Effects of Echinacea Purpurea Polysaccharides on Growth Performance, Serum Biochemistry, and Intestinal Health of Immunosuppressed Broilers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Preparation of Polysaccharides
2.3. Animals and Groups
2.4. Experimental Diet Design
2.5. Growth Performance
2.6. Mahuang Chickens Sample Collection
2.7. Histopathological Staining
2.8. Real-Time Quantitative PCR Analysis
2.9. Antioxidant Kit Assay
2.10. Serum Biochemical Indicators
2.11. Statistical Analysis
3. Results
3.1. Effect of Echinacea Purpurea Polysaccharide on the Body Weight of Immunosuppressed Chickens
3.2. Effect of Echinacea Purpurea Polysaccharides on Organ Index in Immunosuppressed Chickens
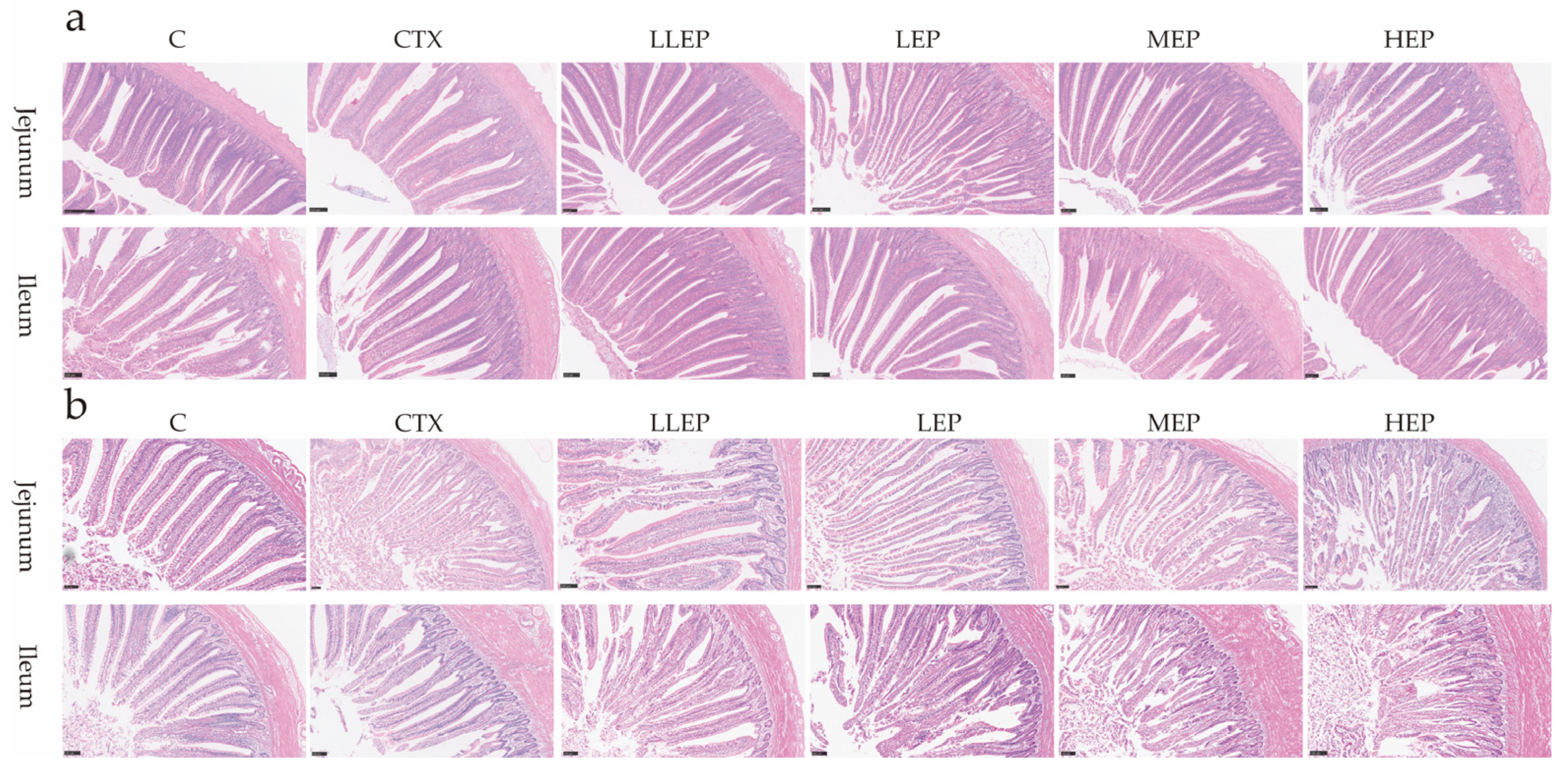
3.3. Effect of Immunosuppressed Chickens on Intestinal Morphology
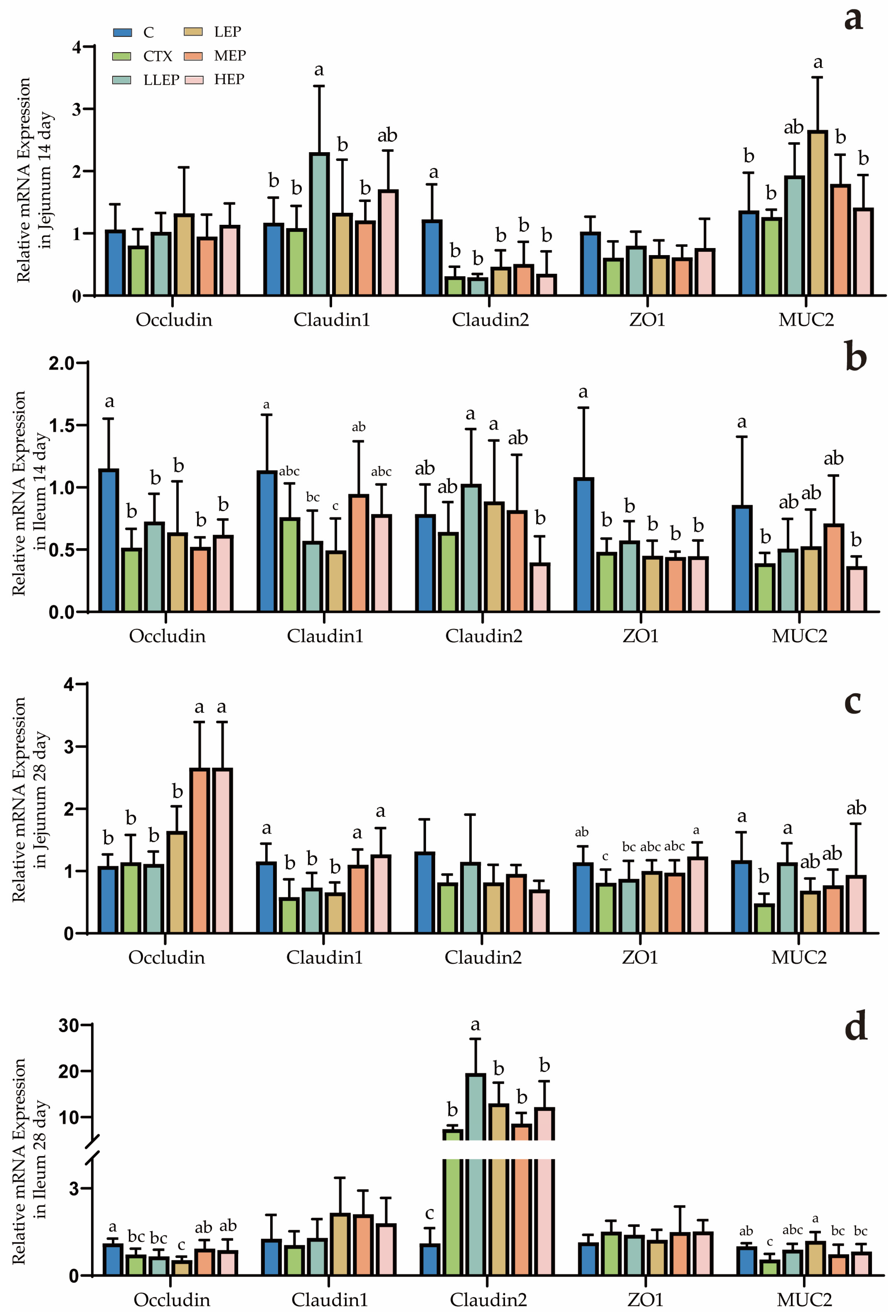
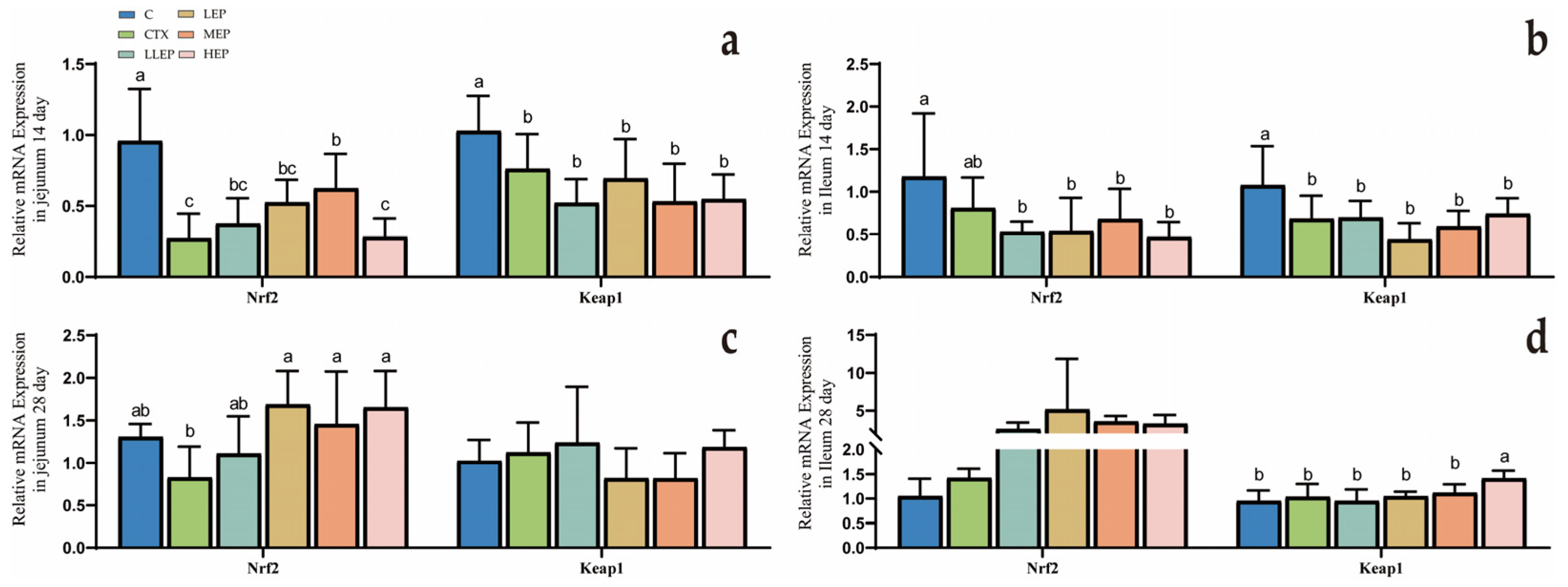
3.4. Effect of Echinacea Purpurea Polysaccharides on Gene Expression in Immunosuppressed Chickens
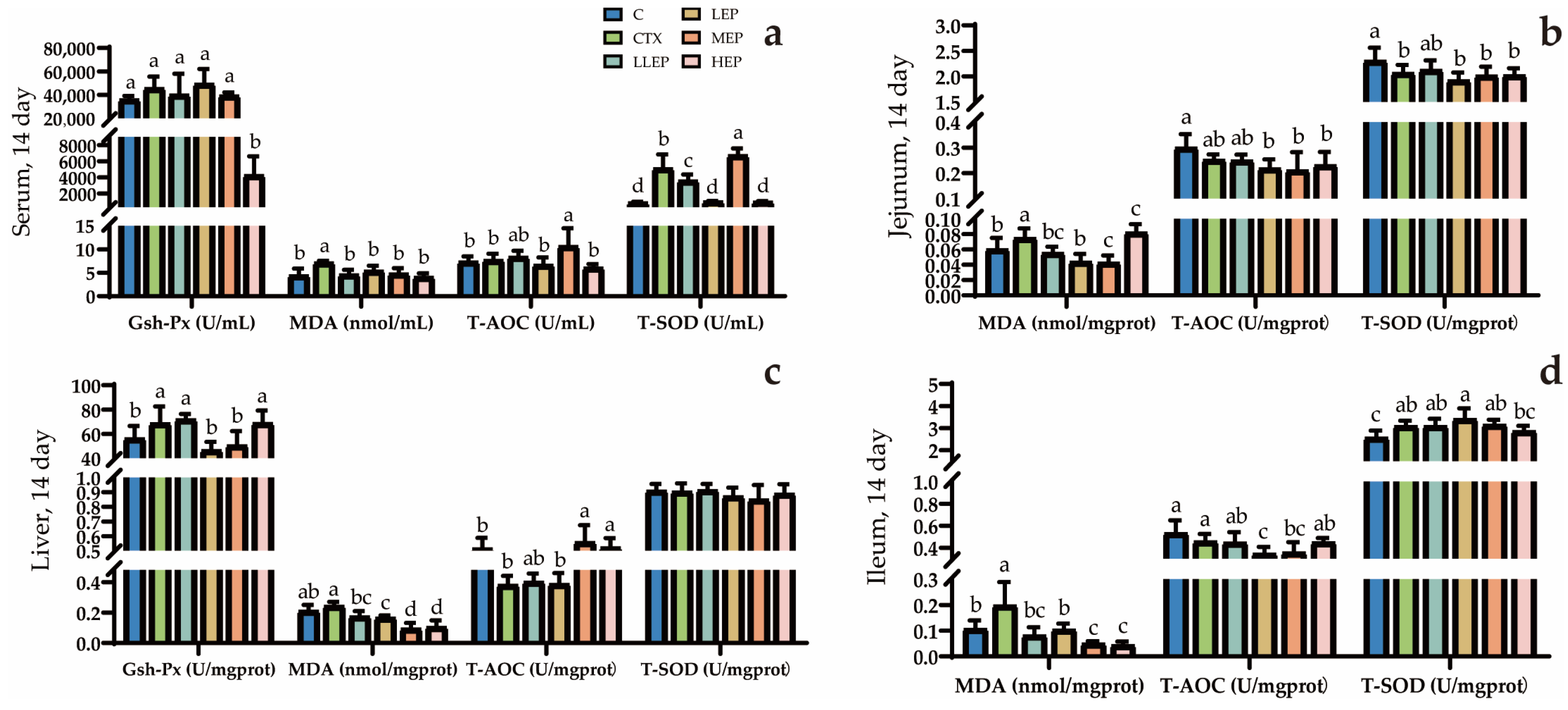
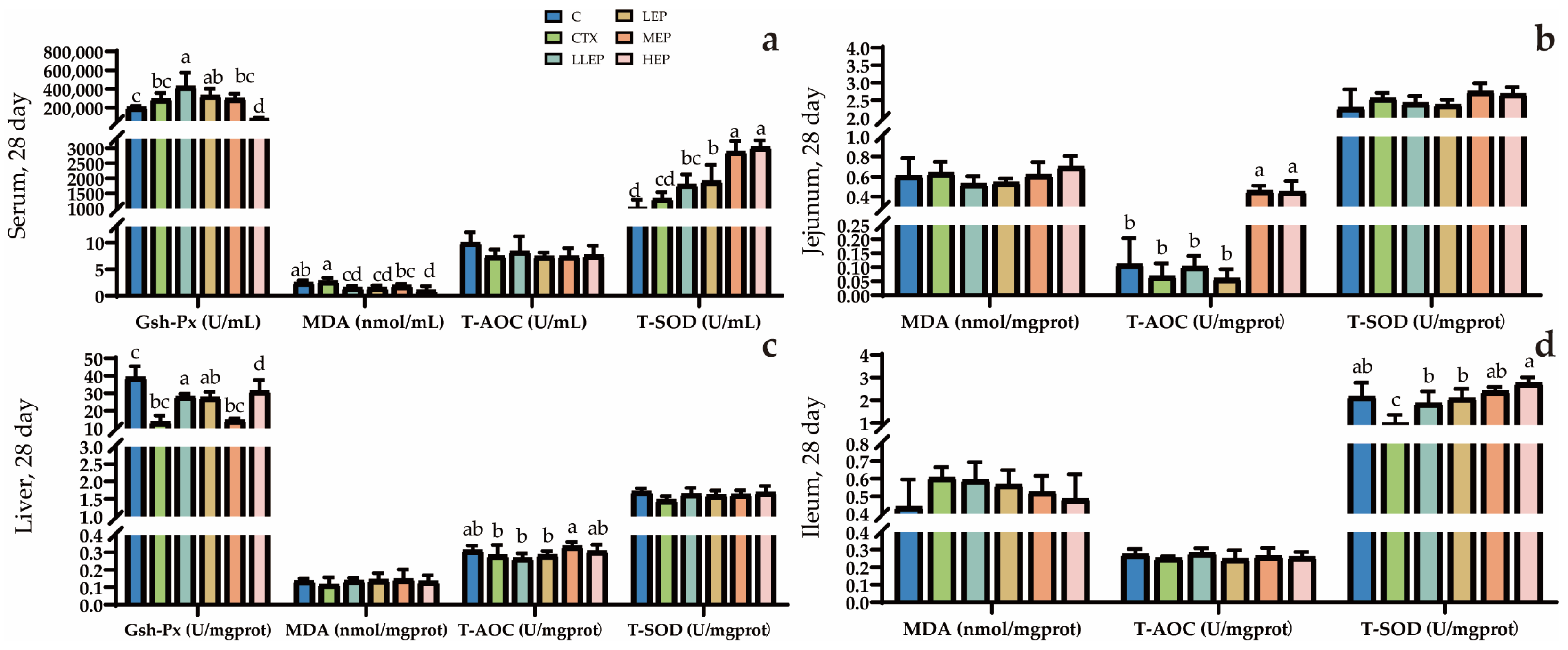
3.5. Effects of Antioxidants in Immunosuppressed Chickens
3.6. Effect of Immunosuppressed Chickens on Biochemical Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbol, B.I.; Alsayeqh, A.F. Use of polyphenols for the control of chicken meat borne zoonotic Campylobacter jejuni serotypes O1/44, O2, and O4 complex. Pak. Vet. J. 2024, 44, 978–987. [Google Scholar]
- Xi, Y.; Li, Y.; Ying, S.; Yan, J.; Shi, Z. Bacterial Lipopolysaccharide with Different Administration Routes Affects Intestinal Mucosal Morphological, Immunological, and Microbial Barrier Functions in Goslings. Poult. Sci. 2023, 102, 102599. [Google Scholar] [CrossRef]
- Hou, J.; Wu, P.; Cai, J.; Xia, B.; Lei, Y.; Huang, C.; Li, Y.; Tareen, M.I.; Tang, Z.; Zhang, H. Gut Microbiota Dysbiosis Amplifies Thiram Hepatotoxicity via a Mitochondrial-Autophagy-Apoptosis Nexus Orchestrated by the Gut-Liver Axis. Cell. Signal. 2025, 136, 112104. [Google Scholar] [CrossRef]
