Lipidomics Techniques Revealed the Adipogenic Differentiation Mechanism of Bovine Adipose-Derived Neural Crest Stem Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Sample Collection
2.2. Lipid Extraction
2.3. Chromatographic Conditions
2.4. Mass Spectrometry Conditions
2.5. Data Processing and Statistical Analysis
3. Results
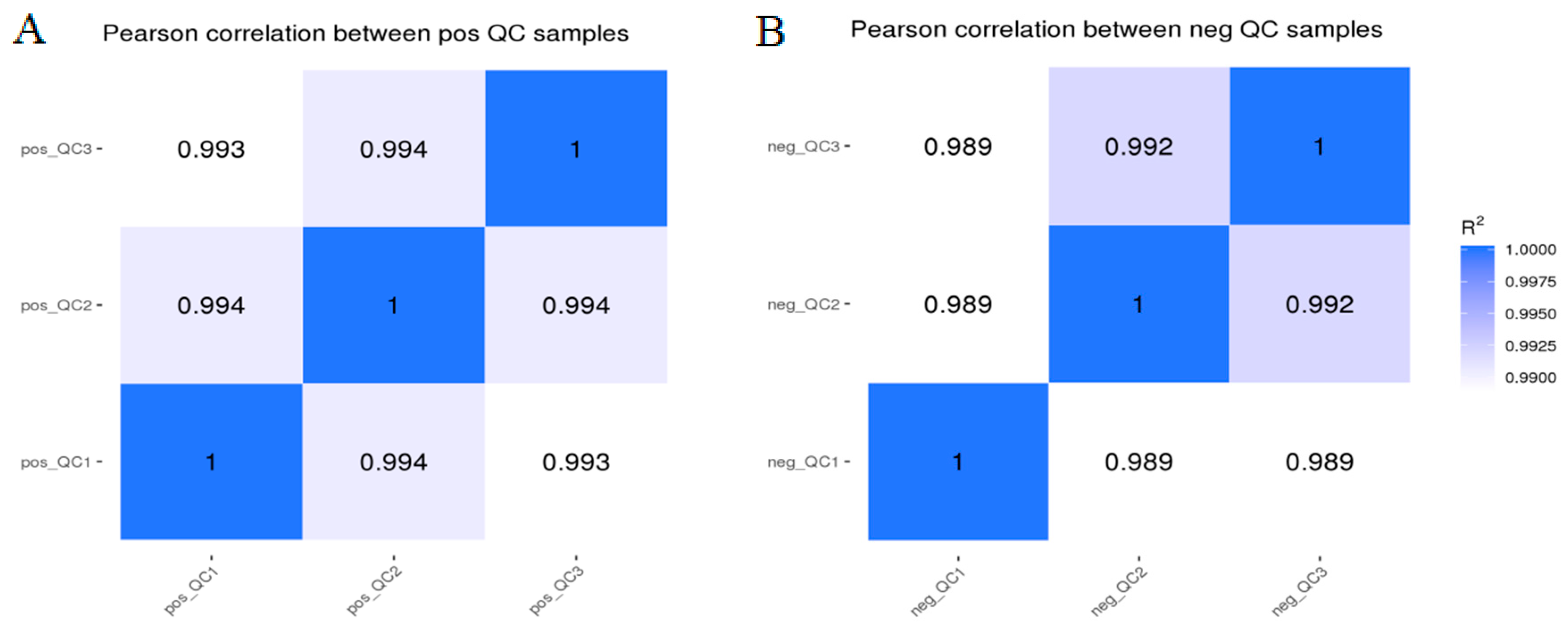
3.1. Sample Quality Control and Method Reliability Evaluation
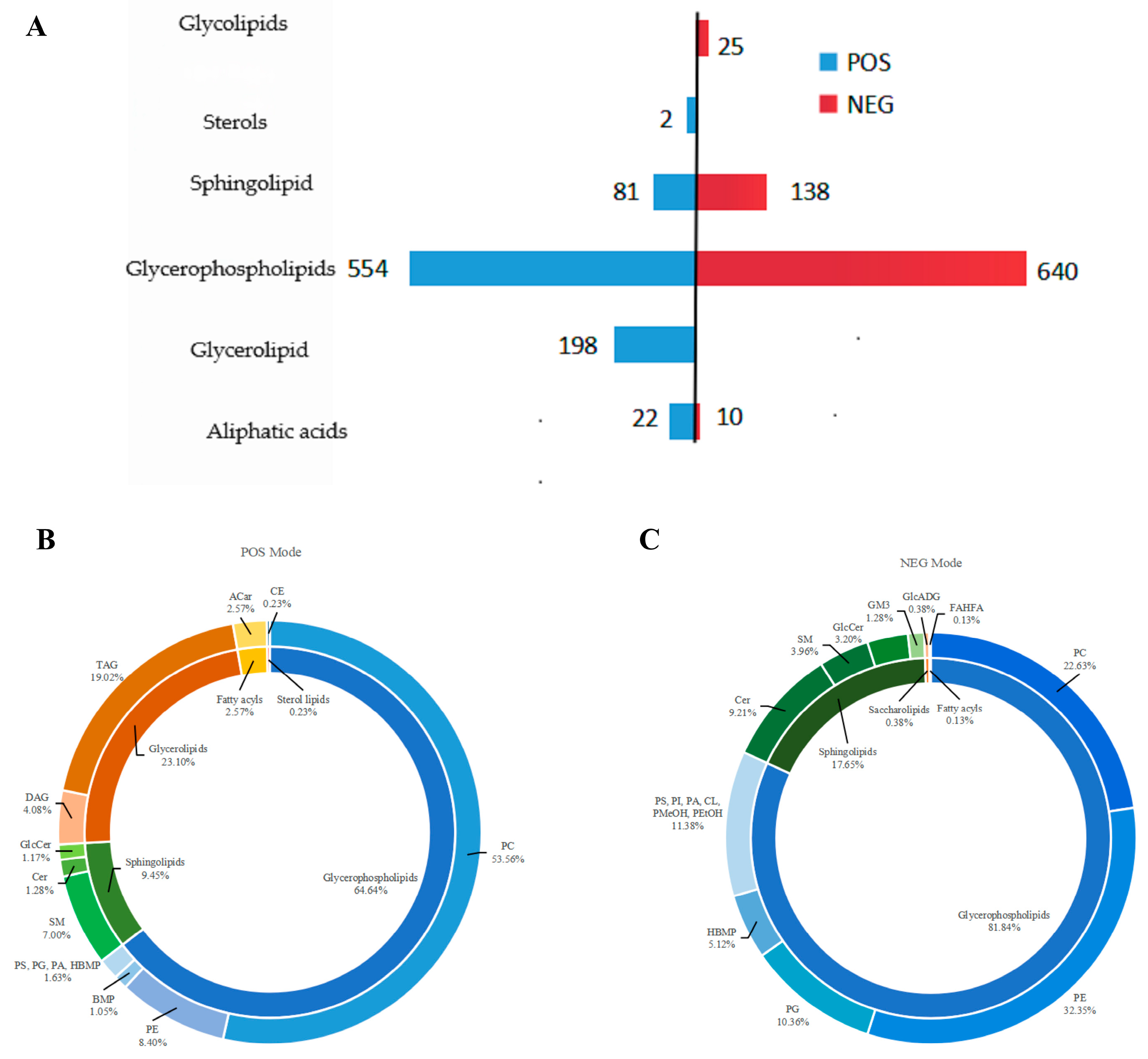
3.2. Intracellular Lipid Identification
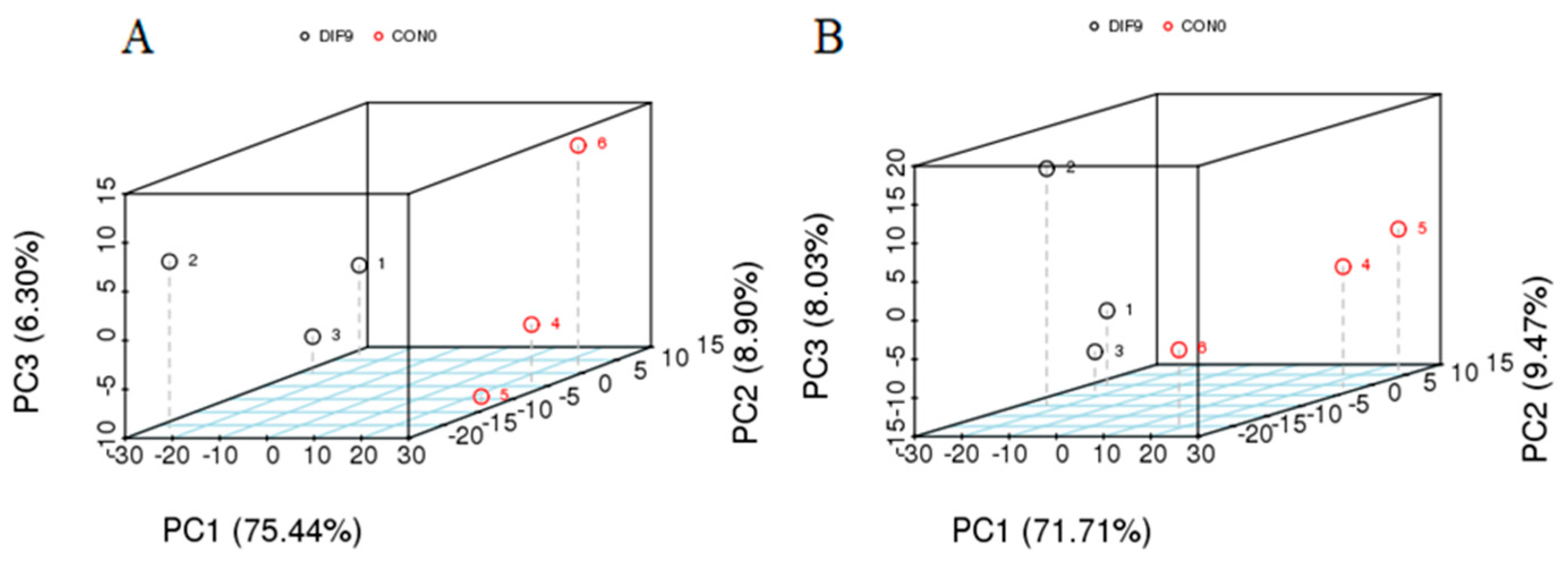
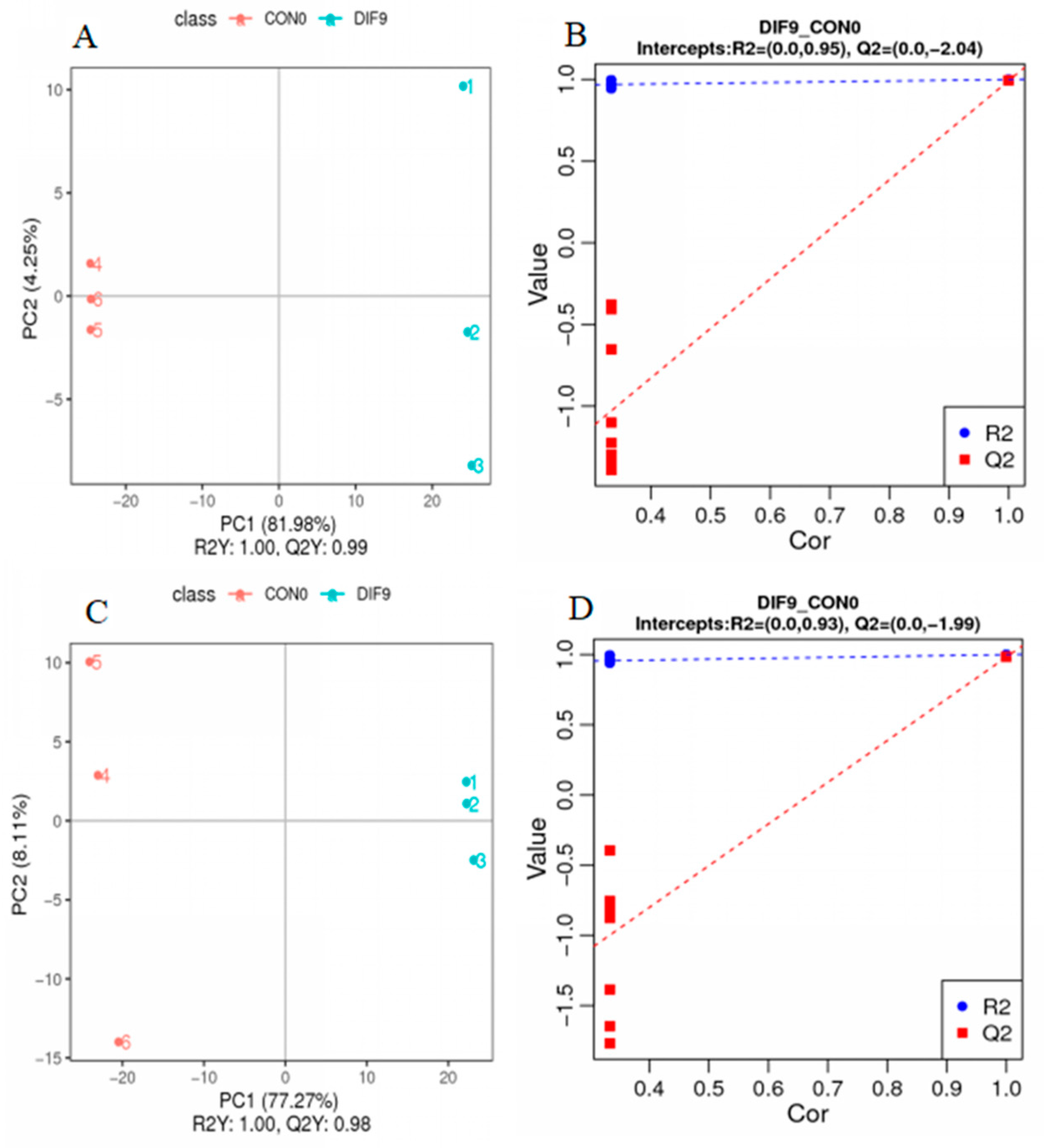
3.3. Principal Component Analysis
3.4. Partial Least Square Discriminant Analysis
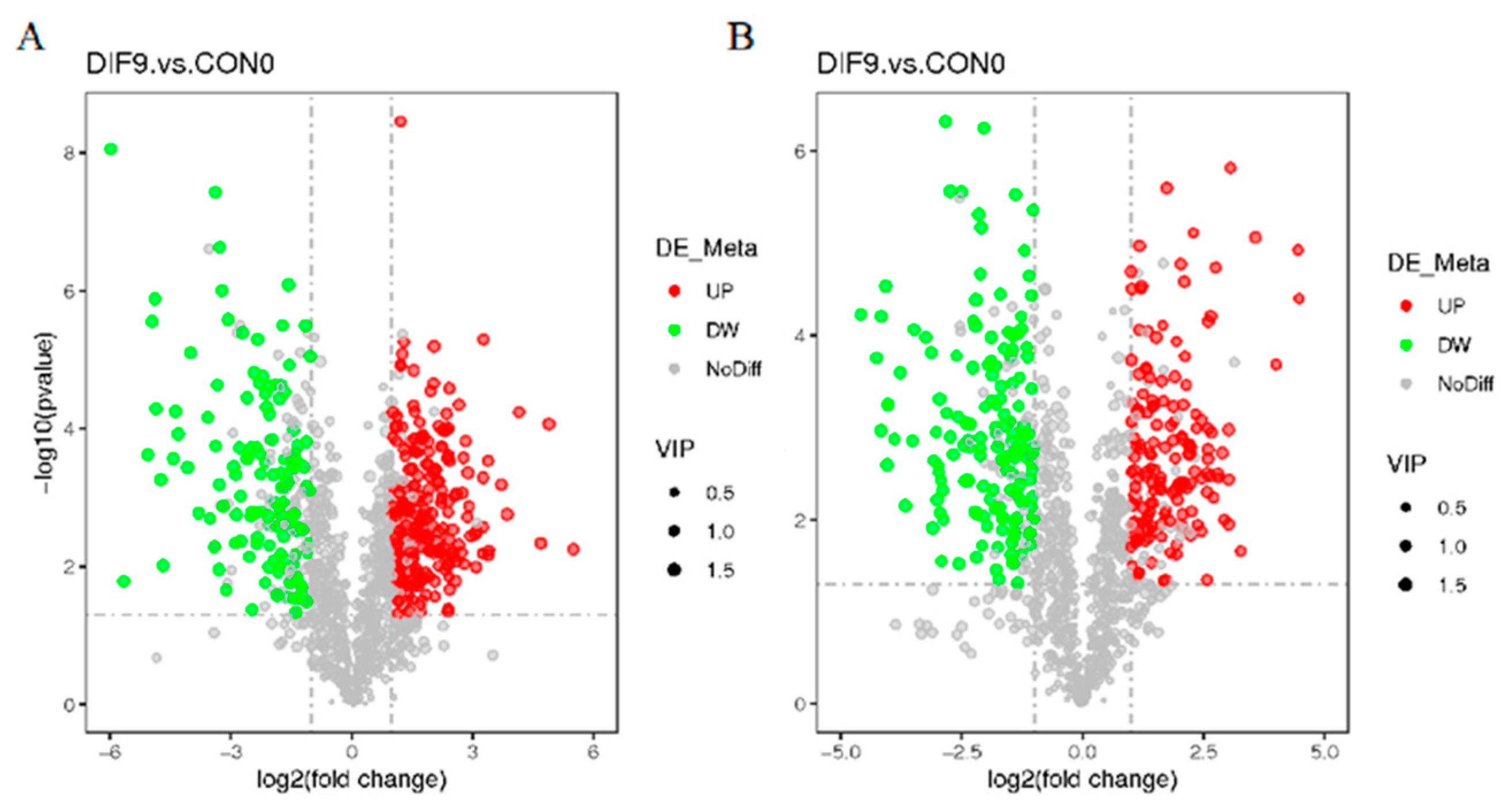
3.5. Volcanic Map of Differential Lipid Compounds
3.6. Analysis of Differential Lipid Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACar | acyl carnitines |
| AKT | serine/threonine kinase |
| baNCSC | bovine adipose-derived neural crest stem cells |
| BMP | bis (monoacyl) glycerophosphate |
| CE | cholesterol esters |
| C/EBPα | CCAAT/enhancer binding protein α |
| Cer | ceramides |
| CHGs | core hub genes |
| CL | cardiolipin |
| DAG | diacylglycerols |
| DEGs | differentially expressed genes |
| EGFR | epidermal growth factor receptor |
| FAHFA | hydroxyfatty acid lipid |
| FAs | aliphatic acids |
| FBS | fetal bovine serum |
| FC | fold change |
| GL | glycerides |
| GlcADG | glucuronic acid diacylglycerol |
| GM3 | gangliosides |
| GP | glycerophospholipids |
| HBMP | hemibi (monoacyl) glycerophosphate |
| HexCer | hexose ceramides |
| IDA | information-dependent acquisition |
| MAPK | mitogen-activated protein kinase |
| PA | phosphatidylic acid |
| PC | phosphatidylcholine |
| PCA | principal component analysis |
| PE | phosphatidylethanolamine |
| PEtOH | phosphatidylethanol |
| PG | phosphatidylglycerol |
| PI | phosphatidylinositol |
| PLS-DA | partial least square discriminant analysis |
| PMeOH | phosphatidylmethanol |
| PI3K | phosphoinositide 3-kinase |
| PPARγ | peroxisome proliferator-activated receptor γ |
| PS | phosphatidylserine |
| QC | quality control |
| SM | sphingomyelin |
| SP | sphingolipids |
| ST | sterols |
| TAG | triacylglycerols |
| UHPLC | ultra-high-performance liquid chromatography |
| VIP | variable importance in the projection |
References
