Take a Look Towards the Stress Response of Working Dogs: Cortisol and Lactate Trend Mismatches During Training
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
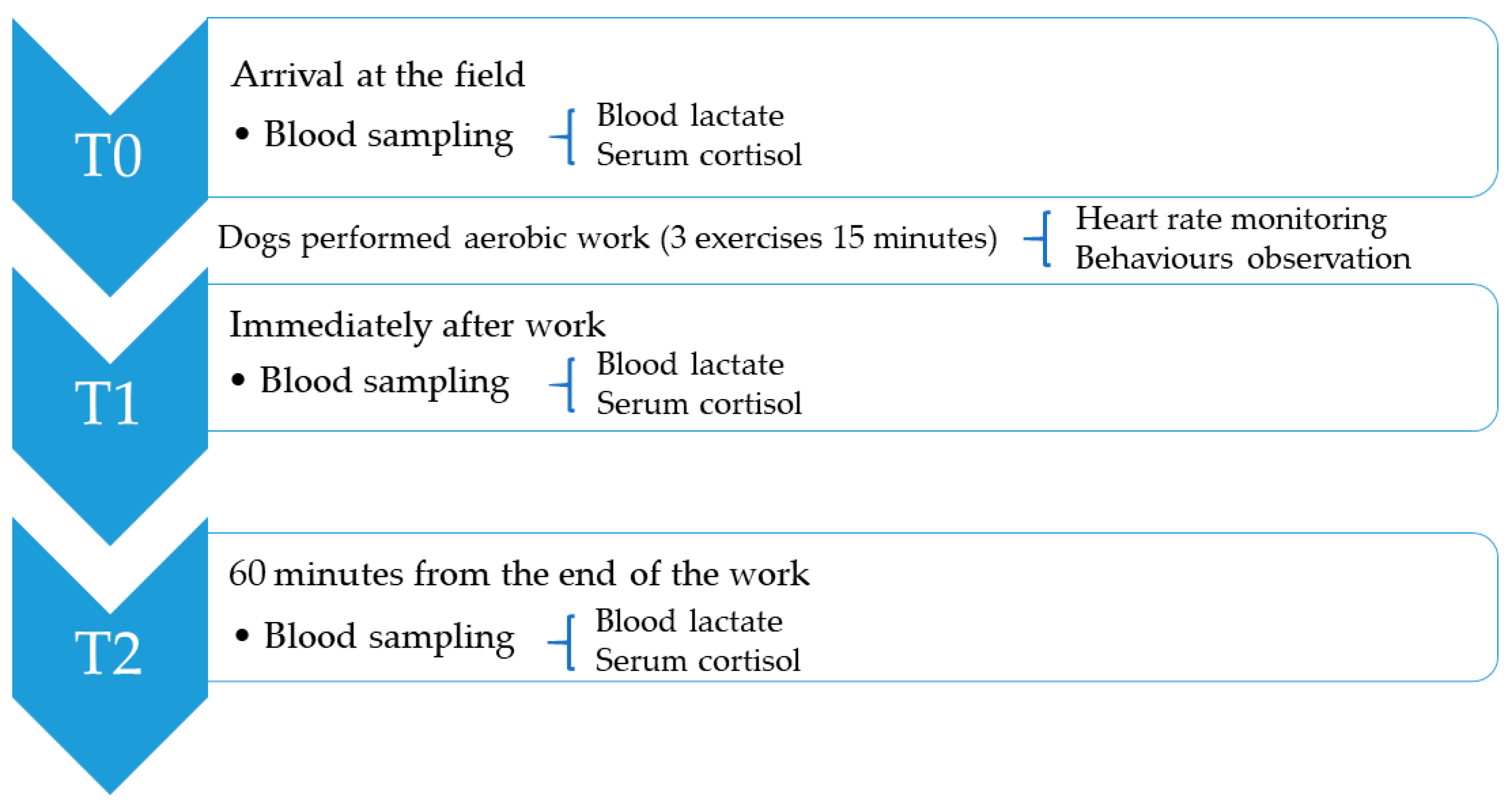
2.2. Blood Sample Collection and Laboratory Analysis
2.3. Statistical Analysis
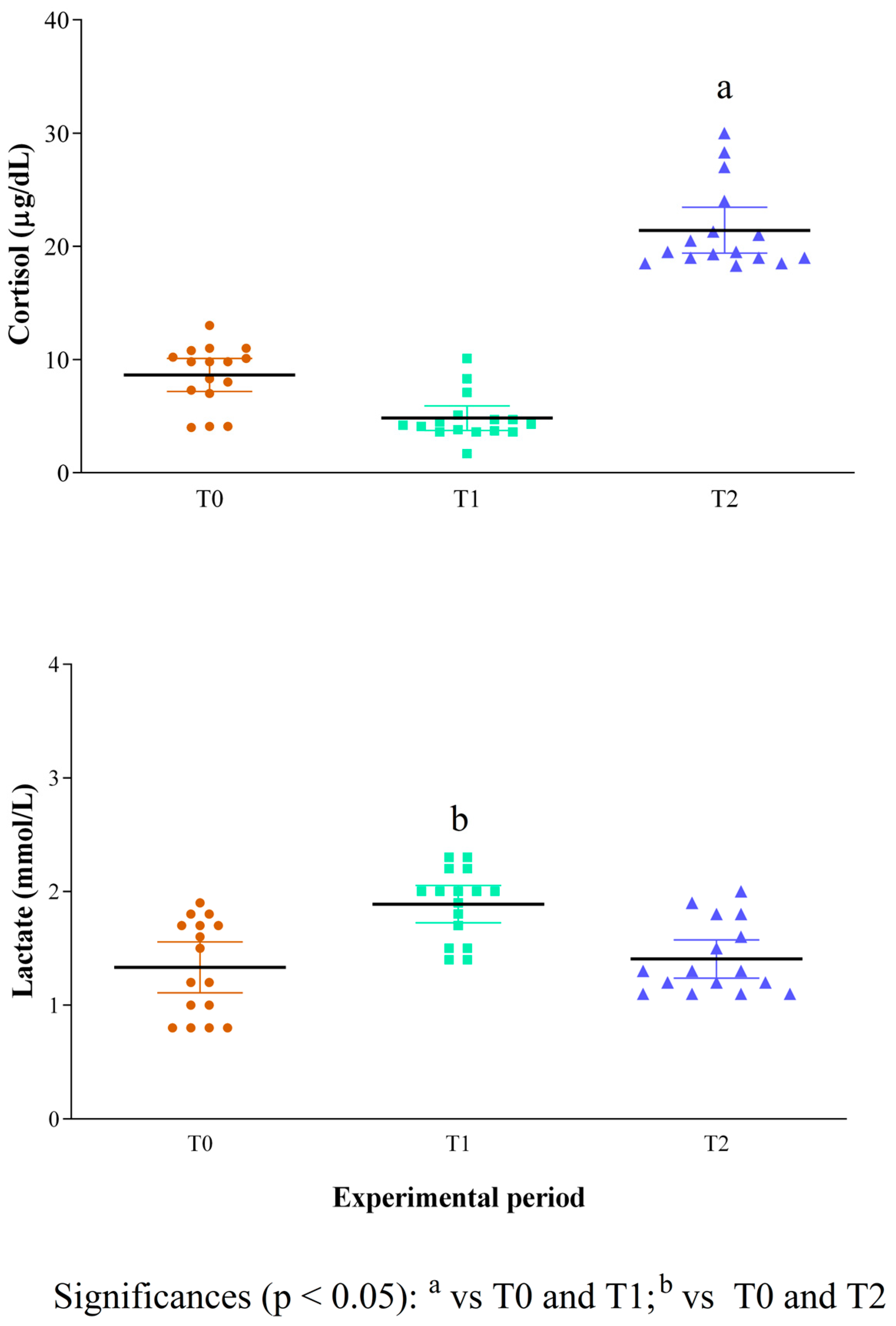
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hall, N.J.; Johnston, A.M.; Bray, E.E.; Otto, C.M.; MacLean, E.L.; Udell, M.A.R. Working Dog Training for the Twenty-First Century. Front. Vet. Sci. 2021, 8, 646022. [Google Scholar] [CrossRef] [PubMed]
- Farhoody, P. Behavior analysis: The science of training. Vet. Clin. N. Am. Exot. Anim. Pract. 2012, 15, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Cobb, M.L.; Otto, C.M.; Fine, A.H. The Animal Welfare Science of Working Dogs: Current Perspectives on Recent Advances and Future Directions. Front. Vet. Sci. 2021, 8, 666898. [Google Scholar] [CrossRef]
- Wilson, C.; Ebbecke, D.; Berger, D.; Otto, C. The Effects of Fitness Training on Working Dog Behavior: Two Case Studies. Vet. Clin. N. Am. Small Anim. Pract. 2024, 54, 87–99. [Google Scholar] [CrossRef]
- Vieira de Castro, A.C.; Araújo, Â.; Fonseca, A.; Olsson, I.A.S. Improving dog training methods: Efficacy and efficiency of reward and mixed training methods. PloS ONE 2021, 16, e0247321. [Google Scholar] [CrossRef]
- Lazarowski, L.; Waggoner, L.P.; Krichbaum, S.; Singletary, M.; Haney, P.; Rogers, B.; Angle, C. Selecting dogs for explosives detection: Behavioral characteristics. Front. Vet. Sci. 2020, 7, 597. [Google Scholar] [CrossRef]
- Rooney, N.J.; Bradshaw, J.W.S.; Almey, H. Attributes of Specialist Search Dogs–Questionnaire Survey of UK Dog Handlers and Trainers. J. Forensic Sci. 2004, 49, 300–306. [Google Scholar] [CrossRef]
- Rocznik, D.; Sinn, D.L.; Thomas, S.; Gosling, S.D. Criterion Analysis and Content Validity for Standardized Behavioral Tests in a Detector-Dog Breeding Program. J. Forensic Sci. 2015, 60, S213–S221. [Google Scholar] [CrossRef]
- Jamieson, L.T.J.; Baxter, G.S.; Murray, P.J. Identifying Suitable Detection Dogs. Appl. Anim. Behav. Sc. 2017, 195, 1–7. [Google Scholar] [CrossRef]
- Troisi, C.A.; Mills, D.S.; Wilkinson, A.; Zulch, H.E. Behavioral and cognitive factors that affect the success of scent detection dogs. Comp. Cogn. Behav. Rev. 2019, 14, 51–76. [Google Scholar] [CrossRef]