- Cui, Y.; Sun, W.; Li, Q.; Wang, K.; Wang, Y.; Lv, F.; Chen, X.; Peng, X.; Wang, Y.; Li, J.; et al. Effects of Caulis Spatholobi Polysaccharide on Immunity, Intestinal Mucosal Barrier Function, and Intestinal Microbiota in Cyclophosphamide-Induced Immunosuppressive Chickens. Front. Vet. Sci. 2022, 9, 833842. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Miao, F. Immunomodulatory and Antioxidant Effects of Hydroxytyrosol in Cyclophosphamide-Induced Immunosuppressed Broilers. Poult. Sci. 2021, 101, 101516. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhong, L.; Chang, X.; Li, Y.; Zhang, H.; Pan, J.; Tang, Z. Echinacea Enhances the Immune Function of Immunosuppressed Chicken by Regulating TLR4-NF-κB Pathway. Acta Vet. Zootech. Sin. 2022, 53, 2354–2363. [Google Scholar]
- Cheng, K.; Song, Z.H.; Zheng, X.C.; Zhang, H.; Zhang, J.F.; Zhang, L.L.; Zhou, Y.M.; Wang, T. Effects of Dietary Vitamin E Type on the Growth Performance and Antioxidant Capacity in Cyclophosphamide Immunosuppressed Broilers. Poult. Sci. 2017, 96, 1159–1166. [Google Scholar] [CrossRef]
- Ma, W.; Sun, H.; Lian, L.; Guo, L.; Wang, Y.; Huang, L. Immunomodulatory Effects of Lactiplantibacillus Plantarum CCFM8661 + Stachyose on Cyclophosphamide-Induced Immunosuppression Mice. Front. Immunol. 2025, 16, 1513531. [Google Scholar] [CrossRef]
- Demicheva, E.; Polanco Espino, F.J.; Vedeneev, P.; Shevyrin, V.; Buhler, A.; Mukhlynina, E.; Berdiugina, O.; Mondragon, A.D.C.; Cepeda Sáez, A.; Lopez-Santamarina, A.; et al. Comparative Study of Lipid Profile for Mice Treated with Cyclophosphamide by HPLC-HRMS and Bioinformatics. Metabolites 2025, 15, 60. [Google Scholar] [CrossRef]