- Sysoeva, V.Y.; Lazarev, M.A.; Kulebyakin, K.Y.; Semina, E.V.; Rubina, K.A. Molecular and Cellular Mechanisms Governing Adipogenic Differentiation. Russ. J. Dev. Biol. 2023, 54, S10–S22. [Google Scholar] [CrossRef]
- Liu, S.S.; Fang, X.; Wen, X.; Liu, J.S.; Alip, M.; Sun, T.; Wang, Y.Y.; Chen, H.W. How mesenchymal stem cells transform into adipocytes: Overview of the current understanding of adipogenic differentiation. World J. Stem Cells 2024, 16, 245–256. [Google Scholar] [CrossRef]
- Wang, G.; Wu, B.; Zhang, L.; Cui, Y.; Zhang, B.; Wang, H. Laquinimod Prevents Adipogenesis and Obesity by Down-Regulating PPAR-γ and C/EBPα through Activating AMPK. ACS Omega 2020, 5, 22958–22965. [Google Scholar] [CrossRef]
- Cristancho, A.G.; Lazar, M.A. Forming Functional Fat: A Growing Understanding of Adipocyte Differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Cui, X.; Zhang, B.; Song, X.; Liu, Q.; Yang, S. Multipotent stem cells with neural crest stem cells characteristics exist in bovine adipose tissue. Biochem. Biophys. Res. Commun. 2020, 522, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Tang, X.; Zhao, R.; Yan, Y.; Song, X. Transcriptomics Analysis of the Adipogenic Differentiation Mechanism of Bovine Adipose-Derived Neural Crest Stem Cells. Animals 2025, 15, 2353. [Google Scholar] [CrossRef]
- Dairi, G.; Al Mahri, S.; Benabdelkamel, H.; Alfadda, A.A.; Alswaji, A.A.; Rashid, M.; Malik, S.S.; Iqbal, J.; Ali, R.; Al Ibrahim, M.; et al. Transcriptomic and Proteomic Analysis Reveals the Potential Role of RBMS1 in Adipogenesis and Adipocyte Metabolism. Int. J. Mol. Sci. 2023, 24, 11300. [Google Scholar] [CrossRef]
- Gire, D.; Inamdar, S.; Acharya, J.; Sadawarte, S.; Kulkarni, A.; Ghaskadbi, S. Dynamic changes in the gene expression during adipogenesis in hMSCs. Gene Rep. 2023, 34, 101860. [Google Scholar] [CrossRef]
- Pei, Y.; Song, Y.; Wang, B.; Lin, C.; Yang, Y.; Li, H.; Feng, Z. Integrated lipidomics and RNA sequencing analysis reveal novel changes during 3T3-L1 cell adipogenesis. PeerJ 2022, 10, e13417. [Google Scholar] [CrossRef]
- Miehle, F.; Möller, G.; Cecil, A.; Lintelmann, J.; Wabitsch, M.; Tokarz, J.; Adamski, J.; Haid, M. Lipidomic Phenotyping Reveals Extensive Lipid Remodeling during Adipogenesis in Human Adipocytes. Metabolites 2020, 10, 217. [Google Scholar] [CrossRef]
