Effects of Tetrabasic Zinc Chloride as Alternative to High Doses of Zinc Oxide on Growth Performance, Nutrient Digestibility, Intestinal Morphology, Immune Function, and Gut Microbiota in Weaned Piglets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Designs
2.2. Growth Performance
2.3. Sample Collections
2.4. Chemical Analysis for Diet and Feces
2.5. Intestinal Morphology Analysis
2.6. Analysis of Serum Antioxidant Parameters and Immune Indexes
2.7. Intestinal Microbiota Community
2.8. Statistical Analysis
3. Results
3.1. Growth Performance and Diarrhea Rate
3.2. The ATTD of Nutrients, the Acid-Binding Capacity, and the pH Value of the Stomach
3.3. Intestinal Morphology
3.4. Serum Antioxidant Parameters
3.5. Serum Immune Indexes
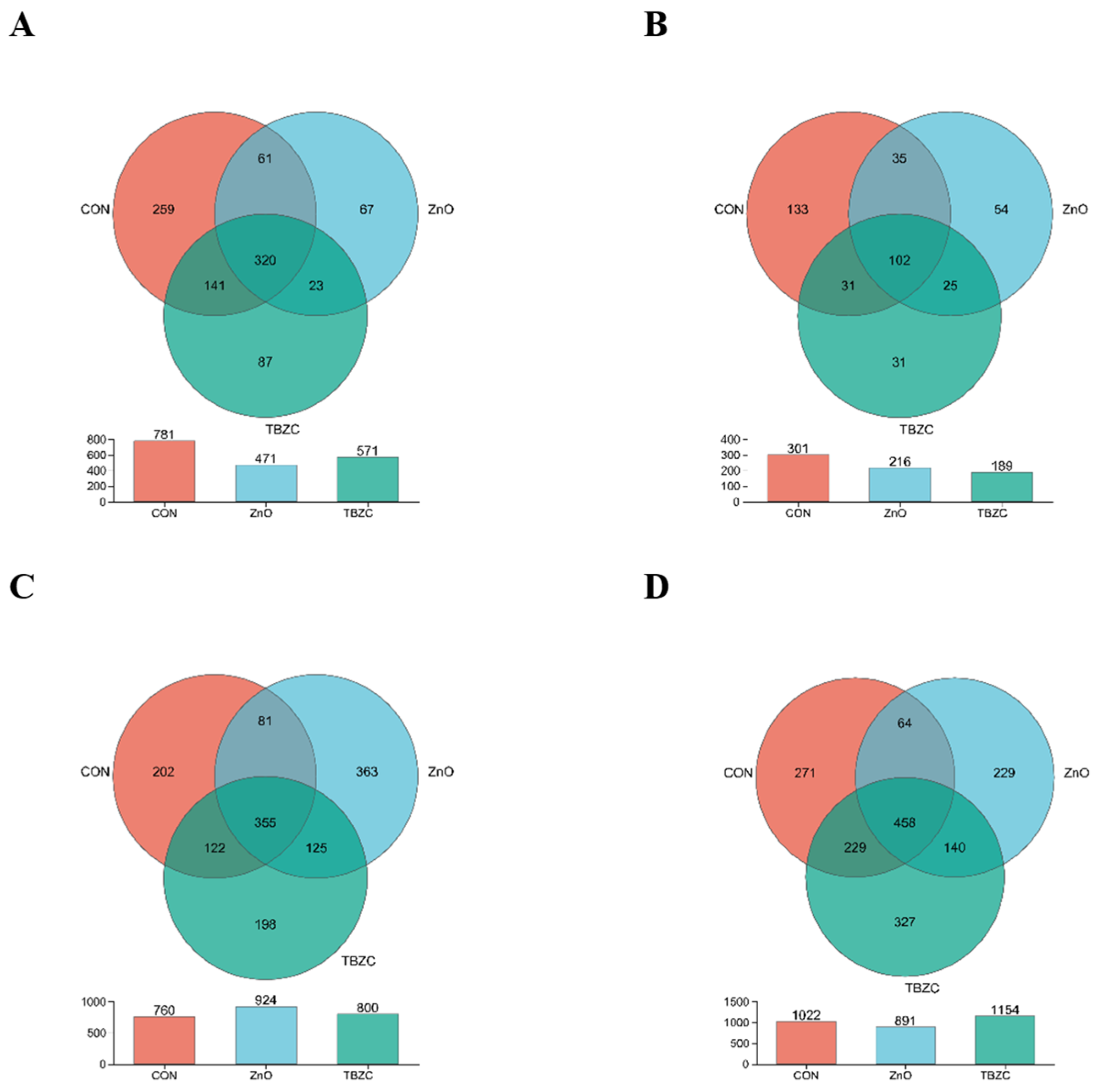
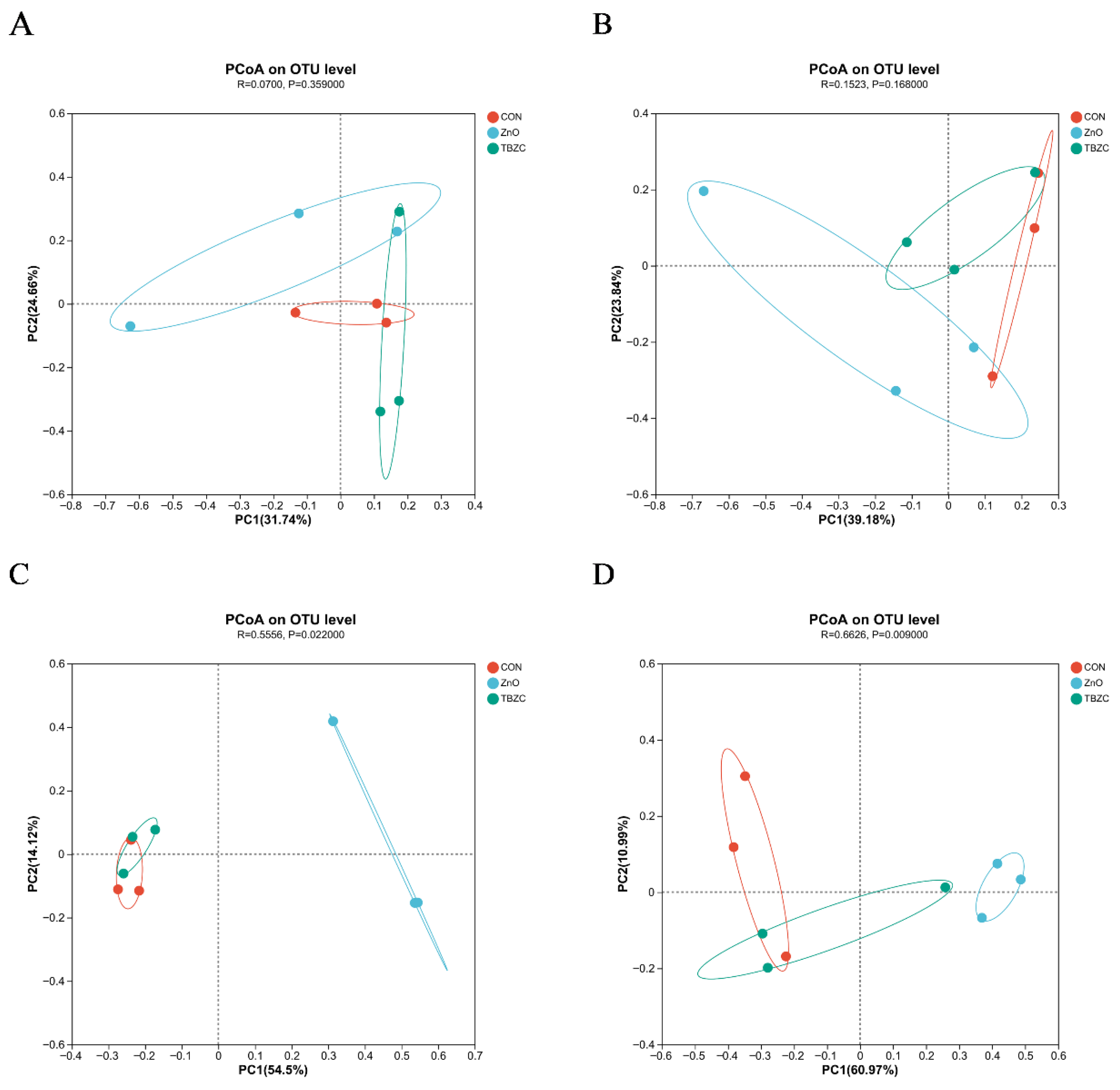
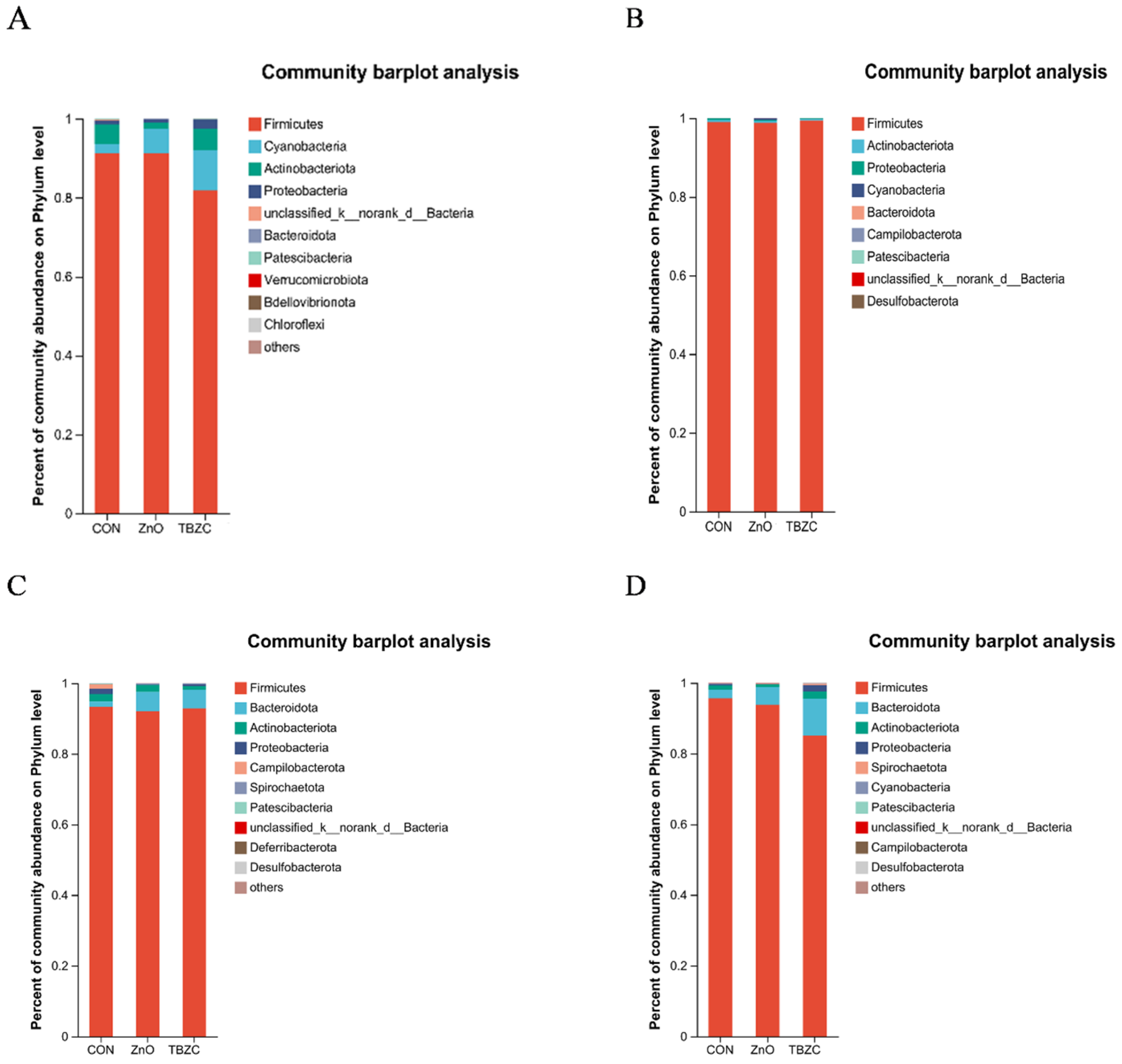
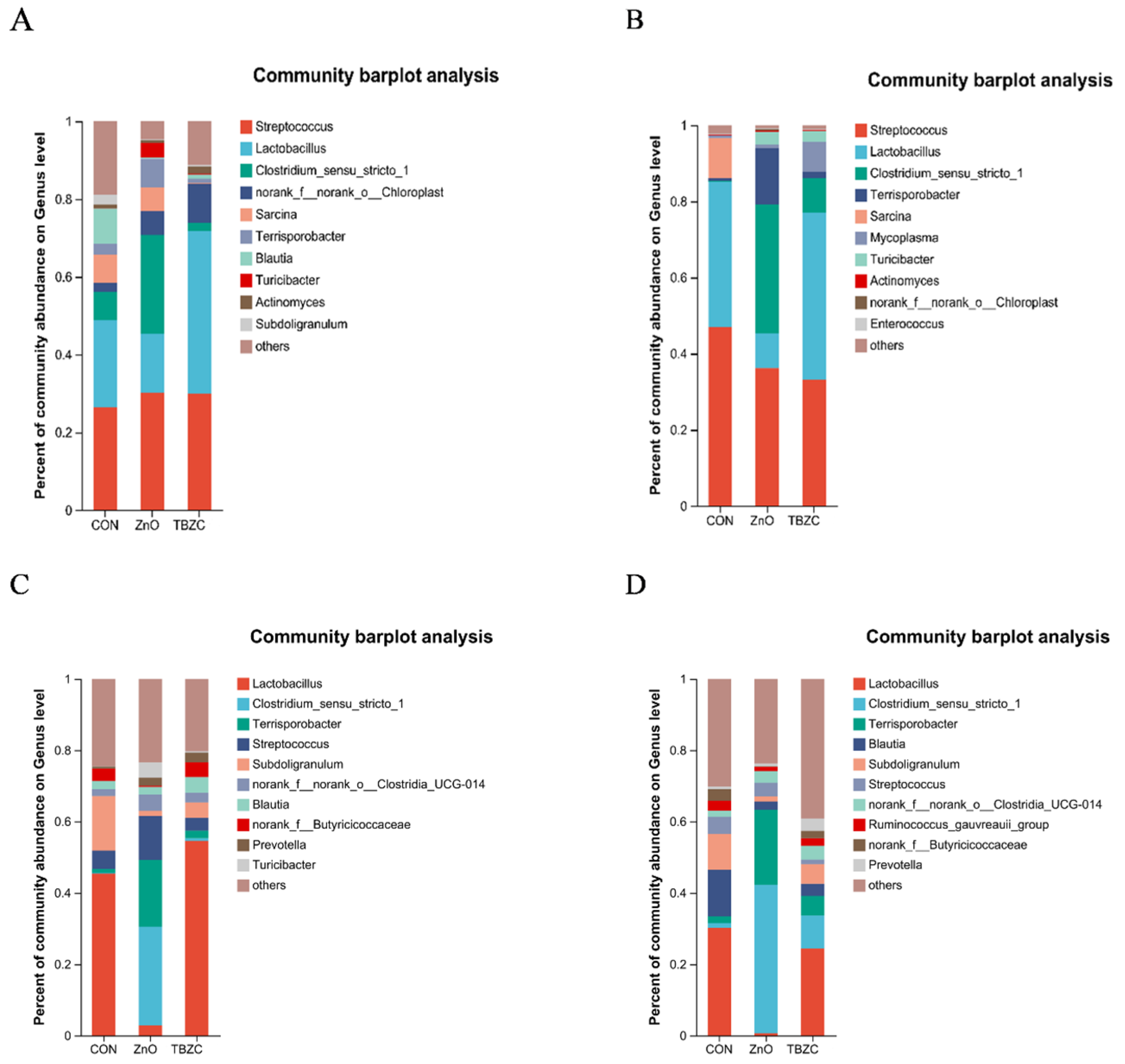
3.6. Gut Microbiota Community Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boston, T.E.; Wang, F.; Lin, X.; Kim, S.W.; Fellner, V.; Scott, M.F.; Ziegler, A.L.; Van Landeghem, L.; Blikslager, A.T.; Odle, J. Prebiotic galactooligosaccharide improves piglet growth performance and intestinal health associated with alterations of the hindgut microbiota during the peri-weaning period. Anim. Sci. Biotechnol. 2024, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, W.; Ma, J.; Zhang, Y.; Liu, M.; Liu, B.; Zhao, J.; Wang, Z.; Shi, Y. Dietary Fiber-Derived Butyrate Alleviates Piglet Weaning Stress by Modulating the TLR4/MyD88/NF-κB Pathway. Nutrients 2024, 16, 1714. [Google Scholar] [CrossRef]
- Sun, Y.B.; Xia, T.; Wu, H.; Zhang, W.J.; Zhu, Y.H.; Xue, J.X.; He, D.T.; Zhang, L.Y. Effects of nano zinc oxide as an alternative to pharmacological dose of zinc oxide on growth performance, diarrhea, immune responses, and intestinal microflora profile in weaned piglets. Anim. Feed. Sci. Technol. 2019, 258, 114312. [Google Scholar] [CrossRef]