- Seker, U.; Uyar, E.; Gokdemir, G.S.; Kavak, D.E.; Irtegun-Kandemir, S. The M1/M2 Macrophage Polarization and Hepatoprotective Activity of Quercetin in Cyclophosphamide—Induced Experimental Liver Toxicity. Vet. Med. Sci. 2025, 11, e70183. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, D.; Zhao, H.; Dou, L.; Chen, Q.; Cheng, Y.; Hu, B.; Tang, Y.; Duan, Y.; Guo, C.; et al. The Pasteurized Weissella Cibaria Alleviates Sepsis-Induced Acute Lung Injury by Modulation of Intestinal Mucus Barrier and Gut Microbiota. J. Transl. Med. 2025, 23, 661. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, X.; Pubu, P.; Ali, M.; Wang, J.; Xu, C.; Almutairi, M.H.; Li, K. Protective effect of lentinan against LPS-induced injury in mice via influencing antioxidant enzyme activity, inflammatory pathways, and gut microbiota. Pak. Vet. J. 2024, 44, 647–656. [Google Scholar]
- Szkopek, D.; Mendel, M.; Kinsner, M.; Ognik, K.; Szyryńska, N.; Lewczuk, B.; Kozłowski, K.; Kos, I.; Konieczka, P. Cannabidiol and Nano-Selenium Mediate Intestinal Barrier Function by Affecting Mucosal Microstructures, and Gut-Associated Immunological and Oxidative Stress Response in the Gut of Chickens Infected with C. Perfringens. Front. Immunol. 2025, 16, 1529449. [Google Scholar] [CrossRef]
- Ye, W.; Shi, H.; Qian, W.; Meng, L.; Wang, M.; Zhou, Y.; Wen, Z.; Han, M.; Peng, Y.; Li, H.; et al. Immunomodulatory Effects of a Prebiotic Formula with 2′-Fucosyllactose and Galacto- and Fructo-Oligosaccharides on Cyclophosphamide (CTX)-Induced Immunosuppressed BALB/c Mice via the Gut–Immune Axis. Nutrients 2024, 16, 3552. [Google Scholar] [CrossRef]
- Moita, V.H.C.; Kim, S.W. Nutritional and Functional Roles of Phytase and Xylanase Enhancing the Intestinal Health and Growth of Nursery Pigs and Broiler Chickens. Animals 2022, 12, 3322. [Google Scholar] [CrossRef]
- Li, Z.; Ahmed, I.; Chen, L.; Ali, S.; Fareed, S.K.; Riaz, R.; Yuan, H. Effect of Selenium Nanoparticles on Intestinal Immunity through Regulation of NLRP3 Signaling Pathway. Sci. Rep. 2025, 15, 19486. [Google Scholar] [CrossRef] [PubMed]
- Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Necrotic Enteritis in Broilers: An Updated Review on the Pathogenesis. Avian Pathol. 2011, 40, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.T.; Ciou, J.Y.; Chen, C.L.; Yu, B. Effect of Echinacea purpurea L. on Oxidative Status and Meat Quality in Arbor Acres Broilers. J. Sci. Food Agric. 2013, 93, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lin, R.; Chen, J.; Hussain, R.; Zhang, S.; Su, Y.; Chan, Y.; Ghaffar, A.; Shi, D. Integrated Gut Microbiota and Metabolomic Analysis Reveals Immunomodulatory Effects of Echinacea Extract and Astragalus Polysaccharides. Front. Vet. Sci. 2022, 9, 971058. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Y.; Zheng, X.; Zheng, X.; Yan, M.; Wang, H.; Liu, C. Echinacea purpurea (L.) Moench Polysaccharide Alleviates DSS-Induced Colitis in Rats by Restoring Th17/Treg Balance and Regulating Intestinal Flora. Foods 2023, 12, 4265. [Google Scholar] [CrossRef]
- Xu, T.; Hou, R.; Li, Q.; Sun, Z.; Kang, S.; Hao, Z. Isolation, Purification and Anti-inflammatory Effect of Echinacea Purpurea Polysaccharide. HeiLongJiang Anim. Sci. Vet. Med. 2019, 22, 133–136. [Google Scholar]
- Yao, L.; Bai, L.; Tan, Y.; Sun, J.; Shi, D.; Guo, S.; Liu, C. Study on Antagonistic Effects of Echinacea purpurea Polysaccharide and Sulfated Echinacea purpurea Polysaccharide on Immunosuppression of Cyclophosphamide in Chickens. China Anim. Husb. Vet. Med. 2019, 46, 3084–3094. [Google Scholar]
- Shi, Q.; Lang, W.; Wang, S.; Li, G.; Bai, X.; Yan, X.; Zhang, H. Echinacea Polysaccharide Attenuates Lipopolysaccharide-Induced Acute Kidney Injury via Inhibiting Inflammation, Oxidative Stress and the MAPK Signaling Pathway. Int. J. Mol. Med. 2021, 47, 243–255. [Google Scholar] [CrossRef]