- Han, L.; Pang, K.; Li, X.; Loor, J.; Yang, G.; Gao, T. Lipidomic profiling analysis of the phospholipid molecules in SCAP-induced lipid droplet formation in bovine mammary epithelial cells. Prostaglandins Other Lipid Mediat. 2020, 149, 106420. [Google Scholar] [CrossRef]
- Padilla-Benavides, T.; Velez-delValle, C.; Marsch-Moreno, M.; Castro-Muñozledo, F.; Kuri-Harcuch, W. Lipogenic Enzymes Complexes and Cytoplasmic Lipid Droplet Formation During Adipogenesis. J. Cell Biochem. 2016, 117, 2315–2326. [Google Scholar] [CrossRef]
- Levental, K.R.; Surma, M.A.; Skinkle, A.D.; Lorent, J.H.; Zhou, Y.; Klose, C.; Chang, J.T.; Hancock, J.F.; Levental, I. ω-3 polyunsaturated fatty acids direct differentiation of the membrane phenotype in mesenchymal stem cells to potentiate osteogenesis. Sci. Adv. 2017, 3, eaao1193. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Mottillo, E.P. Adipocyte Lipolysis: From Molecular Mechanisms of Regulation to Disease and Therapeutics. Biochem. J. 2020, 477, 985–1008. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, X.; Wang, N.; Lu, S.; Dong, J.; Qi, Z.; Zhou, J.; Wang, Q. Evaluation of Changes in Egg Yolk Lipids during Storage Based on Lipidomics through UPLC-MS/MS. Food Chem. 2023, 398, 133931. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.M.; Maciel, E.; Moreira, A.S.P.; Sousa, B.; Melo, T.; Domingues, P.; Curado, L.; Antunes, B.; Domingues, M.R.M.; Santos, F. Lipidomics of Mesenchymal Stromal Cells: Understanding the Adaptation of Phospholipid Profile in Response to Pro-Inflammatory Cytokines. J. Cell Physiol. 2015, 231, 1024–1032. [Google Scholar] [CrossRef]
- Rog, T.; Koivuniemi, A. The biophysical properties of ethanolamine plasmalogens revealed by atomistic molecular dynamics simulations. BBA-Biomembranes 2016, 1858, 97–103. [Google Scholar] [CrossRef]
- Eto, M.; Shindou, H.; Koeberle, A.; Harayama, T.; Yanagida, K.; Shimizu, T. Lysophosphatidylcholine Acyltransferase 3 Is the Key Enzyme for Incorporating Arachidonic Acid into Glycerophospholipids during Adipocyte Differentiation. Int. J. Mol. Sci. 2012, 13, 16267–16280. [Google Scholar] [CrossRef]
- Okumuş, E.B.; Böke, Ö.B.; Turhan, S.Ş.; Doğan, A. From Development to Future Prospects: The Adipose Tissue and Adipose Tissue Organoids. Life Sci. 2024, 351, 122758. [Google Scholar] [CrossRef]
