Melatonin Rescues Triclosan-Disrupted Porcine Oocyte Meiosis via Suppression of p53-Mediated Apoptosis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Antibodies and Chemicals
2.3. In Vitro Culture of Porcine Oocytes
2.4. Assessment of Maturation in Oocytes
2.5. Analysis of Early Apoptosis
2.6. Bioinformatics and Proteomic Analysis
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
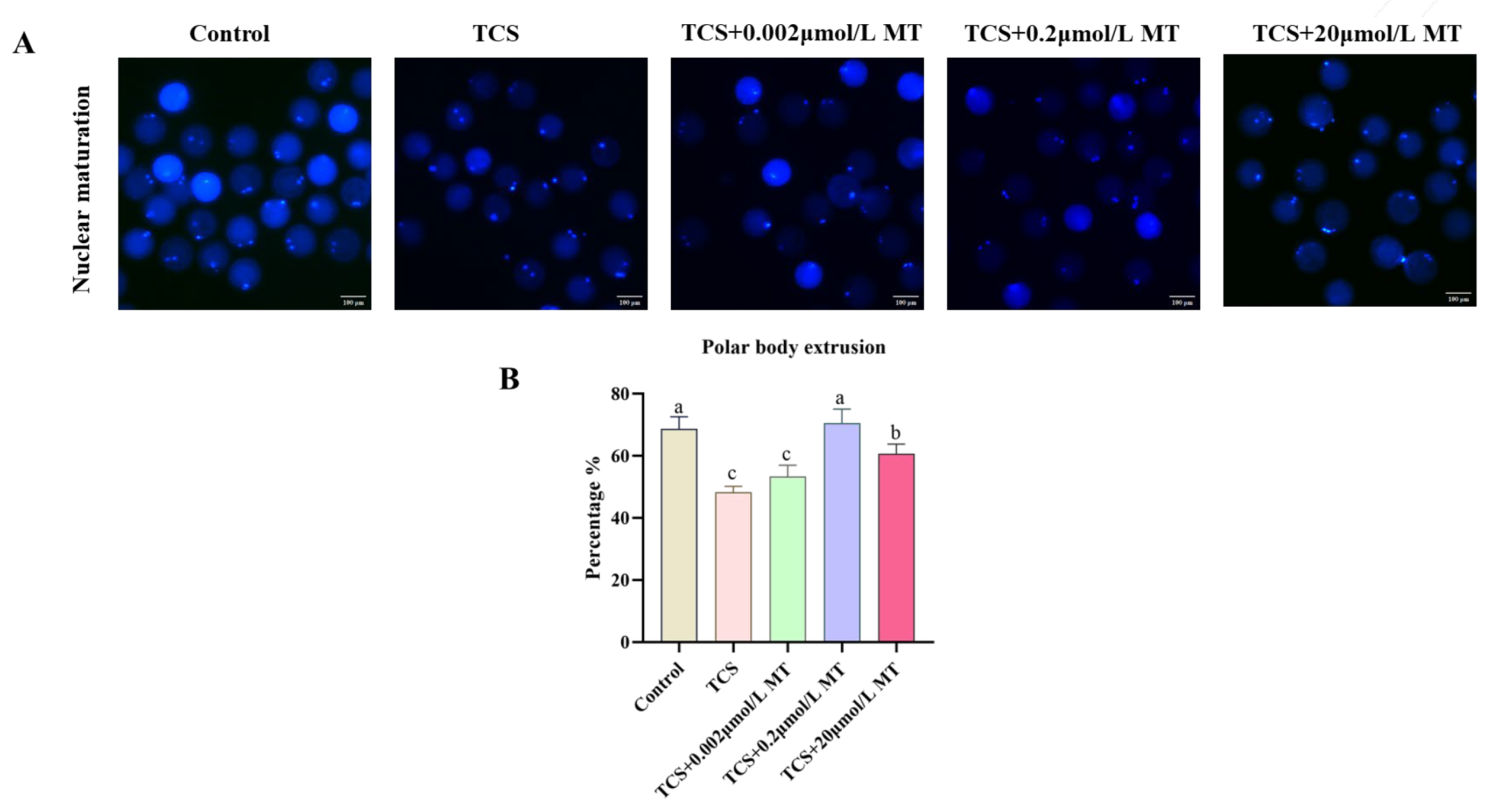
3.1. Melatonin Rescues TCS-Impaired Nuclear Maturation in Porcine Oocytes
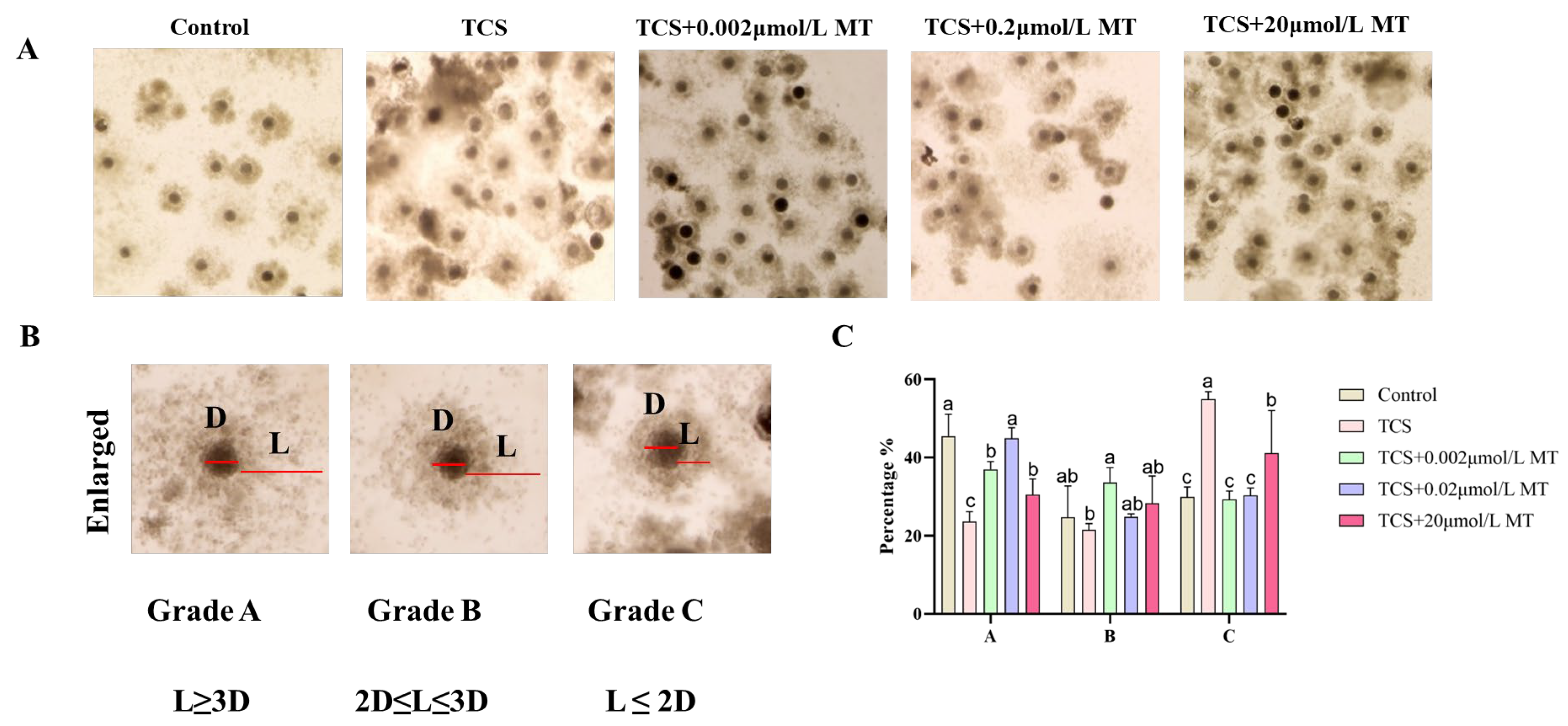
3.2. Melatonin Restores TCS-Suppressed Cumulus Expansion in Porcine Oocytes
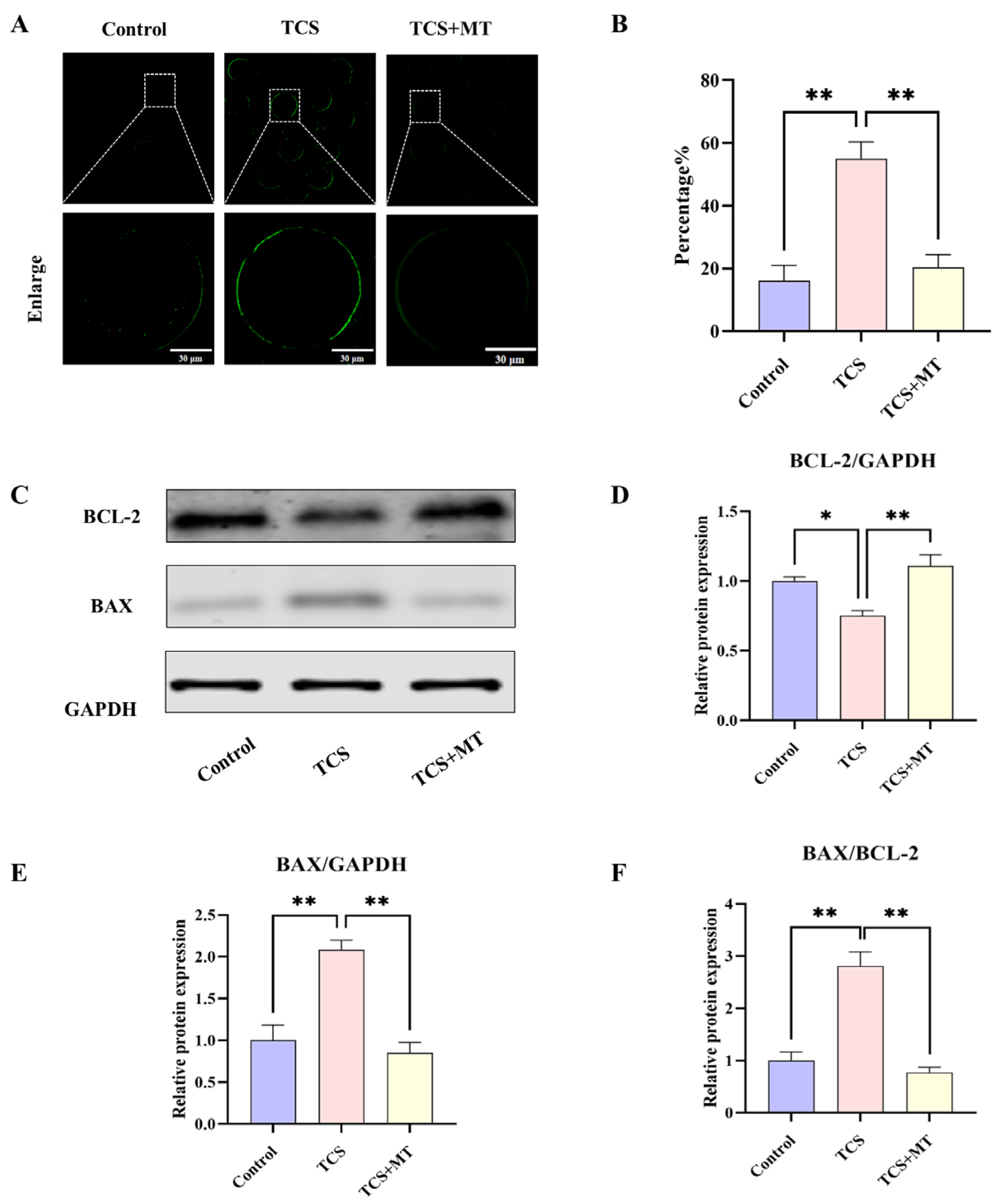
3.3. Melatonin Suppresses Apoptosis in TCS-Exposed Porcine Oocytes
3.4. Proteomic Analysis Implicates p53 Signaling in MT’s Protection
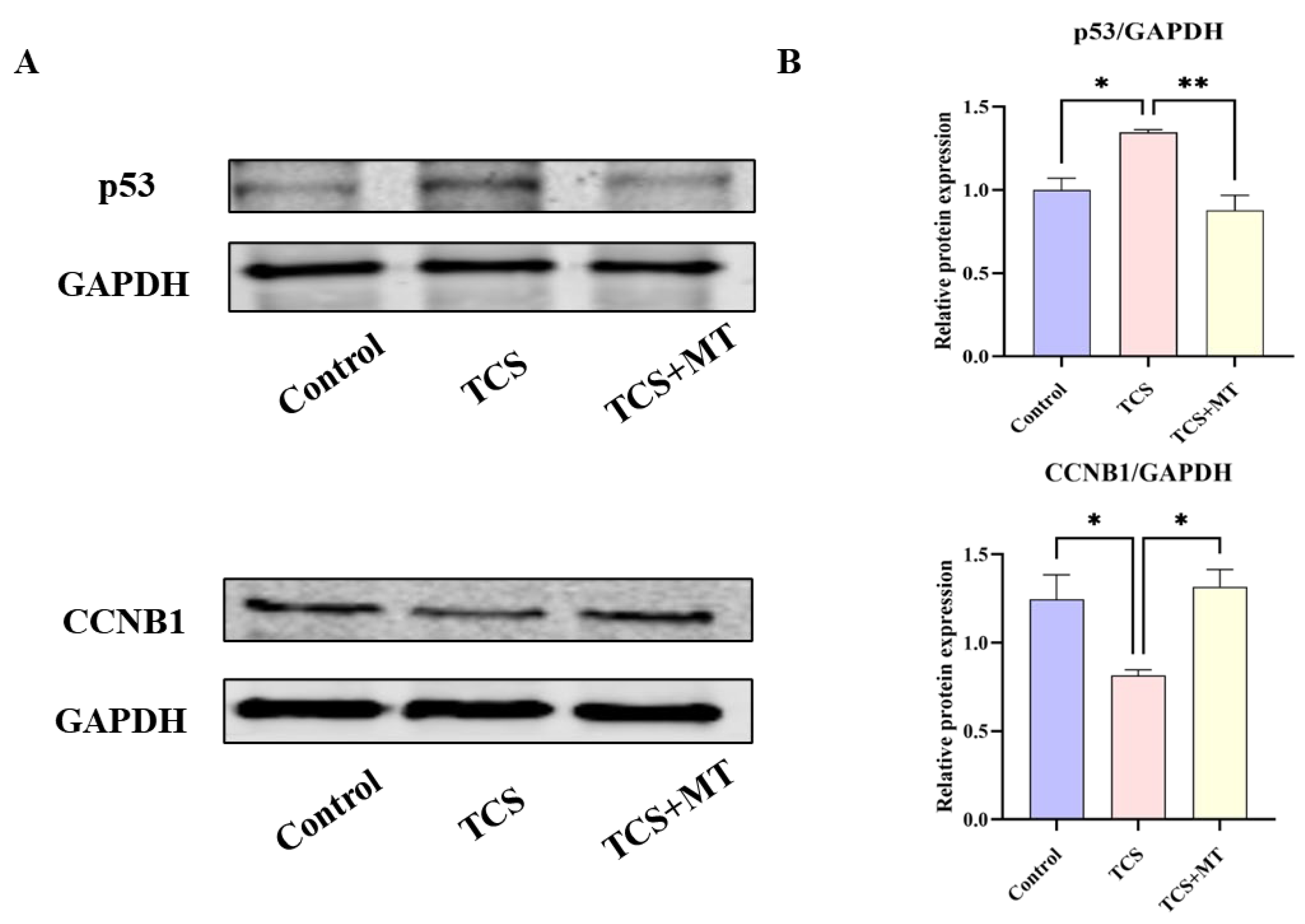
3.5. Melatonin Mitigates TCS Toxicity by Normalizing p53 Pathway Activation
3.6. p53 Inhibition Attenuates TCS-Induced Apoptosis but Abrogates Melatonin’s Protective Effect on Apoptotic Signaling
3.7. Pharmacological Inhibition of p53 Partially Rescues TCS-Impaired Nuclear Maturation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dutta, S.; Sengupta, P.; Bagchi, S.; Chhikara, B.S.; Pavlík, A.; Sláma, P.; Roychoudhury, S. Reproductive toxicity of combined effects of endocrine disruptors on human reproduction. Front. Cell Dev. Biol. 2023, 11, 1162015. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, C.; Yuan, X.-Q.; Cui, F.-P.; Miao, Y.; Yao, W.; Qin, D.-Y.; Deng, Y.-L.; Chen, P.-P.; Zeng, J.-Y.; et al. Individual and joint associations of urinary phthalate metabolites with polycystic ovary and polycystic ovary syndrome: Results from the TREE cohort. Environ. Toxicol. Pharmacol. 2023, 102, 104233. [Google Scholar] [CrossRef] [PubMed]