- Case, C.L.; Carlson, M.S. Effect of feeding organic and inorganic sources of additional zinc on growth performance and zinc balance in nursery pigs. J. Anim. Sci. 2002, 80, 1917–1924. [Google Scholar] [CrossRef]
- Ortiz Sanjuán, J.M.; Manzanilla, E.G.; Cabrera-Rubio, R.; Crispie, F.; Cotter, P.D.; Garrido, J.J.; Ekhlas, D.; O’Neill, L.; Argüello, H. Fine-tuning of post-weaning pig microbiome structure and functionality by in-feed zinc oxide and antibiotics use. Front. Cell. Infect. Microbiol. 2024, 14, 1354449. [Google Scholar] [CrossRef]
- Burrough, E.R.; De Mille, C.; Gabler, N.K. Zinc overload in weaned pigs: Tissue accumulation, pathology, and growth impacts. J. Vet. Diagn. Investig. 2019, 31, 537–545. [Google Scholar] [CrossRef]
- Zhang, B.K.; Guo, Y.M. Beneficial effects of tetrabasic zinc chloride for weanling piglets and the bioavailability of zinc in tetrabasic form relative to ZnO. Anim. Feed. Sci. Technol. 2007, 135, 75–85. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, N.; Zhang, T.; Zhang, Y.; Dong, S.; Wang, H.; Xu, C.; Wang, C. Effects of Tetrabasic Zinc Chloride on the Diarrhea Rate, Intestinal Morphology, Immune Indices and Microflora of Weaned Piglets. Animals 2024, 14, 737. [Google Scholar] [CrossRef]
- Zhang, G.; Xia, T.; Zhao, J.; Liu, L.; He, P.; Zhang, S.; Zhang, L. Moderate tetrabasic zinc chloride supplementation improves growth performance and reduces diarrhea incidence in weaned pigs. Asian-Australas. J. Anim. Sci. 2020, 33, 264–276. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 11th ed.; National Academy Press: Washington, DC, USA, 2012. [Google Scholar]
- Zhang, T.; Zhang, N.; Peng, S.; Zhang, Y.; Wang, H.; Huang, S.; Zhu, M.; Ma, Y. Effects of Dietary Valine Chelated Zinc Supplementation on Growth Performance, Antioxidant Capacity, Immunity, and Intestine Health in Weaned Piglets. Biol. Trace Elem. Res. 2024, 202, 2577–2587. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, J.; Wang, W.; Guo, P.; Lu, W.; Wang, C.; Liu, L.; Johnston, L.J.; Zhao, Y.; Wu, X.; et al. Dietary corn bran altered the diversity of microbial communities and cytokine production in weaned pigs. Front. Microbiol. 2018, 9, 2090. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC International: Arlington, VA, USA, 2007. [Google Scholar]