- Yao, L.; Bai, L.; Tan, Y.; Sun, J.; Shi, D.; Guo, S.; Liu, C. Effect of Svlfated Echinacea Polysaccharide on Dendritic Cells Derived From Bone Marrow in Chicken. HeiLongJiang Anim. Sci. Vet. Med. 2020, 17–22, 26. [Google Scholar]
- Wang, X.; Ding, Y.; Zhang, X.; Feng, Y.; Li, C.; Ge, Y.; Yang, Y.; Su, J.; Chu, X. The Effects of Degraded Polysaccharides from Acanthopanax Senticosus on Growth, Antioxidant and Immune Effects in Broiler Chicks Based on Intestinal Flora. Poult. Sci. 2025, 104, 104933. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Li, Y.; Liu, W.; Li, C.; Zhang, W.; Deng, Z.; Shen, S.; Li, J. Comparison of flavor substances and taste of different varieties of Guangxi yellow-feathered broilers and AA broilers. Feed. Res. 2024, 47, 105–110. [Google Scholar]
- Xiao, L.; Qi, L.; Fu, R.; Nie, Q.; Zhang, X.; Wen, L. A large-scale comparison of the meat quality characteristics of different chicken breeds in South China. Poult. Sci. 2024, 103, 103740. [Google Scholar] [CrossRef]
- Ye, M.; Jiang, W.; Xu, Y.; Chen, J.; He, S.; Luo, W.; Zhang, X.; Zeng, X.; Luo, Q. Comparison and correlation analysis of body size traitsand slaughter performance of two pure strains of mahuang chickens. China Poult. 2023, 45, 107–111. [Google Scholar]
- Liu, C.; Chen, J.; Li, E.; Fan, Q.; Wang, D.; Li, P.; Li, X.; Chen, X.; Qiu, S.; Gao, Z.; et al. The Comparison of Antioxidative and Hepatoprotective Activities of Codonopsis pilosula Polysaccharide (CP) and Sulfated CP. Int. Immunopharmacol. 2015, 24, 299–305. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, L.; Li, J.; Xie, Z.; Li, Y.; Si, H. Selenium Broussonetia Papyrifera Polysaccharide Alleviated Cyclophosphamide-Induced Immune Suppression, Growth Inhibition, Intestinal Damage, and Gut Microbiota Disorder in Yellow-Feather Broilers. Poult. Sci. 2025, 104, 104907. [Google Scholar] [CrossRef]
- NY/T33-2004; Feeding Standard of Chicken. Standards Press of China: Beijing, China, 2004.
