Free Plasma Amino Acid Concentrations in Horses Fed Different Dosing Regimens of Hydrolysed Collagen
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Selection of Horses
2.3. Diet
2.4. Sampling
2.5. Amino Acid Analysis
2.6. Statistical Analysis
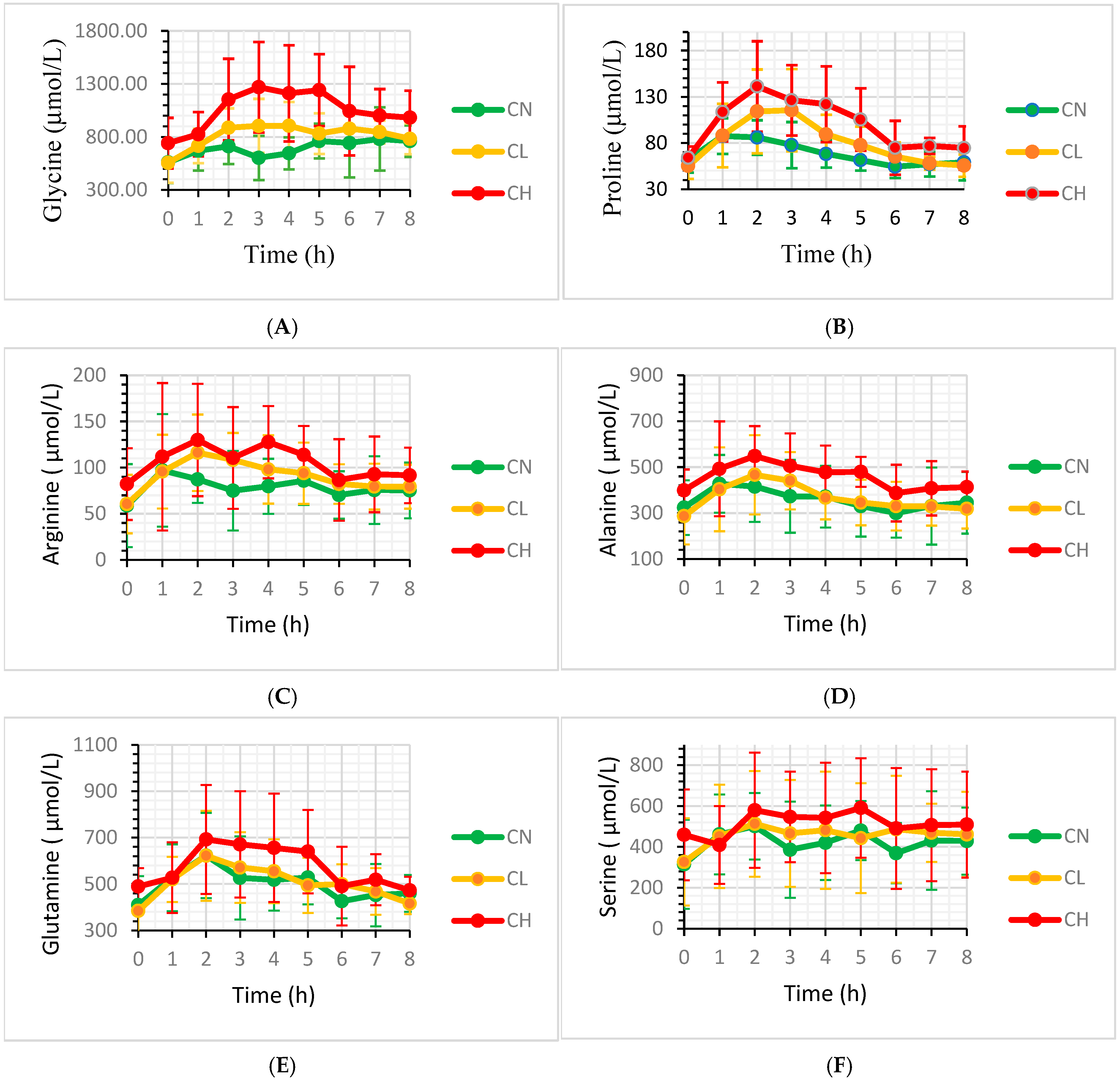
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Amino Acids |
| ALA | Alanine |
| ARG | Arginine |
| ASN | Asparagine |
| ASP | Aspartic acid |
| bwt | Body weight |
| CH | Concentration high hydrolysed collagen |
| CL | Concentration low hydrolysed collagen |
| CN | Concentration none hydrolysed collagen (control) |
| d | Day |
| DoM | Differences of means |
| EGUS | Equine Gastric Ulcer Syndrome |
| EW-pa | Energy value horse (Net Energy) |
| FS | Feeding supplement |
| GLN | Glutamine |
| GLU | Glutamic acid |
| GLY | Glycine |
| HC | Hydrolysed collagen |
| HIS | Histidine |
| HYP | Hydroxyproline |
| ILE | Isoleucine |
| kg | Kilogram |
| LEU | Leucine |
| LYS | Lysine |
| MET | Methionine |
| PHE | Phenylalanine |
| PRO | Proline |
| SER | Serine |
| THR | Threonine |
| TRP | Tryptophan |
| TYR | Tyrosine |
| VAL | Valine |
References
- Bello, A.E.; Oesser, S. Collagen Hydrolysate for the Treatment of Osteoarthritis and Other Joint Disorders:A Review of the Literature. Curr. Med. Res. Opin. 2006, 22, 2221–2232. [Google Scholar] [CrossRef]
- Benito-Ruiz, P.; Camacho-Zambrano, M.M.; Carrillo-Arcentales, J.N.; Mestanza-Peralta, M.A.; Vallejo-Flores, C.A.; Vargas-López, S.V.; Villacís-Tamayo, R.A.; Zurita-Gavilanes, L.A. A Randomized Controlled Trial on the Efficacy and Safety of a Food Ingredient, Collagen Hydrolysate, for Improving Joint Comfort. Int. J. Food Sci. Nutr. 2009, 60, 99–113. [Google Scholar] [CrossRef]
- Crowley, D.C.; Lau, F.C.; Sharma, P.; Evans, M.; Guthrie, N.; Bagchi, M.; Bagchi, D.; Dey, D.K.; Raychaudhuri, S.P. Safety and Efficacy of Undenatured Type II Collagen in the Treatment of Osteoarthritis of the Knee: A Clinical Trial. Int. J. Med. Sci. 2009, 6, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sugihara, F.; Suzuki, K.; Inoue, N.; Venkateswarathirukumara, S. A Double-Blind, Placebo-Controlled, Randomised, Clinical Study on the Effectiveness of Collagen Peptide on Osteoarthritis. J. Sci. Food Agric. 