- Oh, H.J.; Kim, M.H.; Lee, J.H.; Kim, Y.J.; An, J.W.; Chang, S.Y.; Go, Y.B.; Song, D.C.; Cho, H.A.; Jo, M.S.; et al. Effects of different inorganic: Organic zinc ratios or combination of low crude protein diet and mixed feed additive in weaned piglet diets. J. Anim. Sci. Technol. 2022, 64, 23–37. [Google Scholar] [CrossRef]
- Namkung, H.; Gong, J.; Yu, H.; De Lange, C.F.M. Effect of pharmacological intakes of zinc and copper on growth performance, circulating cytokines and gut microbiota of newly weaned piglets challenged with coliform lipopolysaccharides. Can. J. Anim. Sci. 2006, 86, 511–522. [Google Scholar] [CrossRef]
- Ou, D.; Li, D.; Cao, Y.; Li, X.; Yin, J.; Qiao, S.; Wu, G. Dietary supplementation with zinc oxide decreases expression of the stem cell factor in the small intestine of weanling pigs. J. Nutr. Biochem. 2007, 18, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.D.; Baker, D.H. Growth and plasma zinc responses of young pigs fed pharmacologic levels of zinc. J. Anim. Sci. 1993, 71, 3020–3024. [Google Scholar] [CrossRef] [PubMed]
- McCaughey, K.M.; DePeters, E.J.; Robinson, P.H.; Santos, J.E.P.; Taylor, S.J.; Pareas, J.W. Impact of feeding whole upland cottonseed, with or without cracked pima cottonseed with increasing addition of iron sulfate, on milk and milk fat composition of lactating dairy cattle. Anim. Feed. Sci. Technol. 2005, 123–124, 667–685. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, G.; Yang, Z.; Zhao, J. Effects of Tetrabasic Zinc Chloride on Growth Performance, Nutrient Digestibility and Fecal Microbial Community in Weaned Piglets. Front. Vet. Sci. 2022, 9, 905242. [Google Scholar] [CrossRef]
- Stas, E.B.; Warner, A.J.; Post, Z.B.; Hastad, C.W.; Faccin, J.E.G.; Tokach, M.D.; Woodworth, J.C.; DeRouchey, J.M.; Goodband, R.D.; Gebhardt, J.T. Effects of low acid-binding capacity specialty soy protein sources on nursery pig performance in a commercial environment. Transl. Anim. Sci. 2025, 9, txae180. [Google Scholar] [CrossRef]