- Bray, E.E.; Otto, C.M.; Udell, M.A.R.; Hall, N.J.; Johnston, A.M.; MacLean, E.L. Enhancing the Selection and Performance of Working Dogs. Front. Vet. Sci. 2021, 8, 644431. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. The Hypothalamic-Pituitary-Adrenal Axis: A Brief History. Horm. Res. Paediatr. 2018, 89, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, A.B.; Vigors, B.; Sandøe, P. What Is so Positive about Positive Animal Welfare?—A Critical Review of the Literature. Animals 2019, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, M. Preventive Health Care for Working Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 745–764. [Google Scholar] [CrossRef]
- Arfuso, F.; Giannetto, C.; Bazzano, M.; Assenza, A.; Piccione, G. Physiological Correlation between Hypothalamic-Pituitary-Adrenal Axis, Leptin, UCP1 and Lipid Panel in Mares during Late Pregnancy and Early Postpartum Period. Animals 2021, 11, 2051. [Google Scholar] [CrossRef]
- Arfuso, F.; Minuti, A.; Liotta, L.; Giannetto, C.; Trevisi, E.; Piccione, G.; Lopreiato, V. Stress and Inflammatory Response of Cows and Their Calves during Peripartum and Early Neonatal Period. Theriogenology 2023, 196, 157–166. [Google Scholar] [CrossRef]
- Diez-Fraile, A.; Meyer, E.; Burvenich, C. Sympathoadrenal and Immune System Activation during the Periparturient Period and Their Association with Bovine Coliform Mastitis. A Review. Vet. Q. 2003, 25, 31–44. [Google Scholar] [CrossRef]
- Glenk, L.M.; Kothgassner, O.D.; Stetina, B.U.; Palme, R.; Kepplinger, B.; Baran, H. Salivary Cortisol and Behavior in Therapy Dogs during Animal-Assisted Interventions: A Pilot Study. J. Vet. Behav. 2014, 9, 98–106. [Google Scholar] [CrossRef]
- Haverbeke, A.; Diederich, C.; Depiereux, E.; Giffroy, J.M. Cortisol and Behavioral Responses of Working Dogs to Environmental Challenges. Physiol. Behav. 2008, 93, 59–67. [Google Scholar] [CrossRef]
- Gazzano, V.; Curadi, M.C.; Baragli, P.; Mariti, C.; Cecchi, F.; Cavallo, S.; Sacchettino, L.; Gazzano, A. Physiological and Behavioral Evaluation of Shelter Dogs During Veterinary Routine Health Checks. Vet. Sci. 2025, 12, 583. [Google Scholar] [CrossRef]