2015, 95, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Lugo, J.P.; Saiyed, Z.M.; Lane, N.E. Efficacy and Tolerability of an Undenatured Type II Collagen Supplement in Modulating Knee Osteoarthritis Symptoms: A Multicenter Randomized, Double-Blind, Placebo-Controlled Study. Nutr. J. 2016, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Su, W.; Song, Y.; Srivastava, S. Efficacy and Safety of Native Type II Collagen in Modulating Knee Osteoarthritis Symptoms: A Randomised, Double-Blind, Placebo-Controlled Trial. J. Exp. Orthop. 2022, 9, 123. [Google Scholar] [CrossRef]
- Moskowitz, R.W. Role of Collagen Hydrolysate in Bone and Joint Disease. Semin. Arthritis Rheum. 2000, 30, 87–99. [Google Scholar] [CrossRef]
- Clark, K.L.; Sebastianelli, W.; Flechsenhar, K.R.; Aukermann, D.F.; Meza, F.; Millard, R.L.; Deitch, J.R.; Sherbondy, P.S.; Albert, A. 24-Week Study on the Use of Collagen Hydrolysate as a Dietary Supplement in Athletes with Activity-Related Joint Pain. Curr. Med. Res. Opin. 2008, 24, 1485–1496. [Google Scholar] [CrossRef]
- Beynen, A.C.; Geene, H.W.V.; Grim, H.V.; Jacobs, P.; Vlerk, T.V. der Oral Administration of Gelatin Hydrolysate Reduces Clinical Signs of Canine Osteoarthritis in a Double-Blind, Placebo-Controlled Trial. Am. J. Anim. Vet. Sci. 2010, 5, 102–106. [Google Scholar] [CrossRef]
- Blees, N.R.; Teunissen, M.; Dobenecker, B.; Prawitt, J.; Tryfonidou, M.A.; Jan Corbee, R. Collagen Hydrolysates as Nutritional Support in Canine Osteoarthritis: A Narrative Review. J. Anim. Physiol. Anim. Nutr. 2025, 109, 590–600. [Google Scholar] [CrossRef]
- Comblain, F.; Barthélémy, N.; Lefèbvre, M.; Schwartz, C.; Lesponne, I.; Serisier, S.; Feugier, A.; Balligand, M.; Henrotin, Y. A Randomized, Double-Blind, Prospective, Placebo-Controlled Study of the Efficacy of a Diet Supplemented with Curcuminoids Extract, Hydrolyzed Collagen and Green Tea Extract in Owner’s Dogs with Osteoarthritis. BMC Vet. Res. 2017, 13, 395. [Google Scholar] [CrossRef]
- Gupta, R.C.; Canerdy, T.D.; Lindley, J.; Konemann, M.; Minniear, J.; Carroll, B.A.; Hendrick, C.; Goad, J.T.; Rohde, K.; Doss, R.; et al. Comparative Therapeutic Efficacy and Safety of type-II Collagen (uc-II), Glucosamine and Chondroitin in Arthritic Dogs: Pain Evaluation by Ground Force Plate. J. Anim. Physiol. Anim. Nutr. 2012, 96, 770–777. [Google Scholar] [CrossRef]