- Vance, J.E.; Tasseva, G. Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. BBA-Mol. Cell Biol. Lipids 2013, 1831, 543–554. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Liu, C.; Yan, D.; Dong, P. Compositional Differences Between Preterm Milk of Different Gestational Ages with the Term Milk: A Comparative Lipidomic Study by LC-MS/MS. J. Lipid Sci. Tech. 2022, 124, 2100224. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Chen, W.; Li, J.; Shan, T. CRTC3 Regulates the Lipid Metabolism and Adipogenic Differentiation of Porcine Intramuscular and Subcutaneous Adipocytes by Activating the Calcium Pathway. J. Agric. Food Chem. 2021, 69, 7243–7255. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Dou, B.X.; Wang, Y.; Liu, J.J.; Zhang, Z.; Liu, Y.; Zhang, N. Study on the Effect of Crushed Rice-Lotus Seed Starch Reconstituted Rice on Lipid Metabolism Histology in Rats. J. Food Qual. 2022, 2022, 9105936. [Google Scholar] [CrossRef]
- Wenk, M.R. Lipidomics: New Tools and Applications. Cell 2010, 143, 888–895. [Google Scholar] [CrossRef]
- Fei, W.; Shui, G.; Zhang, Y.; Krahmer, N.; Ferguson, C.; Kapterian, T.S.; Lin, R.C.; Dawes, I.W.; Brown, A.J.; Li, P.; et al. A Role for Phosphatidic Acid in the Formation of “Supersized” Lipid Droplets. PLoS Genet. 2011, 7, e1002201. [Google Scholar] [CrossRef]
- Ghosh, S.; Basu Ball, W.; Madaris, T.R.; Srikantan, S.; Madesh, M.; Mootha, V.K.; Gohil, V.M. An Essential Role for Cardiolipin in the Stability and Function of the Mitochondrial Calcium Uniporter. Proc. Natl. Acad. Sci. USA 2020, 117, 16383–16390. [Google Scholar] [CrossRef]
- Blunsom, N.J.; Cockcroft, S. CDP-diacylglycerol synthases (CDS): Gateway to phosphatidylinositol and cardiolipin synthesis. Front. Cell Dev. Biol. 2020, 8, 63. [Google Scholar] [CrossRef]
- Bieberich, E. It’s a lipid’s world: Bioactive lipid metabolism and signaling in neural stem cell differentiation. Neurochem. Res. 2012, 37, 1208–1229. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.Z.; Phillips, P.H.; Chacko, J.G.; Warner, D.B.; Pelaez, D.; Bhattacharya, S.K. Temporal Alterations of Sphingolipids in Optic Nerves After Indirect Traumatic Optic Neuropathy. Ophthalmol. Sci. 2023, 3, 100217. [Google Scholar] [CrossRef] [PubMed]
- Bispo, D.S.C.; Michálková, L.; Correia, M.; Jesus, C.S.H.; Duarte, I.F.; Goodfellow, B.J.; Oliveira, M.B.; Mano, J.F.; Gil, A.M. Endo- and Exometabolome Crosstalk in Mesenchymal Stem Cells Undergoing Osteogenic Differentiation. Cells 2022, 11, 1257. [Google Scholar] [CrossRef]