- Ozga, M.; Jurewicz, J. Environmental exposure to selected non-persistent endocrine disrupting chemicals and polycystic ovary syndrome: A systematic review. Int. J. Occup. Med. Environ. Health 2025, 38, 98–121. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; He, Q.; Gao, Q.; Gu, L.; Miao, Y. Smart RNA Sequencing Reveals the Toxicological Effects of Diisobutyl Phthalate (DiBP) in Porcine Oocytes. Environ. Sci. Technol. 2024, 58, 15017–15026. [Google Scholar] [CrossRef] [PubMed]
- Joan, K.L.; Angelica Van, G.; Kristen, W.; Taylor, H.; Jasmine, F.; Chaohui, D. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef]
- Ge, W.; Niu, Y.-L.; Li, Y.-K.; Li, L.; Wang, H.; Li, W.-W.; Qiao, T.; Feng, Y.-N.; Feng, Y.-Q.; Liu, J.; et al. Spatiotemporal dynamics of early oogenesis in pigs. Genome Biol. 2025, 26, 2. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, L.; Jin, B.; Liu, Y.; Liang, X. Critical review on the environmental behaviors and toxicity of triclosan and its removal technologies. Sci. Total Environ. 2024, 932, 173013. [Google Scholar] [CrossRef]
- Ramires, P.F.; Tavella, R.A.; Escarrone, A.L.; Volcão, L.M.; Honscha, L.C.; Brum, R.d.L.; da Silva, A.B.; Júnior, F.M.R.d.S. Ecotoxicity of triclosan in soil: An approach using different species. Environ. Sci. Pollut. Res. Int. 2021, 28, 41233–41241. [Google Scholar] [CrossRef]
- Pollock, T.; Arbuckle, T.E.; Guth, M.; Bouchard, M.F.; St-Amand, A. Associations among urinary triclosan and bisphenol A concentrations and serum sex steroid hormone measures in the Canadian and US Populations. Environ. Int. 2021, 146, 106229. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, D.; Tan, K.; Wu, M.; Tai, Q.; Zhu, G.; Chen, J.; Zhou, C.; Zhu, Y.; Zhang, Y.; et al. Spermidine supplementation protects porcine oocytes against triclosan-induced defects during maturation in vitro. Anim. Reprod. Sci. 2025, 282, 107999. [Google Scholar] [CrossRef]