- Chen, L.; Li, S.; Zheng, J.; Li, W.; Jiang, X.; Zhao, X.; Li, J.; Che, L.; Lin, Y.; Xu, S.; et al. Effects of dietary Clostridium butyricum supplementation on growth performance, intestinal development, and immune response of weaned piglets challenged with lipopolysaccharide. J. Animal. Sci. Biotechnol. 2018, 9, 62. [Google Scholar] [CrossRef]
- Park, B.C.; Jung, D.Y.; Kang, S.Y.; Ko, Y.H.; Ha, D.M.; Kwon, C.H.; Park, M.J.; Han, J.H.; Jang, I.; Lee, C.Y. Effects of dietary supplementation of a zinc oxide product encapsulated with lipid on growth performance, intestinal morphology, and digestive enzyme activities in weanling pigs. Anim. Feed. Sci. Technol. 2015, 200, 112–117. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Y.; Hao, Y.; Fang, J.; Feng, J. Effects of supplementation with oregano essential oil during late gestation and lactation on serum metabolites, antioxidant capacity and fecal microbiota of sows. Animals 2024, 14, 753. [Google Scholar] [CrossRef]
- Goc, Z.; Szaroma, W.; Kapusta, E.; Dziubek, K. Protective effects of melatonin on the activity of SOD, CAT, GSH-Px and GSH content in organs of mice after administration of SNP. Chin. J. Physiol. 2017, 60, 1–10. [Google Scholar] [CrossRef]
- Knoell, D.L.; Liu, M.J. Impact of zinc metabolism on innate immune function in the setting of sepsis. Int. J. Vitam. Nutr. Res. 2010, 80, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.E.; Kim, S.W. Intestinal microbiota and its interaction to intestinal health in nursery pigs. Anim. Nutr. 2022, 8, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Hao, H.; Liu, Q.; Li, J.; Yao, Y.; Liu, Y.; Zhang, T.; Zhang, Z.; Yi, H. Effect of extracellular vesicles derived from akkermansia muciniphila on intestinal barrier in colitis mice. Nutrients 2023, 15, 4722. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A.; Garaguso, I.; Britti, M.S.; Mengheri, E. Zinc oxide protects cultured enterocytes from the damage induced by escherichia coli. J. Nutr. 2003, 133, 4077–4082. [Google Scholar] [CrossRef]