- Wood, P.L. Fatty acyl esters of hydroxy fatty acid (FAHFA) lipid families. Metabolites 2020, 10, 512. [Google Scholar] [CrossRef]
- Yore, M.M.; Syed, I.; Moraes-Vieira, P.M.; Zhang, T.; Herman, M.A.; Homan, E.A.; Patel, R.T.; Lee, J.; Chen, S.; Peroni, O.D.; et al. Discovery of a Class of Endogenous Mammalian Lipids with Anti-Diabetic and Anti-Inflammatory Effects. Cell 2014, 159, 318–332. [Google Scholar] [CrossRef]
- Moraes-Vieira, P.M.; Saghatelian, A.; Kahn, B.B. GLUT4 Expression in Adipocytes Regulates De Novo Lipogenesis and Levels of a Novel Class of Lipids With Antidiabetic and Anti-inflammatory Effects. Diabetes 2016, 65, 1808–1815. [Google Scholar] [CrossRef]
- Cohen, P.; Kajimura, S. The cellular and functional complexity of thermogenic fat. Nat. Rev. Mol. Cell Biol. 2021, 22, 393–409. [Google Scholar] [CrossRef]
- Gu, D.; Mao, X.; Abouel Azm, F.R.; Zhu, W.; Huang, T.; Wang, X.; Ni, X.; Zhou, M.; Shen, J.; Tan, Q. Optimal Dietary Zinc Inclusion Improved Growth Performance, Serum Antioxidant Capacity, Immune Status, and Liver Lipid and Glucose Metabolism of Largemouth Bass (Micropterus salmoides). Fish. Shellfish. Immun. 2024, 144, 109233. [Google Scholar] [CrossRef]
- Hong, E.; Yang, G.; Oh, S.; Kim, E. Lactiplantibacillus Plantarum L67 Reduces Diet-Induced Obesity by Stimulating Gene Programming for Adipose Lipolysis and Energy Expenditure. J. Funct. Foods 2024, 113, 106028. [Google Scholar] [CrossRef]
- Kim, H.K.; Della-Fera, M.; Lin, J.; Baile, C.A. Docosahexaenoic Acid Inhibits Adipocyte Differentiation and Induces Apoptosis in 3T3-L1 Preadipocytes. J. Nutr. 2006, 136, 2965–2969. [Google Scholar] [CrossRef] [PubMed]
- Noutsi, P.; Gratton, E.; Chaieb, S. Assessment of membrane fluidity fluctuations during cellular development reveals time and cell type specificity. PLoS ONE 2016, 11, e0158313. [Google Scholar] [CrossRef]
- Dwyer, J.R.; Sever, N.; Carlson, M.; Nelson, S.F.; Beachy, P.A.; Parhami, F. Oxysterols Are Novel Activators of the Hedgehog Signaling Pathway in Pluripotent Mesenchymal Cells. J. Biol. Chem. 2007, 282, 8959–8968. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.-M.; Lein, P.J.; et al. Polyunsaturated Fatty Acids and Fatty Acid-Derived Lipid Mediators: Recent Advances in the Understanding of Their Biosynthesis, Structures, and Functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef] [PubMed]