- Wei, F.-H.; Xie, W.-Y.; Zhao, P.-S.; Gao, W.; Gao, F. Echinacea purpurea Polysaccharide Ameliorates Dextran Sulfate Sodium-Induced Colitis by Restoring the Intestinal Microbiota and Inhibiting the TLR4-NF-κB Axis. Nutrients 2024, 16, 1305. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Pan, S.; He, J.; Li, B.; Cao, N.; Fu, X.; Liu, W.; Huang, Y.; Tian, Y.; Xu, D.; et al. Protective Effects of Polysaccharide of Atractylodes Macrocephala Koidz and Jiawei Si-Jun-Zi Decoction on Gut Health and Immune Function in Cyclophosphamide-Treated Chicks. Poult. Sci. 2025, 104, 105160. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, J.; Wang, Y.; Yang, H.; Cao, L.; Gan, S.; Ma, J.; Liu, H. Immuno-Stimulatory Activity of Astragalus Polysaccharides in Cyclophosphamide-Induced Immunosuppressed Mice by Regulating Gut Microbiota. Int. J. Biol. Macromol. 2023, 242, 124789. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liswaniso, S.; Qin, N.; Cao, S.; Wu, X.; Ma, C.; Yan, C.; Xu, R.; Sun, X. Effects of a Novel Synbiotics-Enzyme Complex as a Replacement for Antibiotics on Growth Performance, Slaughter and Meat Characteristics, Immune Organ Index, and Intestinal Morphology of Broilers. Front. Vet. Sci. 2024, 11, 1468847. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Xue, M.; Tang, Y.; Zhang, L.; Hu, P.; Hu, Y.; Cai, D.; Luo, X.; Sun, M. Effects of Zinc Source and Level on the Intestinal Immunity of Xueshan Chickens under Heat Stress. Animals 2023, 13, 3025. [Google Scholar] [CrossRef]
- Zeyneb, H.; Song, Y.; Wang, L.; Zheng, J.; Wang, W.; Pei, H.; Cao, X. Preventive Effect of Quinoa Polysaccharides on Lipopolysaccharide-Induced Inflammation in Mice through Gut Microbiota Regulation. Int. J. Biol. Macromol. 2025, 307, 141899. [Google Scholar] [CrossRef]
- Hou, X.; Sang, Y.; Dong, L. The Improved Effect and Its Mechanism of Phytic Acid on DSS-Induced UC Mice. Life Sci. 2022, 311, 121139. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Xu, W.; Zhao, J.; Liu, K.; Liu, X.; Li, Y.; Tang, Z.; Li, A.; Zhang, H. Chondroitin Sulfate Reverses Tibial Dyschondroplasia, Broiler Chondrocyte Proliferation and Differentiation Dysfunction via the CHST11/β-Catenin Pathway. Int. J. Biol. Macromol. 2025, 315, 144488. [Google Scholar] [CrossRef]
- Jin, X.; Wu, Z.; Chen, H.; Liu, W.; Gu, F.; Li, J. Extraction and Identification of Polysaccharide from Lentinus Edodes and Its Effect on Immunosuppression and Intestinal Barrier Injury Induced by Cyclophosphamide. Int. J. Mol. Sci. 2024, 25, 12432. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, Z.; Huo, Y.; Zhang, H.; Li, T.; Wang, P.; Han, W. CMTM7 Inhibits TLR4 Signaling Pathway via Promoting Rab5 Activation and Alleviates Acute Liver Injury. Cell. Mol. Life Sci. 2025, 82, 229. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, X.; Yu, Y.; Yang, L.; OuYang, J.; Bian, Y.; Liu, Y.; Li, G. Puerarin Alleviates Oxidized Oil-Induced Oxidative Injury and Inflammation via Inhibition of the Nrf2/Keap1 and HMGB1/TLR4/MAPK Signaling Pathways: An Investigation in a Chicken Model. Mol. Nutr. Food Res. 2023, 67, 2200663. [Google Scholar] [CrossRef]
- Kaseda, S.; Horizono, J.; Sannomiya, Y.; Kuwazuru, J.; Suico, M.A.; Sato, R.; Fukiya, H.; Sunamoto, H.; Ogi, S.; Matsushita, T.; et al. Efficacy of Nrf2 Activation in a Proteinuric Alport Syndrome Mouse Model. Life Sci. Alliance 2025, 8, e202503330. [Google Scholar] [CrossRef]
- Rojo, A.I.; Buttari, B.; Cadenas, S.; Carlos, A.R.; Cuadrado, A.; Falcão, A.S.; López, M.G.; Georgiev, M.I.; Grochot-Przeczek, A.; Gumeni, S.; et al. Model Organisms for Investigating the Functional Involvement of NRF2 in Non-Communicable Diseases. Redox Biol. 2024, 79, 103464. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Yang, W.; Huang, G.; Huang, H. The Antioxidant Activities of Balsam Pear Polysaccharide. Int. J. Biol. Macromol. 2020, 142, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Wang, T.; Ou, Y.; Qiu, X.; Li, M.; Zhang, Y.; Lu, S.; Xu, L.; Guan, L.; Yao, H. Structural Characterization and Protective Effects of Camellia Oleifera Fruit Shell Polysaccharides against DSS-Induced Ulcerative Colitis in Mice. J. Agric. Food Chem. 2025, 73, 16860–16878. [Google Scholar] [CrossRef]