- Castillo Zuniga, J.; Rueda, A.M.F.; Samuel, R.S.; St-Pierre, B.; Levesque, C.L. Impact of lactobacillus- and bifidobacterium-based direct-fed microbials on the performance, intestinal morphology, and fecal bacterial populations of nursery pigs. Microorganisms 2024, 12, 1786. [Google Scholar] [CrossRef]
- Rattigan, R.; Lawlor, P.G.; Cormican, P.; Crespo-Piazuelo, D.; Cullen, J.; Phelan, J.P.; Ranjitkar, S.; Crispie, F.; Gardiner, G.E. Maternal and/or post-weaning supplementation with bacillus altitudinis spores modulates the microbial composition of colostrum, digesta and faeces in pigs. Sci. Rep. 2023, 13, 8900. [Google Scholar] [CrossRef] [PubMed]
- Szkopek, D.; Mendel, M.; Kinsner, M.; Fotschki, B.; Juśkiewicz, J.; Kozłowski, K.; Matusevičius, P.; Konieczka, P. Interaction between peroxisome proliferator-activated receptors and cannabidiol in the gut of chickens applied to different challenge conditions. Int. J. Mol. Sci. 2024, 25, 11398. [Google Scholar] [CrossRef]
- López-Martínez, M.J.; Ornelas, M.A.S.; Amarie, R.E.; Manzanilla, E.G.; Martínez-Subiela, S.; Tecles, F.; Tvarijonaviciute, A.; Escribano, D.; González-Bulnes, A.; Cerón, J.J.; et al. Changes in salivary biomarkers of stress, inflammation, redox status, and muscle damage due to streptococcus suis infection in pigs. BMC Vet. Res. 2023, 19, 100. [Google Scholar] [CrossRef] [PubMed]
| Ingredients | Period 1 (d 1 to 14) | Period 2 (d 15 to 42) | Period 3 (d 43 to 70) |
|---|---|---|---|
| Corn | 62.12 | 68.10 | 68.10 |
| Extruded soybeans | 6.00 | 5.00 | 5.00 |
| Peeled soybean meal | 13.00 | 12.00 | 12.00 |
| Fermented soybean meal | 3.50 | 2.50 | 2.50 |
| Fish meal | 4.00 | 3.20 | 3.20 |
| Whey powder | 3.00 | 2.00 | 2.00 |
| Glucose | 4.00 | 3.00 | 3.00 |
| Dicalcium phosphate | 1.20 | 1.20 | 1.20 |
| Limestone | 0.70 | 0.60 | 0.60 |
| Salt | 0.35 | 0.35 | 0.35 |
| Soy oil | 1.00 | 1.00 | 1.00 |
| L-Lys HCI, 78% | 0.40 | 0.35 | 0.35 |
| DL-Methionine, 98% | 0.09 | 0.08 | 0.08 |