- Sambra, V.; Echeverria, F.; Valenzuela, A.; Chouinard-Watkins, R.; Valenzuela, R. Docosahexaenoic and Arachidonic Acids as Neuroprotective Nutrients throughout the Life Cycle. Nutrients 2021, 13, 986. [Google Scholar] [CrossRef] [PubMed]
- Hölzl, G.; Dörmann, P. Chloroplast Lipids and Their Biosynthesis. Annu. Rev. Plant Biol. 2019, 70, 51–81. [Google Scholar] [CrossRef] [PubMed]
- Liaw, L.; Prudovsky, I.; Koza, R.A.; Anunciado-Koza, R.V.; Siviski, M.E.; Lindner, V.; Friesel, R.E.; Rosen, C.J.; Baker, P.R.; Simons, B.; et al. Lipid Profiling of In Vitro Cell Models of Adipogenic Differentiation: Relationships with Mouse Adipose Tissues. J. Cell Biochem. 2016, 117, 2182–2193. [Google Scholar] [CrossRef]
| Lipid Categories | Lipid Subclasses | Up Numbers/Percentage | Down Numbers/Percentage | Total Changes |
|---|---|---|---|---|
| Glycerides | DAG | 11/91.67% | 1/8.33% | 12 |
| TAG | 91/97.85% | 2/2.15% | 93 | |
| Glycerophospholipids | PA | 2/100% | 0/0 | 2 |
| PC | 83/47.98% | 90/52.02% | 173 | |
| PE | 54/62.79% | 32/37.21% | 86 | |
| PG | 21/53.85% | 18/46.15% | 39 | |
| PS | 5/100% | 0/0 | 5 | |
| PI | 4/50% | 4/50% | 8 | |
| CL | 16/100% | 0/0 | 16 | |
| PEtOH | 0/0% | 1/100% | 1 | |
| BMP | 6/100% | 0/0 | 6 | |
| HBMP | 9/40.91% | 13/59.09% | 22 | |
| Sphingolipids | HexCer | 10/83.33% | 2/16.67% | 12 |
| Cer | 4/16% | 21/84% | 25 | |
| SM | 1/1.85% | 53/98.15% | 54 | |
| GM3 | 3/75% | 1/25% | 4 | |
| Aliphatic acids | FAHFA | 1/100% | 0/0 | 1 |
| ACar | 6/100% | 0/0 | 6 | |
| Lycolipids | GlcADG | 0/0 | 1/100% | 1 |
| Sterols | CE | 0/0 | 2/100% | 2 |
| Lipid Categories | Lipid Subclass Metabolites | Ion Mode | FC | Up/ Down | VIP |
|---|---|---|---|---|---|
| GL | DAG (16:1)/18:3) | pos | 5.88 | up | 1.12 |
| DAG (16:1/22:6) | pos | 5.24 | up | 1.25 | |
| DAG (14:0/18:3) | pos | 3.74 | up | 1.28 | |
| 1-O-(1Z-Tetradecenyl)-2-(9Z-octadecenoyl)-sn-glycerol | pos | 3.3 | up | 1.46 | |
| DAG (18:0/22:4) | pos | 3.09 | up | 1.51 | |
| TAG (20:2-22:5-22:6) | pos | 7.73 | up | 1.51 | |
| TAG (18:1-22:5-22:5) | pos | 7.36 | up | 1.25 | |
| TAG (22:1-22:4-22:5) | pos | 7.29 | up | 1.42 | |
| TAG (22:1-22:5-22:5) | pos | 7.08 | up | 1.43 | |
| TAG (16:1-22:5-22:6) | pos | 6.03 | up | 1.51 | |
| GP | PA (16:1/18:1) | neg | 2.35 | up | 1.25 |