- Gupta, R.C.; Canerdy, T.D.; Skaggs, P.; Stocker, A.; Zyrkowski, G.; Burke, R.; Wegford, K.; Goad, J.T.; Rohde, K.; Barnett, D.; et al. Therapeutic Efficacy of Undenatured Type-II Collagen (UC-II) in Comparison to Glucosamine and Chondroitin in Arthritic Horses. J. Vet. Pharmacol. Ther. 2009, 32, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Van De Water, E.; Oosterlinck, M.; Dumoulin, M.; Korthagen, N.M.; Van Weeren, P.R.; Van Den Broek, J.; Everts, H.; Pille, F.; Van Doorn, D.A. The Preventive Effects of Two Nutraceuticals on Experimentally Induced Acute Synovitis. Equine Vet. J. 2017, 49, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Butler, K.D., Jr.; Hintz, H.F. Effect of Level of Feed Intake and Gelatin Supplementation on Growth and Quality of Hoofs of Ponies. J. Anim. Sci. 1977, 44, 257–261. [Google Scholar] [CrossRef]
- Dobenecker, B.; Reese, S.; Jahn, W.; Schunck, M.; Hugenberg, J.; Louton, H.; Oesser, S. Specific Bioactive Collagen Peptides (PETAGILE®) as Supplement for Horses with Osteoarthritis: A Two-Centred Study. J. Anim. Physiol. Anim. Nutr. 2018, 102, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Andrews, F.; Camacho-Luna, P.; Lamp, B.; Olijve, J. Effects of Hydrolyzed Collagen on Equine Gastric Ulcers Scores and Gastric Juice pH. J. Equine Vet. Sci. 2017, 52, 91. [Google Scholar] [CrossRef]
- Camacho-Luna, P.; Andrews, F.M.; Keowen, M.L.; Garza Jr, F.; Liu, C.-C.; Lamp, B.; Olijve, J. The Effect of Porcine Hydrolysed Collagen on Gastric Ulcer Scores, Gastric Juice pH, Gastrin and Amino Acid Concentrations in Horses. Equine Vet. Educ. 2022, 34, 248–257. [Google Scholar] [CrossRef]
- Castro, G.A.; Carvalho, J.E.; Tinti, S.V.; Possenti, A.; Sgarbieri, V.C. Anti-Ulcerogenic Effect of a Whey Protein Isolate and Collagen Hydrolysates against Ethanol Ulcerative Lesions on Oral Administration to Rats. J. Med. Food 2010, 13, 83–90. [Google Scholar] [CrossRef]
- Kumar, D.; Hegde, H.V.; Patil, P.A.; Roy, S.; Kholkute, S.D. Antiulcer Activity of Water Soaked Glycine max L. Grains in Aspirin Induced Model of Gastric Ulcer in Wistar Rats. J. Ayurveda Integr. Med. 2013, 4, 134–137. [Google Scholar] [CrossRef]
- Bakaeva, Z.V.; Sangadzhieva, A.D.; Tani, S.; Myasoedov, N.F.; Andreeva, L.A.; Torshin, V.I.; Wallace, J.L.; Tanaka, T. Glyprolines Exert Protective and Repair-Promoting Effects in the Rat Stomach: Potential Role of the Cytokine GRO/CINC-1. J. Physiol. Pharmacol. 2016, 67, 253–260. [Google Scholar]
- Mok, C.H.; Urschel, K.L. Amino Acid Requirements in Horses. Asian-Australas. J. Anim. Sci. 2020, 33, 679–695. [Google Scholar] [CrossRef]