| L-Threonine, 98% | 0.10 | 0.09 | 0.09 |
| L-Tryptophan, 98% | 0.04 | 0.03 | 0.03 |
| Premix 1 | 0.50 | 0.50 | 0.50 |
| Nutrient levels 2 | |||
| Digestible energy, kcal/kg | 3410 | 3410 | 3410 |
| Crude protein, % | 18.70 | 17.20 | 17.20 |
| Total calcium, % | 0.71 | 0.63 | 0.63 |
| Total phosphorus, % | 0.55 | 0.52 | 0.52 |
| Lysine, % | 1.38 | 1.22 | 1.22 |
| Methionine, % | 0.41 | 0.37 | 0.37 |
| Threonine, % | 0.78 | 0.72 | 0.72 |
| Tryptophan, % | 0.24 | 0.22 | 0.22 |
| Items | CON 1 | ZnO | TBZC | SEM 2 | p-Value |
|---|---|---|---|---|---|
| 0 d BW, kg | 7.12 | 7.12 | 7.12 | 0.25 | 1.00 |
| 14 d BW, kg | 11.08 | 10.92 | 11.41 | 0.20 | 0.61 |
| 28 d BW, kg | 17.50 | 17.12 | 17.61 | 0.31 | 0.80 |
| 42 d BW, kg | 25.61 | 25.74 | 26.18 | 0.42 | 0.85 |
| 70 d BW, kg | 41.25 | 38.57 | 42.75 | 0.75 | 0.07 |
| Days 1 to 14 | |||||
| ADG, g | 269.82 | 271.69 | 305.36 | 13.22 | 0.50 |
| ADFI, g | 393.39 b | 390.89 b | 421.43 a | 18.25 | 0.02 |
| FCR, g/g | 1.46 | 1.44 | 1.38 | 0.02 | 0.27 |
| Diarrhea rate, % | 2.32 | 1.25 | 0.89 | 0.61 | 0.12 |
| Days 15 to 28 | |||||
| ADG, g | 456.87 | 431.53 | 442.3 | 14.77 | 0.80 |
| ADFI, g | 696.96 | 658.36 | 675.83 | 20.91 | 0.85 |
| FCR, g/g | 1.55 | 1.54 | 1.54 | 0.06 | 0.99 |
| Diarrhea rate, % | 0.60 | 0.20 | 0.19 | 0.20 | 0.44 |
| Days 29 to 42 | |||||
| ADG, g | 506.38 | 533.78 | 535.46 | 11.29 | 0.53 |
| ADFI, g | 1076.52 | 1022.68 | 1039.38 | 34.04 | 0.84 |
| FCR, g/g | 2.13 | 1.91 | 1.93 | 0.05 | 0.27 |
| Diarrhea rate, % | 0.99 | 0.20 | 0.39 | 0.34 | 0.19 |
| Days 43 to 70 | |||||
| ADG, g | 558.33 a | 458.16 a | 591.79 b | 14.83 | <0.01 |
| ADFI, g | 1248.16 | 1143.98 | 1244.69 | * | * |
| FCR, g/g | 2.23 | 2.49 | 2.10 | * | * |
| Items | CON 1 | ZnO | TBZC | SEM 2 | p-Value |
|---|---|---|---|---|---|
| ATTD of CP | |||||
| d 14 | 77.51 | 77.25 | 74.69 | 0.72 | 0.22 |
| d 42 | 72.66 | 72.67 | 73.39 | 0.92 | 0.94 |
| pH of stomach contents | 3.40 b | 4.56 a | 4.13 ab | 0.21 | 0.05 |
| Acid-binding capacity | 15.40 c | 19.36 a | 16.37 b | 0.44 | <0.01 |
