Behavioral Plasticity of Rewilding Milu in Mountainous Region of Northern China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
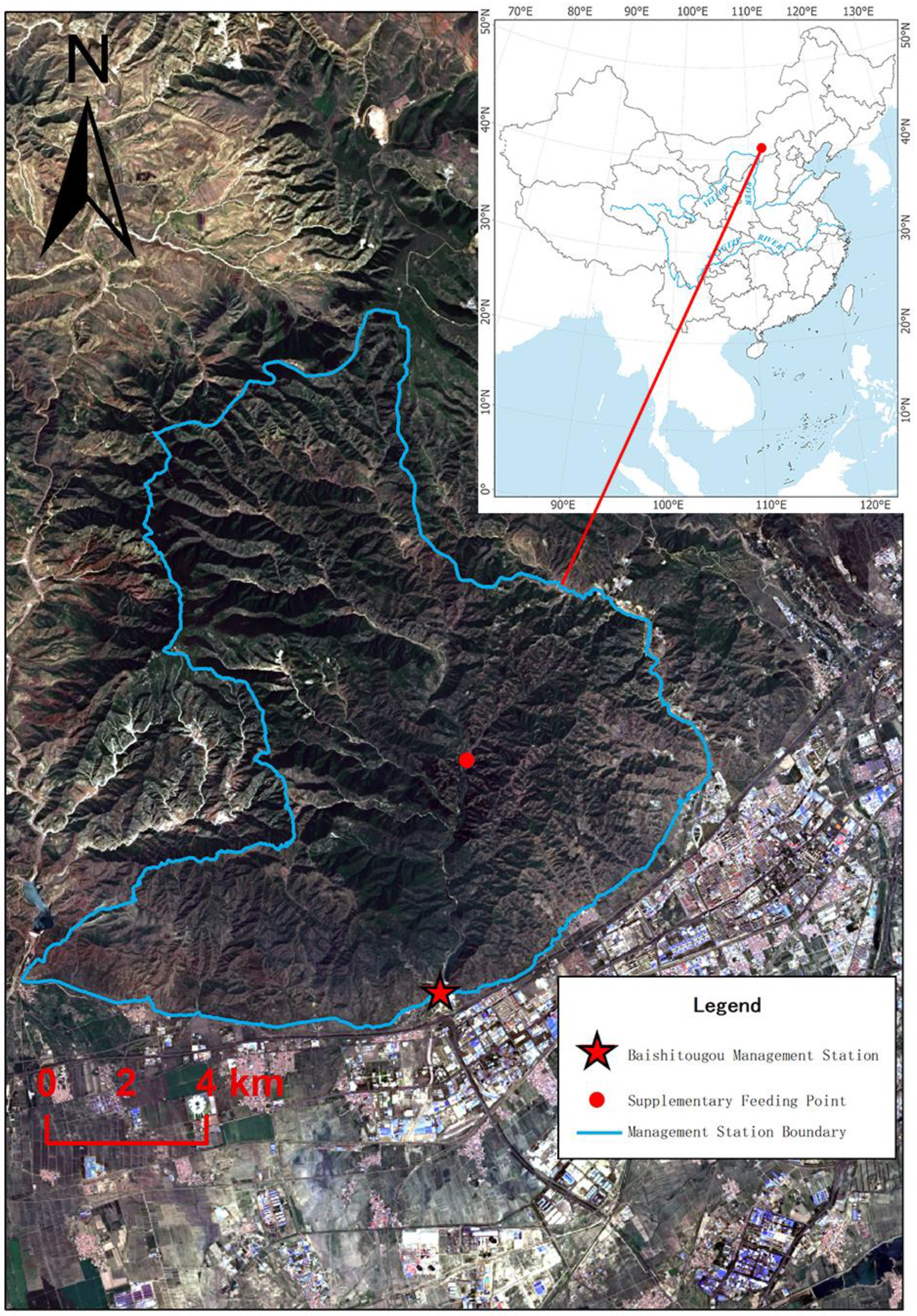