- Riggio, G.; Mariti, C.; Sergi, V.; Diverio, S.; Gazzano, A. Serotonin and Tryptophan Serum Concentrations in Shelter Dogs Showing Different Behavioural Responses to a Potentially Stressful Procedure. Vet. Sci. 2020, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Schüttler, J.; Neumann, S. Interleukin-6 as a Prognostic Marker in Dogs in an Intensive Care Unit. Vet. Clin. Pathol. 2015, 44, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Ogi, A.; Mariti, C.; Baragli, P.; Sergi, V.; Gazzano, A. Effects of Stroking on Salivary Oxytocin and Cortisol in Guide Dogs: Preliminary Results. Animals 2020, 10, 708. [Google Scholar] [CrossRef]
- Chmelíková, E.; Bolechová, P.; Chaloupková, H.; Svobodová, I.; Jovičić, M.; Sedmíková, M. Salivary cortisol as a marker of acute stress in dogs: A review. Domest. Anim. Endocrinol. 2020, 72, 106428. [Google Scholar] [CrossRef]
- Hall, M.M.; Rajasekaran, S.; Thomsen, T.W.; Peterson, A.R. Lactate: Friend or Foe. PM R. 2016, 8 (Suppl. S3), S8–S15. [Google Scholar] [CrossRef]
- Beerda, B.; Schilder, M.B.H.; van Hooff, J.A.R.A.M.; de Vries, H.W.; Mol, J.A. Manifestations of chronic and acute stress in dogs. Appl. Anim. Behav. Sci. 1997, 52, 307–319. [Google Scholar] [CrossRef]
- Beerda, B.; Schilder, M.B.H.; van Hooff, J.A.R.A.M.; de Vries, H.W. Behavioural, saliva cortisol and heart rate responses to different types of stimuli in dogs. Appl. Anim. Behav. Sci. 1998, 58, 365–381. [Google Scholar] [CrossRef]
- Mariti, C.; Ricci, E.; Zilocchi, M.; Sighieri, C.; Gazzano, A. Human-directed social behaviour in dogs: The role of social experience. Appl. Anim. Behav. Sci. 2012, 140, 108–116. [Google Scholar]
- Rugaas, T. On Talking Terms with Dogs: Calming Signals; Dogwise Publishing: Wenatchee, WA, USA, 1997. [Google Scholar]
- De Winkel, T.; van der Steen, S.; Enders-Slegers, M.-J.; Griffioen, R.; Haverbeke, A.; Groenewoud, D.; Hediger, K. Observational behaviors and emotions to assess welfare of dogs: A systematic review. J. Vet. Behav. 2024, 72, 1–17. [Google Scholar] [CrossRef]
- Wafi, A.M.; Gao, L.; Zucker, I.H. Blood lactate as a marker of exercise adaptation and fatigue in male mice with chronic heart failure. Physiol. Rep. 2025, 13, e70485. [Google Scholar] [CrossRef]
- Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. Clinical Biochemistry of Domestic Animals, 5th ed.; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Rizzo, M.; Arfuso, F.; Giannetto, C.; Giudice, E.; Longo, F.; di Pietro, S.; Piccione, G. Cortisol Levels and Leukocyte Population Values in Transported and Exercised Horses after Acupuncture Needle Stimulation. J. Vet. Behav. 2016, 18, 56–61. [Google Scholar] [CrossRef]