| Items | CON 1 | ZnO | TBZC | SEM 2 | p-Value |
|---|---|---|---|---|---|
| Duodenum | |||||
| Villus height, μm | 331.04 | 424.81 | 543.03 | 41.13 | 0.13 |
| Crypt depth, μm | 355.25 | 436.83 | 539.37 | 38.17 | 0.16 |
| Villus height/crypt depth | 0.71 | 1.03 | 1.12 | 0.08 | 0.56 |
| Jejunum | |||||
| Villus height, μm | 389.24 | 473.70 | 540.92 | 32.75 | 0.12 |
| Crypt depth, μm | 286.97 | 353.07 | 295.84 | 10.68 | 0.41 |
| Villus height/crypt depth | 1.23 | 1.22 | 1.91 | 0.19 | 0.22 |
| Ileum | |||||
| Villus height, μm | 269.63 | 286.94 | 302.15 | 13.99 | 0.76 |
| Crypt depth, μm | 303.68 | 364.32 | 359.06 | 17.93 | 0.36 |
| Villus height/crypt depth | 0.95 | 0.88 | 0.94 | 0.08 | 0.92 |
| Items | CON 1 | ZnO | TBZC | SEM 2 | p-Value |
|---|---|---|---|---|---|
| d 14 | |||||
| MDA, nmol/mL | 3.82 a | 3.78 a | 3.06 b | 0.11 | <0.01 |
| SOD, U/mL | 53.76 b | 58.73 ab | 64.29 a | 1.50 | <0.01 |
| GSH-Px, U/mL | 111.62 b | 126.97 b | 146.88 a | 4.53 | <0.01 |
| CAT, U/mL | 34.17 b | 40.02 ab | 46.32 a | 1.65 | <0.01 |
| T-AOC, U/mL | 7.05 | 7.83 | 9.58 | 0.48 | 0.08 |
| d 28 | |||||
| MDA, nmol/mL | 3.75 a | 2.82 b | 2.77 b | 0.14 | <0.01 |
| SOD, U/mL | 68.39 b | 66.59 b | 77.70 a | 1.81 | 0.01 |
| GSH-Px, U/mL | 139.24 | 150.31 | 164.86 | 5.07 | 0.11 |
| CAT, U/mL | 43.50 | 44.51 | 48.88 | 1.11 | 0.10 |
| T-AOC, U/mL | 8.59 | 8.68 | 9.09 | 0.14 | 0.34 |
| d 42 | |||||
| MDA, nmol/mL | 4.02 a | 2.66 b | 2.39 b | 0.22 | <0.01 |
| SOD, U/mL | 74.29 b | 79.03 ab | 82.41 a | 1.31 | 0.02 |
| GSH-Px, U/mL | 153.18 | 163.06 | 178.46 | 5.92 | 0.22 |
| CAT, U/mL | 45.51 b | 48.78 ab | 54.24 a | 1.42 | 0.02 |
| T-AOC, U/mL | 9.15 | 9.19 | 9.58 | 0.19 | 0.63 |
| d 70 | |||||
| MDA, nmol/mL | 3.38 a | 2.48 b | 2.30 b | 0.15 | <0.01 |
| SOD, U/mL | 76.34 | 78.75 | 81.61 | 1.53 | 0.40 |
| GSH-Px, U/mL | 173.41 b | 177.67 b | 210.75 a | 5.66 | <0.01 |
| CAT, U/mL | 53.28 b | 57.02 ab | 66.39 a | 2.14 | 0.02 |
| T-AOC, U/mL | 9.27 | 9.66 | 10.36 | 0.22 | 0.14 |
| Items | CON 1 | ZnO | TBZC | SEM 2 | p-Value |
|---|---|---|---|---|---|