- Woodward, A.D.; Holcombe, S.J.; Steibel, J.P.; Staniar, W.B.; Colvin, C.; Trottier, N.L. Cationic and Neutral Amino Acid Transporter Transcript Abundances Are Differentially Expressed in the Equine Intestinal Tract. J. Anim. Sci. 2010, 88, 1028–1033. [Google Scholar] [CrossRef]
- Iwai, K.; Hasegawa, T.; Taguchi, Y.; Morimatsu, F.; Sato, K.; Nakamura, Y.; Higashi, A.; Kido, Y.; Nakabo, Y.; Ohtsuki, K. Identification of Food-Derived Collagen Peptides in Human Blood after Oral Ingestion of Gelatin Hydrolysates. J. Agric. Food Chem. 2005, 53, 6531–6536. [Google Scholar] [CrossRef] [PubMed]
- Coenen, M.; Appelt, K.; Niemeyer, A.; Vervuert, I. Study of Gelatin Supplemented Diet on Amino Acid Homeostasis in the Horse. Equine Vet. J. 2006, 38, 606–610. [Google Scholar] [CrossRef]
- Henneke, D.R.; Potter, G.D.; Kreider, J.L.; Yeates, B.F. Relationship between Condition Score, Physical Measurements and Body Fat Percentage in Mares. Equine Vet. J. 1983, 15, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Sykes, B.W.; Hewetson, M.; Hepburn, R.J.; Luthersson, N.; Tamzali, Y. European College of Equine Internal Medicine Consensus Statement—Equine Gastric Ulcer Syndrome in Adult Horses. J. Vet. Intern. Med. 2015, 29, 1288–1299. [Google Scholar] [CrossRef]
- Centraal Veevoederbureau. Het EW-Pa en VREp Systeem; Productschap Diervoeder: Lelystad, The Netherlands, 2004. [Google Scholar]
- Harris, P.A.; Ellis, A.D.; Fradinho, M.J.; Jansson, A.; Julliand, V.; Luthersson, N.; Santos, A.S.; Vervuert, I. Review: Feeding Conserved Forage to Horses: Recent Advances and Recommendations. Animal 2017, 11, 958–967. [Google Scholar] [CrossRef]
- Métayer, N.; Lhôte, M.; Bahr, A.; Cohen, N.D.; Kim, I.; Roussel, A.J.; Julliand, V. Meal Size and Starch Content Affect Gastric Emptying in Horses. Equine Vet. J. 2004, 36, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.A.; Rodiek, A.V.; Lawrence, L.M. Effect of Meal Schedules and Fasting on Selected Plasma Free Amino Acids in Horses. J. Anim. Sci. 1986, 63, 1428–1431. [Google Scholar] [CrossRef]
- Grasset, E.; Briand, F.; Virgilio, N.; Schön, C.; Wilhelm, M.; Cudennec, B.; Ravallec, R.; Aboubacar, H.; Vleminckx, S.; Prawitt, J.; et al. A Specific Collagen Hydrolysate Improves Postprandial Glucose Tolerance in Normoglycemic and Prediabetic Mice and in a First Proof of Concept Study in Healthy, Normoglycemic and Prediabetic Humans. Food Sci. Nutr. 2024, 12, 9607–9620. [Google Scholar] [CrossRef]