| 1-arachidoyl-sn-glycero-3-phosphate | pos | 2.01 | up | 1.4 | |
| PC (16:2/22:6) | pos | 45.08 | up | 1.43 | |
| PC (16:2/20:5) | pos | 29.68 | up | 1.53 | |
| PC (14:0/16:3) | pos | 25.78 | up | 1.49 | |
| PC (17:2/17:2) | pos | 16.88 | up | 1.49 | |
| OxPC (18:0-20:3+1O(1Cyc)) | neg | 16.79 | up | 1.49 | |
| PE (20:5/22:6) | pos | 9.57 | up | 1.47 | |
| PE (16:1e/16:2) | neg | 8.34 | up | 1.21 | |
| PE (16:1e/16:0) | neg | 8.11 | up | 1.54 | |
| 2-linoleoyl-sn-glycero-3-phosphoethanolamine | neg | 7.61 | up | 1.25 | |
| PE (16:0/18:2) | neg | 6.73 | up | 1.34 | |
| PG (20:5/20:5) | neg | 8.1 | up | 1.56 | |
| PG (18:1/19:1) | neg | 0.13 | down | 1.6 | |
| PG (14:0/16:0) | neg | 0.15 | down | 1.44 | |
| 1,2-dioleoyl-sn-glycero-3-phospho-(1′-sn-glycerol) | pos | 6.04 | up | 1.25 | |
| PG (16:1(9Z)/20:4(5Z,8Z,11Z,14Z)) | neg | 5.5 | up | 1.43 | |
| PS (18:0/22:4) | neg | 3.04 | up | 1.07 | |
| 1-palmitoyl-2-stearoyl-sn-glycero-3-phosphoserine | neg | 2.18 | up | 1.13 | |
| PS (16:1(9Z)/18:1(9Z)) | pos | 2.31 | up | 1.25 | |
| 1-stearoyl-2-oleoyl-sn-glycero-3-phosphoserine | neg | 2.25 | up | 1.16 | |
| PS (18:0/22:4) | pos | 2.22 | up | 1.22 | |
| PI (18:1/20:5) | neg | 9.65 | up | 1.18 | |
| PI (16:1/20:4) | neg | 5.2 | up | 1.15 | |
| PI (18:0/20:2) | neg | 0.19 | down | 1.52 | |
| PI (17:0/20:4) | neg | 0.22 | down | 1.31 | |
| PI (16:0/22:6) | neg | 0.29 | down | 1.44 | |
| CL (16:0-18:2-16:1-18:1) | neg | 6.97 | up | 1.08 | |
| CL (16:1-18:1-16:1-18:2) | neg | 5.11 | up | 1.04 | |
| CL (16:1-18:1-18:2-18:2) | neg | 5.04 | up | 1.04 | |
| CL (14:0-18:2-16:1-18:1) | neg | 4.63 | up | 1.26 | |
| CL (16:1-18:1-18:2-20:4) | neg | 4.15 | up | 1.03 | |
| PEtOH (18:0-20:4) | neg | 0.47 | down | 1.5 | |
| BMP (22:6/22:6) | pos | 6.56 | up | 1.53 | |
| BMP (22:4/22:6) | pos | 6.31 | up | 1.52 | |
| BMP (22:5/22:5) | pos | 5.24 | up | 1.54 | |
| BMP (20:4/22:5) | pos | 4.12 | up | 1.5 | |
| BMP (20:4/22:6) | pos | 2.95 | up | 1.51 | |
| HBMP (22:5-22:6-16:1) | neg | 11.9 | up | 1.58 | |
| HBMP (18:0-20:1-18:1) | neg | 0.11 | down | 1.4 | |
| HBMP (18:0-18:1-18:0) | neg | 0.13 | down | 1.12 | |
| HBMP (16:0-22:6-16:0) | neg | 0.13 | down | 1.14 | |
| HBMP (18:0-18:1-16:0) | neg | 0.14 | down | 1.3 | |
| SP | HexCer-NDS (d18:0/16:0) | neg | 0.05 | down | 1.27 |
| HexCer-NS (d18:2/24:1) | pos | 4.12 | up | 1.55 | |