- Peng, S.; Cui, Y.; Yu, M.; Song, M.; Tian, Z.; Deng, D.; Liu, Z.; Ma, X. Effect of Fermented Mulberry Leaves on Gut Health of Finishing Pigs. Animals 2024, 14, 2911. [Google Scholar] [CrossRef]
- Ogunro, O.B.; Ofeniforo, B.E. Fertility Protective Effects of Brillantaisia Patula Leaf Extract against Cyclophosphamide-Induced Ovarian Damage in Wistar Rats. BMC Biotechnol. 2024, 24, 88. [Google Scholar] [CrossRef]
- Li, J.; Zhao, G.; Liu, J.; Hu, X.; Yu, W.; Wang, J.; Zhong, S.; Zhu, W.; Yang, T.; Zhou, Y.; et al. Effect of Continuous Lipopolysaccharide Induction on Oxidative Stress and Heart Injury in Weaned Piglets. Vet. Sci. 2025, 12, 330. [Google Scholar] [CrossRef]
- Alharbi, N.; Nour, O.; Makled, M.N.; Nader, M. Agmatine Abrogates Tacrolimus-Induced Testicular Injury in Rats. Pharmaceutics 2025, 17, 703. [Google Scholar] [CrossRef]
- Rofaeil, R.R.; Sharata, E.E.; Attya, M.E.; Abo-Youssef, A.M.; Hemeida, R.A.M.; Khalaf, M.M. Repurposing Levomilnacipran to Attenuate Premature Ovarian Insufficiency Induced by Cyclophosphamide in Female Wistar Albino Rats through Modulation of TLR4/P38-MAPK/NF-κB P65, Caspase-3-Driven Apoptosis, and Klotho Protein Expression. Food Chem. Toxicol. 2025, 200, 115406. [Google Scholar] [CrossRef]
- Tian, B.; Wang, P.; Xu, T.; Cai, M.; Mao, R.; Huang, L.; Sun, P.; Yang, K. Ameliorating Effects of Hericium Erinaceus Polysaccharides on Intestinal Barrier Injury in Immunocompromised Mice Induced by Cyclophosphamide. Food Funct. 2023, 14, 2921–2932. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, S.; Shen, D.; Han, G.; Li, Y.; Dou, X.; Li, C. Effects of Dietary Different Whole-Plant Corn Silage Levels on Growth Performance, Slaughter Performance, Meat Quality and Serum Biochemical Parameters of Geese. Acta Vet. Zootech. Sin. 2021, 52, 3501–3511. [Google Scholar]
- Wu, Z.; Zeng, L.; Li, Y.; Zhong, Z.; Lin, J.; Tan, W. Effects of Dietary Broussonetia papyrifera Twig Leaves on Growth Performance, Slaughter Performance and Serum Biochemical Indexes of Magang Goose. China Poult. 2021, 43, 44–49. [Google Scholar]

| Item 1 | C | CTX | LLEP | LEP | MEP | HEP | p-Value |
|---|---|---|---|---|---|---|---|
| ADG | |||||||
| 7 days old | 6.27 ± 0.24 | 6.37 ± 0.54 | 6.14 ± 0.28 | 6.74 ± 0.28 | 6.40 ± 0.15 | 6.52 ± 0.84 | 0.308 |
| 14 days old | 10.16 ± 0.19 a | 7.44 ± 0.45 b | 7.29 ± 1.66 b | 6.98 ± 0.76 b | 7.31 ± 0.35 a | 7.70 ± 0.29 b | <0.001 |
| 21 days old | 11.57 ± 0.60 a | 9.45 ± 1.07 b | 10.48 ± 1.45 ab | 11.21 ± 0.46 a | 11.46 ± 0.62 a | 11.41 ± 0.72 a | 0.002 |
| 28 days old | 14.64 ± 0.40 | 12.49 ± 1.13 | 13.56 ± 1.75 | 13.10 ± 1.83 | 13.57 ± 2.07 | 14.00 ± 0.98 | 0.221 |
| ADFI | |||||||
| 7 days old | 7.65 ± 1.04 | 9.50 ± 3.06 | 9.10 ± 1.53 | 7.35 ± 1.81 | 7.30 ± 1.86 | 7.85 ± 1.15 | 0.212 |
| 14 days old | 15.60 ± 0.11 ab | 16.15 ± 4.66 a | 15.40 ± 2.41 ab | 12.05 ± 0.60 c | 12.80 ± 1.10 bc | 13.40 ± 0.22 abc | 0.012 |
| 21 days old | 24.35 ± 0.05 c | 28.50 ± 3.72 a | 27.45 ± 2.46 ab | 24.45 ± 1.48 c | 25.55 ± 0.93 bc | 24.90 ± 1.75 bc | 0.006 |
| 28 days old | 31.05 ± 0.27 ab | 34.15 ± 3.56 a | 33.35 ± 1.91 ab | 29.60 ± 1.31 b | 31.25 ± 0.60 bc | 29.05 ± 5.09 bc | 0.018 |
| F/G | |||||||
| 7 days old | 1.23 ± 0.17 | 1.48 ± 0.47 | 1.48 ± 0.26 | 1.10 ± 0.28 | 1.14 ± 0.30 | 1.21 ± 0.20 | 0.118 |
| 14 days old | 1.54 ± 0.03 b | 2.18 ± 0.67 a | 2.19 ± 0.52 a | 1.75 ± 0.20 ab | 1.76 ± 0.21 ab | 1.75 ± 0.07 ab | 0.021 |
| 21 days old | 2.11 ± 0.11 c | 3.04 ± 0.42 a | 2.67 ± 0.47 b | 2.118 ± 0.08 c | 2.24 ± 0.14 c | 2.19 ± 0.18 c | <0.001 |
| 28 days old | 2.12 ± 0.05 bc | 2.75 ± 0.27 a | 2.49 ± 0.33 b | 2.30 ± 0.36 bc | 2.35 ± 0.39 bc | 2.08 ± 0.36 c | 0.010 |
| Item | C | CTX | LLEP | LEP | MEP | HEP | p-Value |
|---|---|---|---|---|---|---|---|
| 14 days old | |||||||
| Splenic index | 0.10 ± 0.02 a | 0.07 ± 0.03 bc | 0.06 ± 0.02 c | 0.07 ± 0.02 abc | 0.08 ± 0.02 abc | 0.09 ± 0.01 ab | 0.026 |
| Thymic index | 0.34 ± 0.08 a | 0.08 ± 0.05 b | 0.15 ± 0.09 b | 0.12 ± 0.04 b | 0.14 ± 0.08 b | 0.08 ± 0.02 b | <0.001 |
| Bursal index | 0.38 ± 0.06 a | 0.17 ± 0.09 c | 0.22 ± 0.10 bc | 0.20 ± 0.06 bc | 0.20 ± 0.11 bc | 0.31 ± 0.14 ab | 0.006 |