- Urschel, K.L.; Lawrence, L.M. Amino Acids and Protein. In Equine Applied and Clinical Nutrition; Elsevier: Amsterdam, The Netherlands, 2013; pp. 113–135. ISBN 978-0-7020-3422-0. [Google Scholar]
- Bochröder, B.; Schubert, R.; Bödeker, D. Studies on the Transport in Vitro of Lysine, Histidine, Arginine and Ammonia across the Mucosa of the Equine Colon. Equine Vet. J. 1994, 26, 131–133. [Google Scholar] [CrossRef]
- Bockisch, F.; Taubert, J.; Coenen, M.; Vervuert, I. Protein Evaluation of Feedstuffs for Horses. Animals 2023, 13, 2624. [Google Scholar] [CrossRef]
- Hulmes, D.J.S. Collagen Diversity, Synthesis and Assembly. In Collagen; Fratzl, P., Ed.; Springer: Boston, MA, USA, 2008; pp. 15–47. ISBN 978-0-387-73905-2. [Google Scholar]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and Bioactive Properties of Collagen and Gelatin from Alternative Sources: A Review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Schroeder, W.A.; Kay, L.M.; LeGette, J.; Honnen, L.; Green, F.C. The Constitution of Gelatin. Separation and Estimation of Peptides in Partial Hydrolysates. J. Am. Chem. Soc. 1954, 76, 3556–3564. [Google Scholar] [CrossRef]
- Ao, J.; Li, B. Amino Acid Composition and Antioxidant Activities of Hydrolysates and Peptide Fractions from Porcine Collagen. Food Sci. Technol. Int. 2012, 18, 425–434. [Google Scholar] [CrossRef] [PubMed]
| Day | Activity | Details |
|---|---|---|
| 1–6 | Dose regime (CN, CL, CH) 1 | Normal feeding schedule 3 IV catheters placed evening day 6 |
| 7 | Blood sampling day 2 | Blood sample T = 0 Feeding HC dose regime only (CN, CL, CH) 1 Blood samples T = 1–8 h after HC supplement |
| 8–13 | Dose regime 2 (CN, CL, CH) 1 | Normal feeding schedule 3 IV catheters placed evening day 13 |
| 14 | Blood sampling day 2 | Blood sample T = 0 Feeding HC dose regime only (CN, CL, CH) 1 Blood samples T = 1–8 h after HC supplement |
| 15–20 | Dose regime 3 (CN, CL, CH) 1 | Normal feeding schedule 3 IV catheters placed evening day 20 |
| 21 | Blood sampling day 2 | Blood sample T = 0 Feeding HC dose regime only (CN, CL, CH) 1 Blood samples T = 1–8 h after HC supplement |
| AA | Pellet I 1 g/kg | Pellet II 1 g/kg | Hay 1 g/kg | HC 2 g/16 gN |
|---|---|---|---|---|
| Ala | 5.3 | 11.9 | 3.2 | 8.9 |
| Arg | 8.3 | 13.0 | 2.6 | 7.6 |
| Asp | 8.7 | 12.1 | 4.4 | 5.8 |
| Cys | 2.1 | 1.9 | 0.6 | 0.1 |
| Glu | 23.0 | 28.9 | 5.3 | 10.5 |
| Gly | 5.7 | 21.6 | 2.8 | 19.9 |
| His | 2.6 | 2.9 | 0.9 | 0,8 |