- Park, H.J.; Song, B.S.; Kim, J.W.; Yang, S.G.; Kim, S.U.; Koo, D.B. Exposure of Triclosan in Porcine Oocyte Leads to Superoxide Production and Mitochondrial-Mediated Apoptosis During In Vitro Maturation. Int. J. Mol. Sci. 2020, 21, 3050. [Google Scholar] [CrossRef]
- Zhao, N.; Xu, A.; Yang, J.; Zhao, J.; Xie, J.; Li, B.; Duan, J.; Cao, G. Triclosan Caused Oocyte Meiotic Arrest by Modulating Oxidative Stress, Organelle Dysfunctions, Autophagy, and Apoptosis in Pigs. Animals 2025, 15, 802. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.H.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, N.; Hao, H.; Li, C.; Zhao, Y.; Yan, C.; Wang, H.; Du, W.; Wang, D.; Liu, Y.; et al. Melatonin improves the fertilization capacity and developmental ability of bovine oocytes by regulating cytoplasmic maturation events. J. Pineal Res. 2018, 64, e12445. [Google Scholar] [CrossRef]
- Li, W.D.; Yu, S.; Luo, S.M.; Shen, W.; Yin, S.; Sun, Q.Y. Melatonin defends mouse oocyte quality from benzo[ghi]perylene-induced deterioration. J. Cell Physiol. 2019, 234, 6220–6229. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.-Q.; Liu, R.-P.; Li, Y.-H.; Yao, X.-R.; Kim, N.-H.; Xu, Y.-N. Melatonin Supplementation during In Vitro Maturation of Porcine Oocytes Alleviates Oxidative Stress and Endoplasmic Reticulum Stress Induced by Imidacloprid Exposure. Animals 2023, 13, 2596. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, C.H.; Zhang, H.L.; Pan, Z.N.; Sun, S.C. Exposure to nivalenol declines mouse oocyte quality via inducing oxidative stress-related apoptosis and DNA damage. Biol. Reprod. 2021, 105, 1474–1483. [Google Scholar] [CrossRef]
- Ishtiaq, A.; Ali, T.; Bakhtiar, A.; Bibi, R.; Bibi, K.; Mushtaq, I.; Li, S.; Khan, W.; Khan, U.; Anis, R.A.; et al. Melatonin abated Bisphenol A-induced neurotoxicity via p53/PUMA/Drp-1 signaling. Environ. Sci. Pollut. Res. Int. 2021, 28, 17789–17801. [Google Scholar] [CrossRef]
- Carlsen, L.; Zhang, S.; Tian, X.; De La Cruz, A.; George, A.; Arnoff, T.E.; El-Deiry, W.S. The role of p53 in anti-tumor immunity and response to immunotherapy. Front. Mol. Biosci. 2023, 10, 1148389. [Google Scholar] [CrossRef]