| d 14 | |||||
| IgA, g/L | 0.87 | 1.06 | 0.98 | 0.06 | 0.52 |
| IgM, g/L | 1.74 | 2.07 | 1.85 | 0.11 | 0.50 |
| IgG, g/L | 15.03 | 17.17 | 15.62 | 0.96 | 0.67 |
| d 28 | |||||
| IgA, g/L | 1.19 | 1.20 | 1.52 | 0.09 | 0.22 |
| IgM, g/L | 2.15 | 2.22 | 2.38 | 0.11 | 0.70 |
| IgG, g/L | 17.69 | 18.45 | 20.11 | 0.99 | 0.63 |
| d 42 | |||||
| IgA, g/L | 1.56 | 1.31 | 1.21 | 0.08 | 0.16 |
| IgM, g/L | 2.62 | 2.45 | 2.24 | 0.10 | 0.30 |
| IgG, g/L | 20.85 | 20.87 | 21.03 | 0.76 | 0.99 |
| d 70 | |||||
| IgA, g/L | 1.58 | 1.68 | 1.38 | 0.08 | 0.28 |
| IgM, g/L | 2.83 | 2.93 | 2.51 | 0.10 | 0.20 |
| IgG, g/L | 21.89 | 21.78 | 20.55 | 0.66 | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, S.; Fang, J.; Zhang, N.; Chen, Y.; Ma, Y.; Wang, C. Effects of Tetrabasic Zinc Chloride as Alternative to High Doses of Zinc Oxide on Growth Performance, Nutrient Digestibility, Intestinal Morphology, Immune Function, and Gut Microbiota in Weaned Piglets. Animals 2025, 15, 3071. https://doi.org/10.3390/ani15213071
Peng S, Fang J, Zhang N, Chen Y, Ma Y, Wang C. Effects of Tetrabasic Zinc Chloride as Alternative to High Doses of Zinc Oxide on Growth Performance, Nutrient Digestibility, Intestinal Morphology, Immune Function, and Gut Microbiota in Weaned Piglets. Animals. 2025; 15(21):3071. https://doi.org/10.3390/ani15213071
Chicago/Turabian StylePeng, Shuyu, Jingzi Fang, Nan Zhang, Yi Chen, Yongxi Ma, and Chunlin Wang. 2025. "Effects of Tetrabasic Zinc Chloride as Alternative to High Doses of Zinc Oxide on Growth Performance, Nutrient Digestibility, Intestinal Morphology, Immune Function, and Gut Microbiota in Weaned Piglets" Animals 15, no. 21: 3071. https://doi.org/10.3390/ani15213071
APA StylePeng, S., Fang, J., Zhang, N., Chen, Y., Ma, Y., & Wang, C. (2025). Effects of Tetrabasic Zinc Chloride as Alternative to High Doses of Zinc Oxide on Growth Performance, Nutrient Digestibility, Intestinal Morphology, Immune Function, and Gut Microbiota in Weaned Piglets. Animals, 15(21), 3071. https://doi.org/10.3390/ani15213071