| Ile | 4.0 | 4.6 | 2.2 | 1.3 |
| Leu | 7.5 | 9.2 | 4.0 | 3.4 |
| Lys | 4.6 | 6.8 | 2.6 | 3.5 |
| Met | 1.9 | 2.3 | 1.5 | 8.0 |
| Phe | 4.8 | 5.9 | 2.4 | 2.3 |
| Pro | 7.1 | 15.8 | 2.8 | 10.9 |
| Ser | 5.0 | 6.8 | 2.1 | 3.2 |
| Thr | 3.9 | 5.0 | 2.4 | 2.0 |
| Tyr | 3.4 | 3.7 | 1.6 | 0.9 |
| Val | 5.8 | 7.3 | 2.9 | 2.7 |
| Hyp | <0.5 | 9.8 | <0.5 | 11.2 |
| Trp | 0.1 |
| AA | CN Dose g/day | CL Dose g/day | CH Dose g/day |
|---|---|---|---|
| Ala | 5.3 | 9.2 | 13.1 |
| Arg | 8.3 | 11.3 | 14.3 |
| Asp | 8.7 | 11.0 | 13.3 |
| Cys | 2.1 | 2.1 | 2.1 |
| Glu | 23 | 27.4 | 31.8 |
| Gly | 5.7 | 14.7 | 23.8 |
| His | 2.6 | 2.9 | 3.2 |
| Ile | 4.0 | 4.5 | 5.1 |
| Leu | 7.5 | 8.8 | 10.1 |
| Lys | 4.6 | 6.0 | 7.5 |
| Met | 1.9 | 2.2 | 2.5 |
| Phe | 4.8 | 5.7 | 6.5 |
| Pro | 7.1 | 12.2 | 17.4 |
| Ser | 5.0 | 6.2 | 7.5 |
| Thr | 3.9 | 4.7 | 5.5 |
| Tyr | 3.4 | 3.7 | 4.1 |
| Val | 5.8 | 6.9 | 8.0 |
| Hyp | <0.5 | 5.4 | 10.8 |
| AA | Dose CH 1 | Dose CL 1 |
|---|---|---|
| Ala | 99.27 (76.00–122.54) | - |
| Arg | 26.86 (16.32–37.40) | 12.08 (1.54–22.62) |
| Gln | 76.24 (39.86–112.62) | - |
| Gly | 359.10 (288.86–429.33) | 118.97 (48.74–189.21) |
| Pro | 0.35 (0.27–0.43) | 0.12 (0.04–0.21) |
| Ser | 93.49 (51.19–135.79) | - |
| Hyp 3 | 59.66 (49.73–69.59) | 36.68 (26.75–46.61) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kranenburg, L.C.; Reinke, K.S.; van den Broek, J.; Zaal, E.A.; van den Boom, R.; van Doorn, D.A. Free Plasma Amino Acid Concentrations in Horses Fed Different Dosing Regimens of Hydrolysed Collagen. Animals 2025, 15, 3195. https://doi.org/10.3390/ani15213195
Kranenburg LC, Reinke KS, van den Broek J, Zaal EA, van den Boom R, van Doorn DA. Free Plasma Amino Acid Concentrations in Horses Fed Different Dosing Regimens of Hydrolysed Collagen. Animals. 2025; 15(21):3195. https://doi.org/10.3390/ani15213195
Chicago/Turabian StyleKranenburg, Lieuwke C., Katharina S. Reinke, Jan van den Broek, Esther A. Zaal, Robin van den Boom, and David A. van Doorn. 2025. "Free Plasma Amino Acid Concentrations in Horses Fed Different Dosing Regimens of Hydrolysed Collagen" Animals 15, no. 21: 3195. https://doi.org/10.3390/ani15213195
APA StyleKranenburg, L. C., Reinke, K. S., van den Broek, J., Zaal, E. A., van den Boom, R., & van Doorn, D. A. (2025). Free Plasma Amino Acid Concentrations in Horses Fed Different Dosing Regimens of Hydrolysed Collagen. Animals, 15(21), 3195. https://doi.org/10.3390/ani15213195