- Zhang, J.; Li, F.; Zhang, X.; Xie, T.; Qin, H.; Lv, J.; Gao, Y.; Li, M.; Gao, Y.; Jia, Y. Melatonin Improves Turbot Oocyte Meiotic Maturation and Antioxidant Capacity, Inhibits Apoptosis-Related Genes mRNAs In Vitro. Antioxidants 2023, 12, 1389. [Google Scholar] [CrossRef]
- Wang, C.; Chen, C.; Wang, M.; Rahman, S.U.; Wei, B.; Ding, H.; Huang, W.; Wang, X. Rutin attenuates zearalenone-induced ferroptosis of endometrial stromal cells in piglets through the p53 signaling pathway. Ecotoxicol. Environ. Saf. 2025, 290, 117546. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Pei, X.; Qin, X.; Liu, X.; Li, C.; Li, L.; Dai, C.; Xiao, X.; Tang, S. Olaquindox-Induced Liver Damage Involved the Crosstalk of Oxidative Stress and p53 In Vivo and In Vitro. Oxid. Med. Cell Longev. 2020, 2020, 8835207. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, W.; Wang, Y.; Wu, R.; Dai, Y.; Deng, Y.; Wang, S.; Yuan, J.; Tan, R. p53 contributes to cardiovascular diseases via mitochondria dysfunction: A new paradigm. Free Radic. Biol. Med. 2023, 208, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Chen, H.; Xu, D.; Li, Y.; Li, X.; Cheng, J.; Hua, R.; Zhang, Z.; Yang, L.; Li, Q. 17β-estradiol improves the developmental ability, inhibits reactive oxygen species levels and apoptosis of porcine oocytes by regulating autophagy events. J. Steroid Biochem. Mol. Biol. 2021, 209, 105826. [Google Scholar] [CrossRef]
- Xiang, D.-C.; Jia, B.-Y.; Fu, X.-W.; Guo, J.-X.; Hong, Q.-H.; Quan, G.-B.; Wu, G.-Q. Role of astaxanthin as an efficient antioxidant on the in vitro maturation and vitrification of porcine oocytes. Theriogenology 2021, 167, 13–23. [Google Scholar] [CrossRef]
