 2014, 157, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, J.J.; Murphy, P.J.; Gaillard, S.; Zhao, X.; Wu, J.-T.; Nicchitta, C.V.; Yoshida, M.; Toft, D.O.; Pratt, W.B.; Yao, T.-P. HDAC6 Regulates Hsp90 Acetylation and Chaperone-Dependent Activation of Glucocorticoid Receptor. Mol. Cell 2005, 18, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Yudt, M.R.; Cidlowski, J.A. Molecular Identification and Characterization of a and b Forms of the Glucocorticoid Receptor. Mol. Endocrinol. 2001, 15, 1093–1103. [Google Scholar] [CrossRef]
- Kwok, A.; Wang, Y.; Wang, C.; Leung, F. Cloning of Chicken Glucocorticoid Receptor (GR) and Characterization of Its Expression in Pituitary and Extrapituitary Tissues. Poult. Sci. 2007, 86, 423–430. [Google Scholar] [CrossRef]
- Jenkins, S.; Porter, T. Ontogeny of the Hypothalamo–Pituitary–Adrenocortical Axis in the Chicken Embryo: A Review. Domest. Anim. Endocrinol. 2004, 26, 267–275. [Google Scholar] [CrossRef]
- Leach, S.; Suzuki, K. Adrenergic Signaling in Circadian Control of Immunity. Front. Immunol. 2020, 11, 1235. [Google Scholar] [CrossRef]
- Romero, L.M.; Dickens, M.J.; Cyr, N.E. The Reactive Scope Model—A New Model Integrating Homeostasis, Allostasis, and Stress. Horm. Behav. 2009, 55, 375–389. [Google Scholar] [CrossRef]
- Hau, M.; Goymann, W. Endocrine Mechanisms, Behavioral Phenotypes and Plasticity: Known Relationships and Open Questions. Front. Zool. 2015, 12, S7. [Google Scholar] [CrossRef]
- Hau, M.; Casagrande, S.; Ouyang, J.Q.; Baugh, A.T. Glucocorticoid-Mediated Phenotypes in Vertebrates: Multilevel Variation and Evolution. Adv. Study Behav. 2016, 48, 41–115. [Google Scholar]