| 28 days old | |||||||
| Splenic index | 0.11 ± 0.03 | 0.12 ± 0.05 | 0.12 ± 0.03 | 0.11 ± 0.02 | 0.10 ± 0.04 | 0.10 ± 0.04 | 0.995 |
| Thymic index | 0.39 ± 0.20 a | 0.14 ± 0.07 b | 0.11 ± 0.08 b | 0.14 ± 0.06 b | 0.14 ± 0.09 b | 0.12 ± 0.05 b | 0.002 |
| Bursal index | 0.44 ± 0.11 | 0.57 ± 0.20 | 0.57 ± 0.18 | 0.59 ± 0.12 | 0.68 ± 0.12 | 0.61 ± 0.24 | 0.283 |
| Item 1 | C | CTX | LLEP | LEP | MEP | HEP | p-Value |
|---|---|---|---|---|---|---|---|
| 14 days old | |||||||
| Jejunum | |||||||
| VH (μm) | 748.68 ± 40.02 a | 688.53 ± 17.68 b | 807.76 ± 31.48 ab | 737.43 ± 51.16 ab | 804.29 ± 56.62 a | 731.86 ± 36.35 ab | 0.028 |
| CD (μm) | 146.35 ± 16.81 b | 158.08 ± 7.05 ab | 164.81 ± 3.64 a | 152.33 ± 4.82 ab | 155.76 ± 3.57 ab | 127.86 ± 6.59 c | 0.003 |
| VH/CD | 5.58 ± 1.47 | 4.50 ± 0.72 | 4.91 ± 0.33 | 4.84 ± 0.71 | 5.17 ± 0.72 | 5.89 ± 1.16 | 0.137 |
| Ileum | |||||||
| VH (μm) | 620.40 ± 51.88 b | 600.23 ± 28.19 b | 657.30 ± 57.85 ab | 701.22 ± 6.67 a | 637.78 ± 28.85 b | 642.53 ± 16.29 ab | 0.037 |
| CD (μm) | 154.23 ± 23.60 | 162.40 ± 10.20 | 163.01 ± 8.54 | 163.04 ± 5.29 | 164.57 ± 7.53 | 158.37 ± 2.54 | 0.885 |
| VH/CD | 4.29 ± 1.03 | 3.72 ± 0.40 | 4.03 ± 0.38 | 4.26 ± 0.36 | 3.89 ± 0.37 | 4.06 ± 0.24 | 0.422 |
| 28 days old | |||||||
| Jejunum | |||||||
| VH (μm) | 756.77 ± 37.11 c | 711.08 ± 8.29 c | 902.78 ± 6.23 b | 974.20 ± 1.51 a | 990.53 ± 12.78 a | 891.96 ± 23.39 b | <0.001 |
| CD (μm) | 129.47 ± 15.70 b | 125.92 ± 19.58 b | 126.50 ± 8.37 b | 143.67 ± 16.78 ab | 143.01 ± 7.82 ab | 183.17 ± 9.46 a | 0.016 |
| VH/CD | 4.79 ± 1.17 | 3.63 ± 0.62 | 4.98 ± 1.11 | 5.08 ± 2.09 | 4.09 ± 0.24 | 4.38 ± 0.15 | 0.205 |
| Ileum | |||||||
| VH (μm) | 650.19 ± 20.99 bc | 614.84 ± 26.55 c | 756.11 ± 57.57 a | 727.05 ± 16.00 ab | 649.37 ± 14.25 bc | 657.99 ± 19.07 bc | 0.017 |
| CD (μm) | 139.95 ± 4.93 | 173.38 ± 10.87 | 152.28 ± 5.11 | 149.16 ± 10.10 | 156.83 ± 5.13 | 151.82 ± 6.55 | 0.086 |
| VH/CD | 5.83 ± 1.04 | 5.99 ± 1.50 | 7.23 ± 1.04 | 6.99 ± 1.08 | 7.61 ± 2.00 | 5.95 ± 2.12 | 0.213 |
| Item 1 | C | CTX | LLEP | LEP | MEP | HEP | p-Value |
|---|---|---|---|---|---|---|---|
| 14 days old | |||||||
| ALB | 9.93 ± 0.97 b | 10.62 ± 1.07 b | 11.32 ± 0.91 ab | 11.28 ± 1.33 ab | 12.30 ± 1.88 a | 10.66 ± 1.48 b | 0.037 |
| TP | 24.70 ± 2.48 | 23.38 ± 2.92 | 26.50 ± 1.98 | 25.93 ± 2.93 | 27.83 ± 3.59 | 24.81 ± 2.99 | 0.107 |
| GLB | 14.75 ± 1.75 | 13.33 ± 1.67 | 15.17 ± 1.17 | 14.75 ± 1.75 | 15.57 ± 1.90 | 14.25 ± 1.58 | 0.227 |
| 28 days old | |||||||
| ALB | 8.58 ± 0.86 b | 8.05 ± 1.80 b | 10.55 ± 1.87 a | 10.67 ± 1.77 a | 10.83 ± 0.59 a | 9.15 ± 0.66 ab | 0.012 |
| TP | 21.85 ± 2.24 bc | 20.80 ± 4.09 c | 26.82 ± 3.760 ab | 26.60 ± 4.31 ab | 28.00 ± 2.75 a | 26.12 ± 4.82 ab | 0.033 |
| GLB | 13.33 ± 1.86 | 12.75 ± 2.36 | 16.33 ± 2.07 | 15.83 ± 2.93 | 17.25 ± 2.87 | 16.17 ± 4.58 | 0.157 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Peng, S.; Jung, H.; She, P.; Li, W.; Xiao, Y.; Shan, A.; Huang, X.; Shi, D. Effects of Echinacea Purpurea Polysaccharides on Growth Performance, Serum Biochemistry, and Intestinal Health of Immunosuppressed Broilers. Animals 2025, 15, 3036. https://doi.org/10.3390/ani15203036
Zhang Z, Peng S, Jung H, She P, Li W, Xiao Y, Shan A, Huang X, Shi D. Effects of Echinacea Purpurea Polysaccharides on Growth Performance, Serum Biochemistry, and Intestinal Health of Immunosuppressed Broilers. Animals. 2025; 15(20):3036. https://doi.org/10.3390/ani15203036
Chicago/Turabian StyleZhang, Zhiying, Su Peng, Hyerin Jung, Peining She, Wanqi Li, Yang Xiao, Aiting Shan, Xiaojie Huang, and Dayou Shi. 2025. "Effects of Echinacea Purpurea Polysaccharides on Growth Performance, Serum Biochemistry, and Intestinal Health of Immunosuppressed Broilers" Animals 15, no. 20: 3036. https://doi.org/10.3390/ani15203036
APA StyleZhang, Z., Peng, S., Jung, H., She, P., Li, W., Xiao, Y., Shan, A., Huang, X., & Shi, D. (2025). Effects of Echinacea Purpurea Polysaccharides on Growth Performance, Serum Biochemistry, and Intestinal Health of Immunosuppressed Broilers. Animals, 15(20), 3036. https://doi.org/10.3390/ani15203036