- Duan, J.; Chen, H.; Li, Y.; Xu, D.; Li, X.; Zhang, Z.; Cheng, J.; Yang, L.; Li, Q. 17β-Estradiol Enhances Porcine Meiosis Resumption from Autophagy-Induced Gap Junction Intercellular Communications and Connexin 43 Phosphorylation via the MEK/ERK Signaling Pathway. J. Agric. Food Chem. 2021, 69, 11847–11855. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, M.; Li, X.; Ju, J.; Chen, L.; Sun, Y.; Sun, S. Modified hydrated sodium calcium aluminosilicate-supplemented diet protects porcine oocyte quality from zearalenone toxicity. Environ. Mol. Mutagen. 2021, 62, 124–132. [Google Scholar] [CrossRef]
- Xing, C.; Chen, S.; Wang, Y.; Pan, Z.; Zou, Y.; Sun, S.; Ren, Z.; Zhang, Y. Glyphosate exposure deteriorates oocyte meiotic maturation via induction of organelle dysfunctions in pigs. J. Anim. Sci. Biotechnol. 2022, 13, 80–93. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bandla, C.; Kundu, D.J.; Kamatchinathan, S.; Bai, J.; Hewapathirana, S.; John, N.S.; Prakash, A.; Walzer, M.; Wang, S.; et al. The PRIDE database at 20 years: 2025 update. Nucleic Acids Res. 2025, 53, D543–D553. [Google Scholar] [CrossRef]
- Balbi, T.; Miglioli, A.; Montagna, M.; Piazza, D.; Risso, B.; Dumollard, R.; Canesi, L. The biocide triclosan as a potential developmental disruptor in Mytilus early larvae. Environ. Sci. Pollut. Res. 2023, 30, 106342–106354. [Google Scholar] [CrossRef]
- Forte, M.; Mita, L.; Cobellis, L.; Merafina, V.; Specchio, R.; Rossi, S.; Mita, D.G.; Mosca, L.; Castaldi, M.A.; De Falco, M.; et al. Triclosan and bisphenol a affect decidualization of human endometrial stromal cells. Mol. Cell Endocrinol. 2016, 422, 74–83. [Google Scholar] [CrossRef]
- Tammy, E.S.; Emily, K.G.; Leah, M.Z. Triclosan Exposure Modulates Estrogen-Dependent Responses in the Female Wistar Rat. Toxicol. Sci. 2010, 117, 45–53. [Google Scholar] [CrossRef]