- Hesham, M.; El Shereen, A.; Enas, S. Impact of Different Light Colors in Behavior, Welfare Parameters and Growth Performance of Fayoumi Broiler Chickens Strain. J. Hell. Vet. Med. Soc. 2018, 69, 951–958. [Google Scholar] [CrossRef]
- Archer, G. Exposing Broiler Eggs to Green, Red and White Light during Incubation. Animal 2017, 11, 1203–1209. [Google Scholar] [CrossRef]
- Olanrewaju, H.; Purswell, J.; Collier, S.; Branton, S. Effects of Color Temperatures (Kelvin) of LED Bulbs on Blood Physiological Variables of Broilers Grown to Heavy Weights. Poult. Sci. 2015, 94, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, H.; Miller, W.; Maslin, W.; Collier, S.; Purswell, J.; Branton, S. Influence of Photoperiod, Light Intensity and Their Interaction on Health Indices of Modern Broilers Grown to Heavy Weights. Int. J. Poult. Sci. 2015, 14, 183. [Google Scholar] [CrossRef]
- Xie, D.; Wang, Z.; Dong, Y.; Cao, J.; Wang, J.; Chen, J.; Chen, Y. Effects of Monochromatic Light on Immune Response of Broilers. Poult. Sci. 2008, 87, 1535–1539. [Google Scholar] [CrossRef] [PubMed]
- Franco, B.R.; Shynkaruk, T.; Crowe, T.; Fancher, B.; French, N.; Gillingham, S.; Schwean-Lardner, K. Light Color and the Commercial Broiler: Effect on Behavior, Fear, and Stress. Poult. Sci. 2022, 101, 102052. [Google Scholar] [CrossRef]
- Mehaisen, G.M.; Eshak, M.G.; Elkaiaty, A.M.; Atta, A.-R.M.; Mashaly, M.M.; Abass, A.O. Comprehensive Growth Performance, Immune Function, Plasma Biochemistry, Gene Expressions and Cell Death Morphology Responses to a Daily Corticosterone Injection Course in Broiler Chickens. PLoS ONE 2017, 12, e0172684. [Google Scholar] [CrossRef]
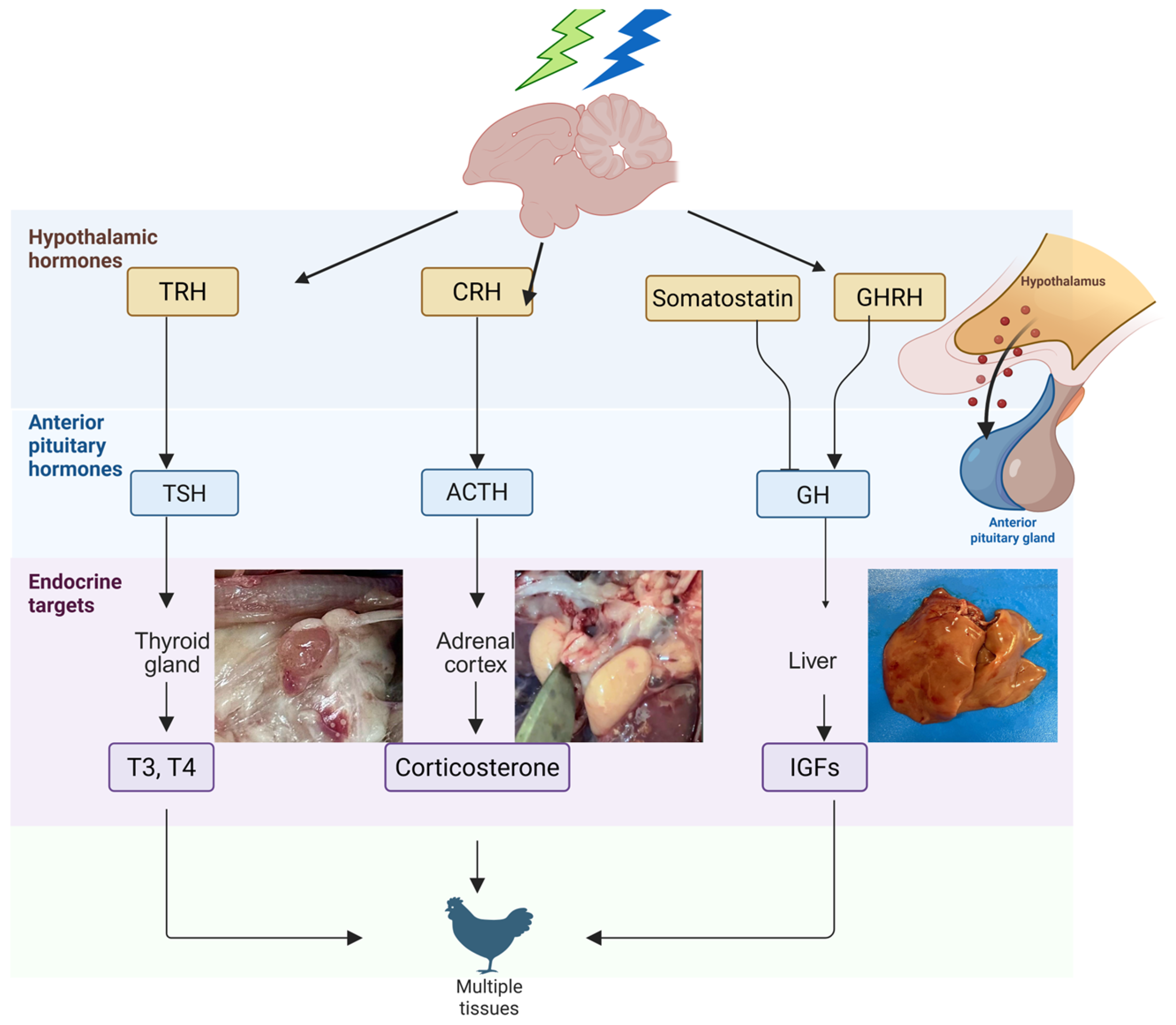
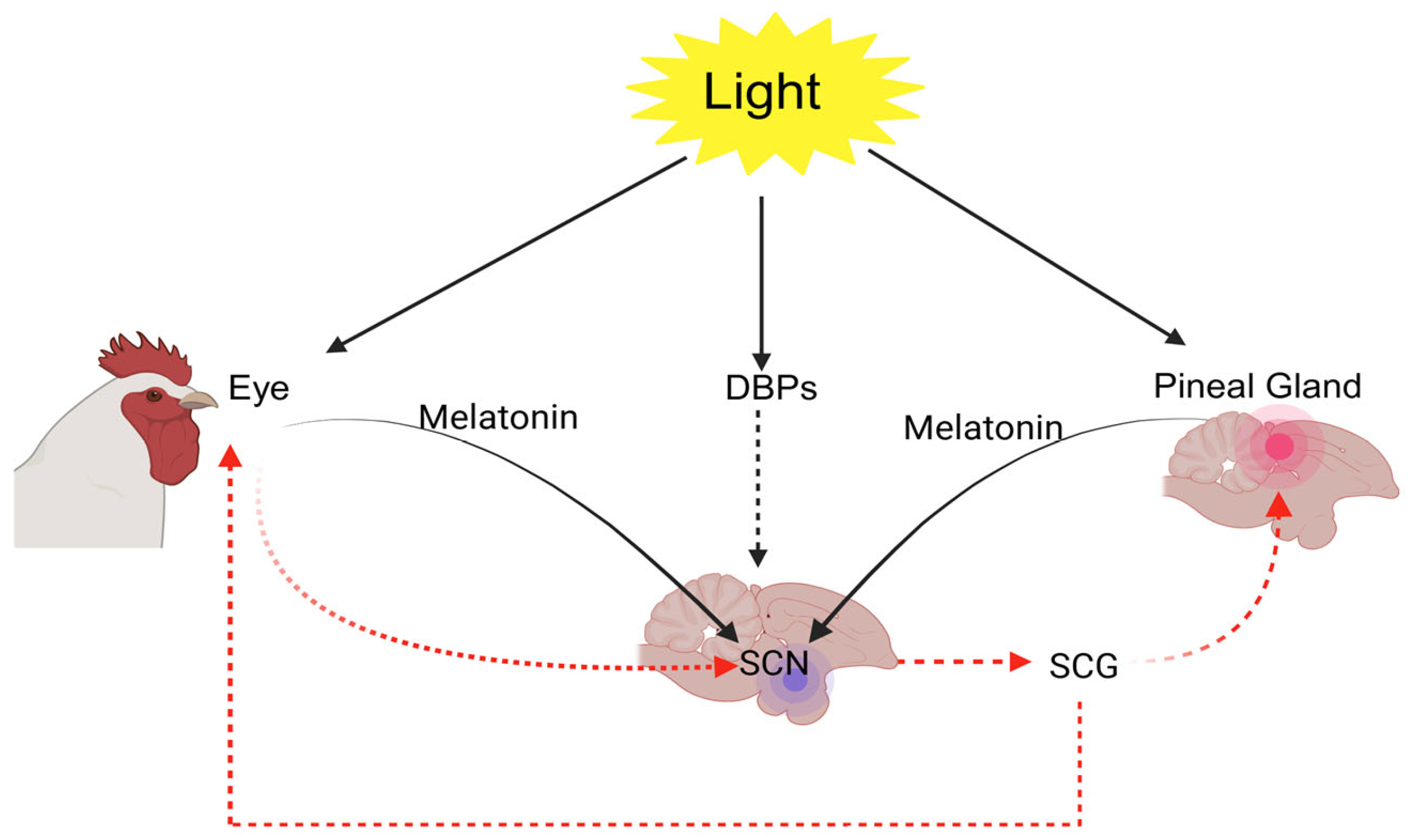
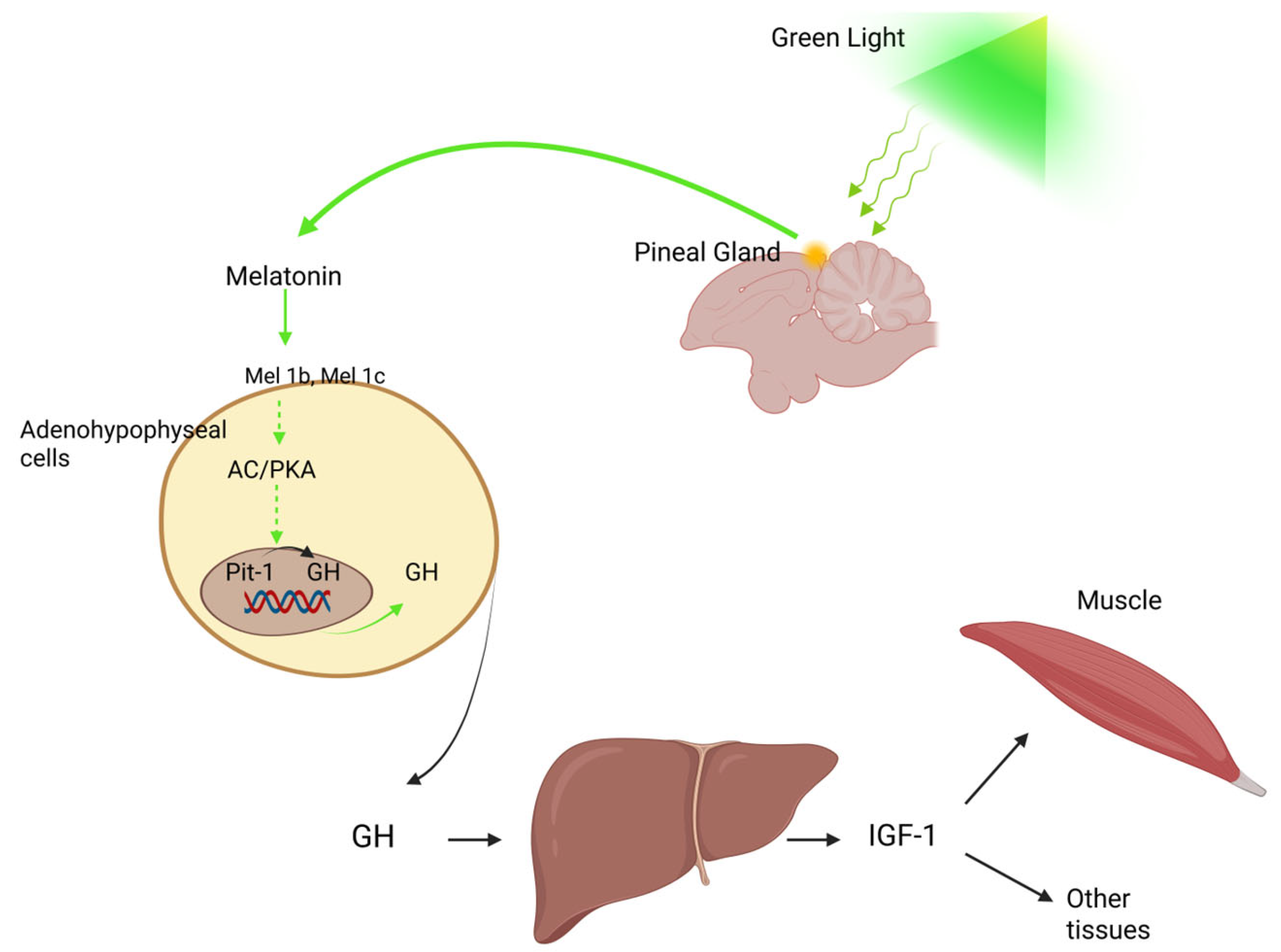
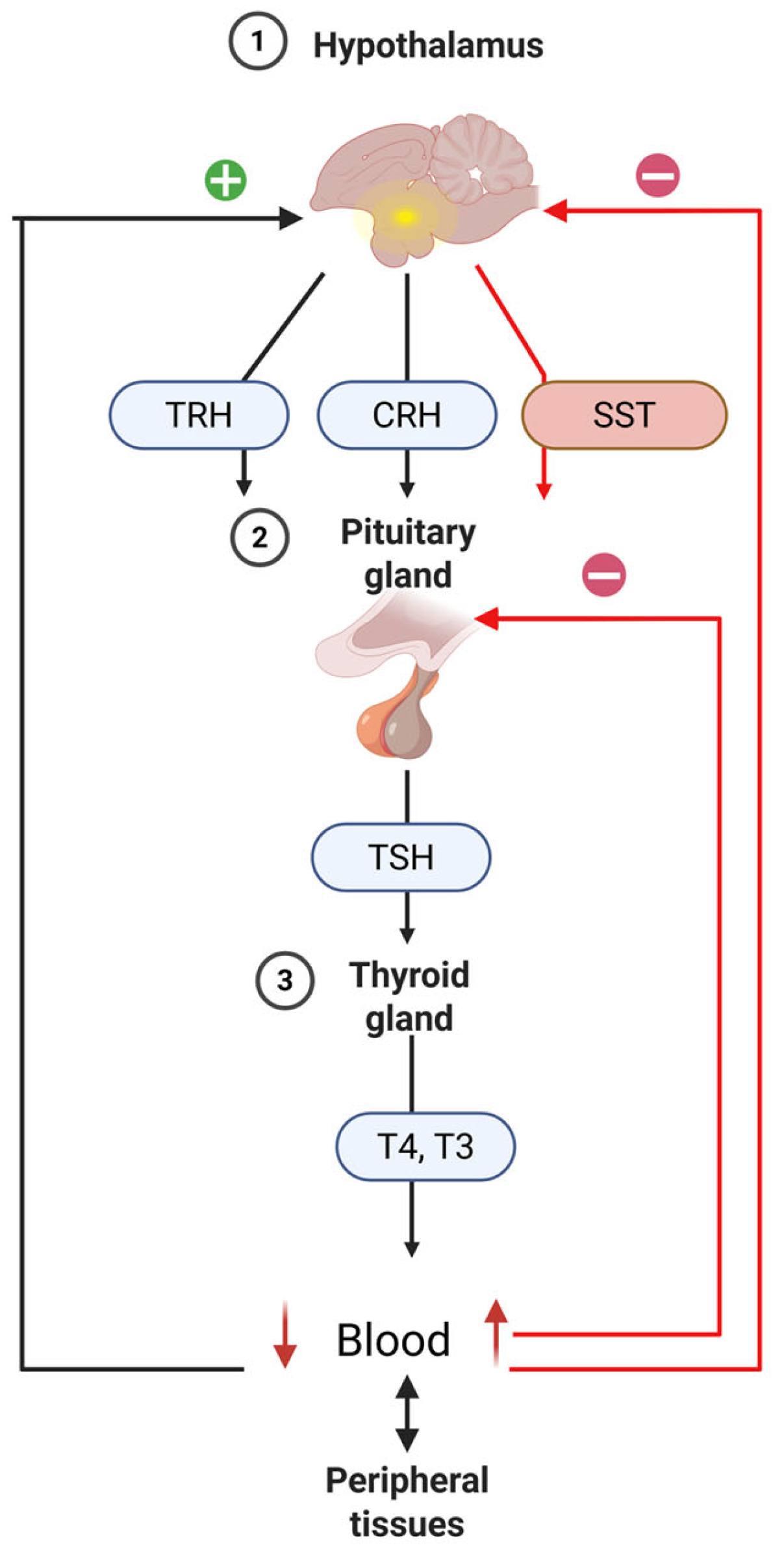
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galan, L.; Solcan, G.; Solcan, C. The Influence of Different Light Spectra on Broiler Chicken Endocrine Systems and Productivity. Animals 2025, 15, 3209. https://doi.org/10.3390/ani15213209
Galan L, Solcan G, Solcan C. The Influence of Different Light Spectra on Broiler Chicken Endocrine Systems and Productivity. Animals. 2025; 15(21):3209. https://doi.org/10.3390/ani15213209
Chicago/Turabian StyleGalan, Lenuța, Gheorghe Solcan, and Carmen Solcan. 2025. "The Influence of Different Light Spectra on Broiler Chicken Endocrine Systems and Productivity" Animals 15, no. 21: 3209. https://doi.org/10.3390/ani15213209
APA StyleGalan, L., Solcan, G., & Solcan, C. (2025). The Influence of Different Light Spectra on Broiler Chicken Endocrine Systems and Productivity. Animals, 15(21), 3209. https://doi.org/10.3390/ani15213209