- Arfuso, F.; Giudice, E.; Panzera, M.; Rizzo, M.; Fazio, F.; Piccione, G.; Giannetto, C. Interleukin-1Ra (Il-1Ra) and Serum Cortisol Level Relationship in Horse as Dynamic Adaptive Response during Physical Exercise. Vet. Immunol. Immunopathol. 2022, 243, 110368. [Google Scholar] [CrossRef]
- Clark, S.D.; Martin, F.; McGowan, R.T.S.; Smidt, J.M.; Anderson, R.; Wang, L.; Turpin, T.; Langenfeld-McCoy, N.; Bauer, B.A.; Mohabbat, A.B. Physiological State of Therapy Dogs during Animal-Assisted Activities in an Outpatient Setting. Animals 2020, 10, 819. [Google Scholar] [CrossRef]
- Di Natale, A. Preliminary study on the psychophysical stress of dogs in law enforcement dog units during off-duty period. Dog Behav. 2022, 2, 29–44. [Google Scholar]
- Hare, E.; Kelsey, K.M.; Niedermeyer, G.M.; Otto, C.M. Long-Term Behavioral Resilience in Search-and-Rescue Dogs Responding to the September 11, 2001 Terrorist Attacks. Appl. Anim. Behav. Sci. 2021, 234, 105173. [Google Scholar] [CrossRef]
- Wojtaś, J.; Karpiński, M.; Zieliński, D. Salivary Cortisol Levels in Search and Rescue (SAR) Dogs under Rescue Examination Conditions. J. Vet. Behav. 2021, 42, 11–15. [Google Scholar] [CrossRef]
- Etim, N.N.; Evans, E.I.; Offiong, E.E.A.; Williams, M.E. Stress and the neuroendocrine system: Implications for animal well-being. Am. J. Biol. Life Sci. 2013, 1, 20–26. [Google Scholar]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [PubMed]
- Puglisi, I.; Masucci, M.; Cozzi, A.; Teruel, E.; Navarra, M.; Cirmi, S.; Pennisi, M.G.; Siracusa, C. Effects of a Novel Gel Formulation of Dog Appeasing Pheromone (DAP) on Behavioral and Physiological Stress Responses in Dogs Undergoing Clinical Examination. Animals 2022, 12, 2472. [Google Scholar] [CrossRef]
- Menor-Campos, D.J.; Molleda-Carbonell, J.M.; López-Rodríguez, R. Effects of exercise and human contact on animal welfare in a dog shelter. Vet. Rec. 2011, 169, 388. [Google Scholar] [CrossRef]
- Duan, H.; Wang, L.; Zhang, L.; Liu, J.; Zhang, K.; Wu, J. The Relationship between Cortisol Activity during Cognitive Task and Posttraumatic Stress Symptom Clusters. PloS ONE 2015, 10, e0144315. [Google Scholar] [CrossRef]
- Piccione, G.; Casella, S.; Panzera, M.; Giannetto, C.; Fazio, F. Effect of Moderate Treadmill Exercise on Some Physiological Parameters in Untrained Beagle Dogs. Exp. Anim. 2012, 61, 511–515. [Google Scholar] [CrossRef]
- Hogan, M.C.; Gladden, L.B.; Kurdak, S.S.; Poole, D.C. Increased [Lactate] in Working Dog Muscle Reduces Tension Development Independent of pH. Med. Sci. Sports Exerc. 1995, 27, 371–377. [Google Scholar] [CrossRef]