- He, Y.; Yu, T.; Li, H.; Sun, Q.; Chen, M.; Lin, Y.; Dai, J.; Wang, W.; Li, Q.; Ju, S. Polystyrene nanoplastic exposure actives ferroptosis by oxidative stress-induced lipid peroxidation in porcine oocytes during maturation. J. Anim. Sci. Biotechnol. 2024, 15, 117. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, B.; Miao, Y.; Gao, Q. Silibinin supplementation ameliorates the toxic effects of butyl benzyl phthalate on porcine oocytes by eliminating oxidative stress and autophagy. Environ. Pollut. 2023, 329, 121734. [Google Scholar] [CrossRef]
- Joel Cox Menka, B.; Bernard Tawiah, O.; Paul Ayiku, A.; Isaac Williams, O.; Herman, L.; William Otoo, E. Ochratoxin a in Food Commodities: A Review of Occurrence, Toxicity, and Management Strategies. Heliyon 2024, 10, e39313. [Google Scholar] [CrossRef]
- Mesbah, F.; Kafi, M.; Nili, H. Cumulus cell expansion and first polar body extrusion during in vitro oocyte maturation in relation to morphological and morphometric characteristics of the dromedary camel ovary. Reprod. Domest. Anim. 2016, 51, 916–923. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, F.; Zhao, T.; Du, J.; Li, N.; Qiao, X.; Yao, Y.; Wu, D.; Peng, F.; Wang, D.; et al. Melatonin improves mouse oocyte quality from 2-ethylhexyl diphenyl phosphate-induced toxicity by enhancing mitochondrial function. Ecotoxicol. Environ. Saf. 2024, 280, 116559. [Google Scholar] [CrossRef] [PubMed]
- Kosińska, K.; Szychowski, K.A. Current state of knowledge of triclosan (TCS)-dependent reactive oxygen species (ROS) production. Environ. Res. 2024, 250, 118532–118545. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, K.A.; Skóra, B.; Bar, M.; Piechowiak, T. Triclosan (TCS) affects the level of DNA methylation in the human oral squamous cell carcinoma (SCC-15) cell line in a nontoxic concentration. Biomed. Pharmacother. 2022, 149, 112815. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Hua, R.; Zhao, B.; Qiu, D.; Zhang, C.; Huang, S.; Pan, Y. Melatonin protects TEGDMA-induced preodontoblast mitochondrial apoptosis via the JNK/MAPK signaling pathway. Acta Biochim. Biophys. Sin. 2024, 56, 393–404. [Google Scholar] [CrossRef]