| HexCer-NS (d30:2/14:1) | neg | 0.3 | down | 1.5 | |
| HexCer-NS (d18:1/18:0) | pos | 2.89 | up | 1.03 | |
| HexCer-NS (d18:1/24:1) | neg | 2.62 | up | 1.49 | |
| Cer-NDS (d18:0/20:0) | neg | 0.04 | down | 1.29 | |
| Cer-NDS (d18:0/18:0) | neg | 0.06 | down | 1.51 | |
| Cer-NDS (d18:0/22:0) | neg | 0.06 | down | 1.15 | |
| Cer-NDS (d18:0/14:0) | neg | 0.08 | down | 1.51 | |
| Cer-NDS (d18:0/15:0) | neg | 0.09 | down | 1.36 | |
| N-[(15Z)-tetracosenoyl]sphinganine-1-phosphocholine | pos | 0.02 | down | 1.22 | |
| SM(d18:0/14:0) | neg | 0.06 | down | 1.42 | |
| SM (d14:0/18:0) | neg | 0.07 | down | 1.42 | |
| SM (d26:0/12:0) | pos | 0.08 | down | 1.17 | |
| SM (d14:0/22:0) | neg | 0.09 | down | 1.34 | |
| GM3 d34:0 | neg | 0.25 | down | 1.04 | |
| GM3 d42:3 | neg | 2.76 | up | 1.15 | |
| GM3 d42:1 | neg | 2.73 | up | 1.4 | |
| GM3 d42:2 | neg | 2.22 | up | 1.21 | |
| FAs | FAHFA (22:6/22:5) | neg | 7.39 | up | 1.59 |
| ACar 24:1 | pos | 9.63 | up | 1.39 | |
| ACar 22:1 | pos | 6.92 | up | 1.21 | |
| ACar 20:2 | pos | 3.85 | up | 1.35 | |
| ACar 16:2 | pos | 3.57 | up | 1.04 | |
| ACar 20:1 | pos | 2.96 | up | 1.32 | |
| SL | GlcADG (18:2-20:3) | neg | 0.3 | down | 1.45 |
| ST | CE 18:1 | pos | 0.03 | down | 1.35 |
| CE 20:3 | pos | 0.04 | down | 1.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Liang, Z.; Ding, Y.; Song, X.; Zhao, R.; Yan, Y.; Tang, X. Lipidomics Techniques Revealed the Adipogenic Differentiation Mechanism of Bovine Adipose-Derived Neural Crest Stem Cells. Animals 2025, 15, 3191. https://doi.org/10.3390/ani15213191
Zhang K, Liang Z, Ding Y, Song X, Zhao R, Yan Y, Tang X. Lipidomics Techniques Revealed the Adipogenic Differentiation Mechanism of Bovine Adipose-Derived Neural Crest Stem Cells. Animals. 2025; 15(21):3191. https://doi.org/10.3390/ani15213191
Chicago/Turabian StyleZhang, Kai, Zhaotong Liang, Yilin Ding, Xianyi Song, Rui Zhao, Yibo Yan, and Xiaopeng Tang. 2025. "Lipidomics Techniques Revealed the Adipogenic Differentiation Mechanism of Bovine Adipose-Derived Neural Crest Stem Cells" Animals 15, no. 21: 3191. https://doi.org/10.3390/ani15213191
APA StyleZhang, K., Liang, Z., Ding, Y., Song, X., Zhao, R., Yan, Y., & Tang, X. (2025). Lipidomics Techniques Revealed the Adipogenic Differentiation Mechanism of Bovine Adipose-Derived Neural Crest Stem Cells. Animals, 15(21), 3191. https://doi.org/10.3390/ani15213191