- Friedrich, J.; Strandberg, E.; Arvelius, P.; Sánchez-Molano, E.; Pong-Wong, R.; Hickey, J.M.; Haskell, M.J.; Wiener, P. Genetic dissection of complex behaviour traits in German Shepherd dogs. Heredity 2019, 123, 746–758. [Google Scholar] [CrossRef]

| n | Breed | Age | Gender | n |
|---|---|---|---|---|
| 11 | German Shepherd | Median 2 years | Males | 7 |
| Range 1–6 | Females | 4 | ||
| 5 | Rottweiler | Median 2 years | Males | 2 |
| Range 2–6 | Females | 3 |
| Observed Behaviour | n (%) | Behaviour Manifestation Time |
|---|---|---|
| Body expression | ||
| Scratching/Sniffing/Shivering/Shaking | 0 (0.0%) | - |
| Mouth expression | ||
| The dog licks his lips | 14 (87.5%) | Sit, lie down, approach the owner |
| Tail language | ||
| The dog’s tail is between the legs | 2 (12.5%) | Sit, lie down, approach the owner |
| Ear appearance | ||
| The dog’ ears are turned back | 14 (87.5%) | Sit, lie down, approach the owner |
| Vocalization | ||
| The dog barked or whined | 10 (62.5%) | Sit, lie down, approach the owner |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocco, R.; Sechi, S.; Sisia, G.; Pinna Parpaglia, M.L.; Rizzo, M.; Arrigo, F.; Giannetto, C.; Piccione, G.; Arfuso, F. Take a Look Towards the Stress Response of Working Dogs: Cortisol and Lactate Trend Mismatches During Training. Animals 2025, 15, 3175. https://doi.org/10.3390/ani15213175
Cocco R, Sechi S, Sisia G, Pinna Parpaglia ML, Rizzo M, Arrigo F, Giannetto C, Piccione G, Arfuso F. Take a Look Towards the Stress Response of Working Dogs: Cortisol and Lactate Trend Mismatches During Training. Animals. 2025; 15(21):3175. https://doi.org/10.3390/ani15213175
Chicago/Turabian StyleCocco, Raffaella, Sara Sechi, Giulia Sisia, Maria Luisa Pinna Parpaglia, Maria Rizzo, Federica Arrigo, Claudia Giannetto, Giuseppe Piccione, and Francesca Arfuso. 2025. "Take a Look Towards the Stress Response of Working Dogs: Cortisol and Lactate Trend Mismatches During Training" Animals 15, no. 21: 3175. https://doi.org/10.3390/ani15213175
APA StyleCocco, R., Sechi, S., Sisia, G., Pinna Parpaglia, M. L., Rizzo, M., Arrigo, F., Giannetto, C., Piccione, G., & Arfuso, F. (2025). Take a Look Towards the Stress Response of Working Dogs: Cortisol and Lactate Trend Mismatches During Training. Animals, 15(21), 3175. https://doi.org/10.3390/ani15213175