- Pourbarkhordar, V.; Rahmani, S.; Roohbakhsh, A.; Hayes, A.W.; Karimi, G. Melatonin effect on breast and ovarian cancers by targeting the PI3K/Akt/mTOR pathway. IUBMB Life 2024, 76, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Guo, J.; Wang, S.; Min, C.; Wang, J.; Liu, H.; Fang, Y.; Ding, H.; Zhao, J.; Ma, X.; et al. Melatonin protects bovine oocyte from βHB-induced oxidative stress through the Nrf2 pathway. Theriogenology 2025, 234, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wang, S.; Wang, H.; Sun, M.; Wu, S.; Bao, W. Melatonin Ameliorates the Toxicity Induced by Deoxynivalenol in Murine Ovary Granulosa Cells by Antioxidative and Anti-Inflammatory Effects. Antioxidants 2021, 10, 1045. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, J.; Luo, X.; Li, F.; Cong, L.; Wang, Y.; Sun, Y. Melatonin attenuates cadmium-induced ovulatory dysfunction by suppressing endoplasmic reticulum stress and cell apoptosis. Reprod. Biol. Endocrinol. 2019, 17, 61. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, C.; Yu, D.; She, D.; Yu, Y.; Cai, Y.; Chen, N. Melatonin protects oogenesis from hypobaric hypoxia-induced fertility damage in mice. Zygote 2024, 32, 161–169. [Google Scholar] [CrossRef]
- Tornesello, M.L. TP53 mutations in cancer: Molecular features and therapeutic opportunities (Review). Int. J. Mol. Med. 2025, 55, 7. [Google Scholar] [CrossRef]
- Wu, H.H.; Leng, S.; Eisenstat, D.D.; Sergi, C.; Leng, R. Targeting p53 for immune modulation: Exploring its functions in tumor immunity and inflammation. Cancer Lett. 2025, 617, 217614. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, Z.; Fu, Y.; Hou, Y.; Sun, J.; Hu, F.; Yu, S.; Gong, K.; Liu, Y.; Zhao, G. An overview of the functions of p53 and drugs acting either on wild- or mutant-type p53. Eur. J. Med. Chem. 2024, 265, 116121. [Google Scholar] [CrossRef]
- Toro, A.; Anselmino, N.; Solari, C.; Francia, M.; Oses, C.; Sanchis, P.; Bizzotto, J.; Echegaray, C.V.; Petrone, M.V.; Levi, V.; et al. Novel Interplay between p53 and HO-1 in Embryonic Stem Cells. Cells 2020, 10, 35. [Google Scholar] [CrossRef]
- Yue, W.; Zhang, H.Y.; Schatten, H.; Meng, T.G.; Sun, Q.Y. CtIP regulates G2/M transition and bipolar spindle assembly during mouse oocyte meiosis. J. Genet. Genom. 2024, 51, 1435–1446. [Google Scholar] [CrossRef]
- Chen, L.; Ouyang, Y.; Gu, L.; Guo, J.; Han, Z.; Wang, Z.; Hou, Y.; Schatten, H.; Sun, Q. Septin 9 controls CCNB1 stabilization via APC/C(CDC20) during meiotic metaphase I/anaphase I transition in mouse oocytes. Cell Prolif. 2023, 56, e13359. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; van Soest, D.M.K.; Polderman, P.E.; Burgering, B.M.T.; Dansen, T.B. DNA damage and oxidant stress activate p53 through differential upstream signaling pathways. Free Radic. Biol. Med. 2021, 172, 298–311. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, J.; Zhao, N.; Wang, S.; Li, X.; Li, B.; Cao, G. Melatonin Rescues Triclosan-Disrupted Porcine Oocyte Meiosis via Suppression of p53-Mediated Apoptosis. Animals 2025, 15, 3193. https://doi.org/10.3390/ani15213193
Duan J, Zhao N, Wang S, Li X, Li B, Cao G. Melatonin Rescues Triclosan-Disrupted Porcine Oocyte Meiosis via Suppression of p53-Mediated Apoptosis. Animals. 2025; 15(21):3193. https://doi.org/10.3390/ani15213193
Chicago/Turabian StyleDuan, Jiaxin, Ning Zhao, Shibin Wang, Xinyu Li, Bugao Li, and Guoqing Cao. 2025. "Melatonin Rescues Triclosan-Disrupted Porcine Oocyte Meiosis via Suppression of p53-Mediated Apoptosis" Animals 15, no. 21: 3193. https://doi.org/10.3390/ani15213193
APA StyleDuan, J., Zhao, N., Wang, S., Li, X., Li, B., & Cao, G. (2025). Melatonin Rescues Triclosan-Disrupted Porcine Oocyte Meiosis via Suppression of p53-Mediated Apoptosis. Animals, 15(21), 3193. https://doi.org/10.3390/ani15213193