2.1. Study Area
2.2. Animals and Data Collection
2.3. Data Analysis
3. Results
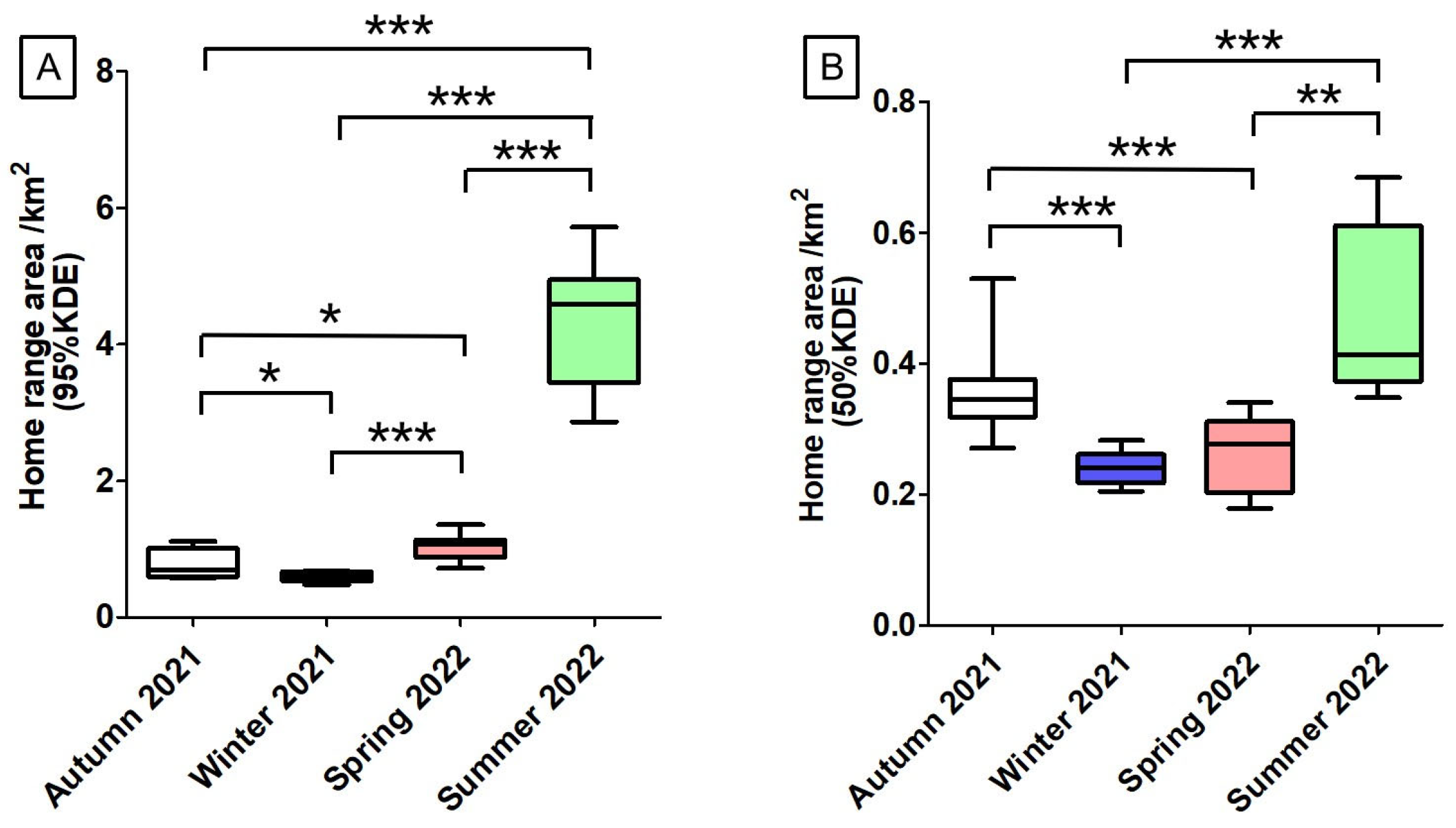
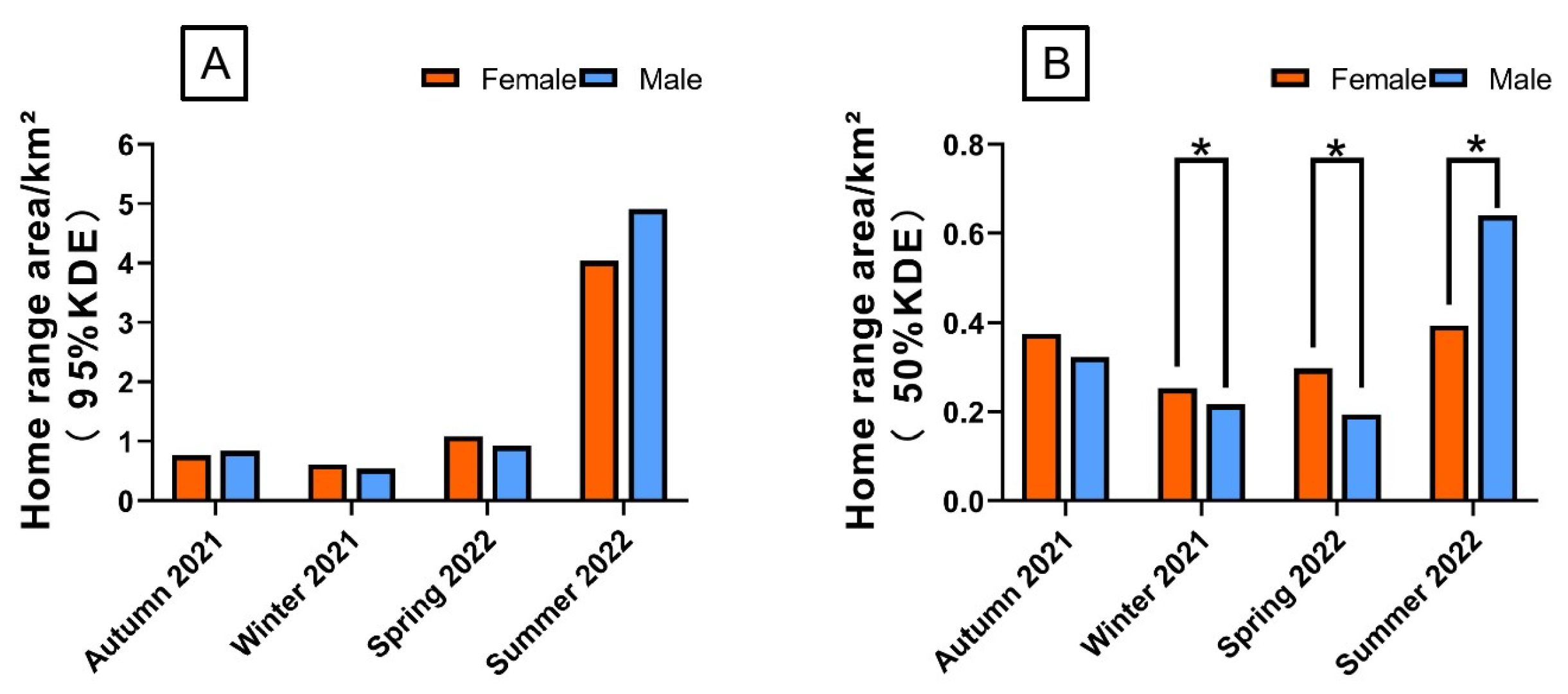
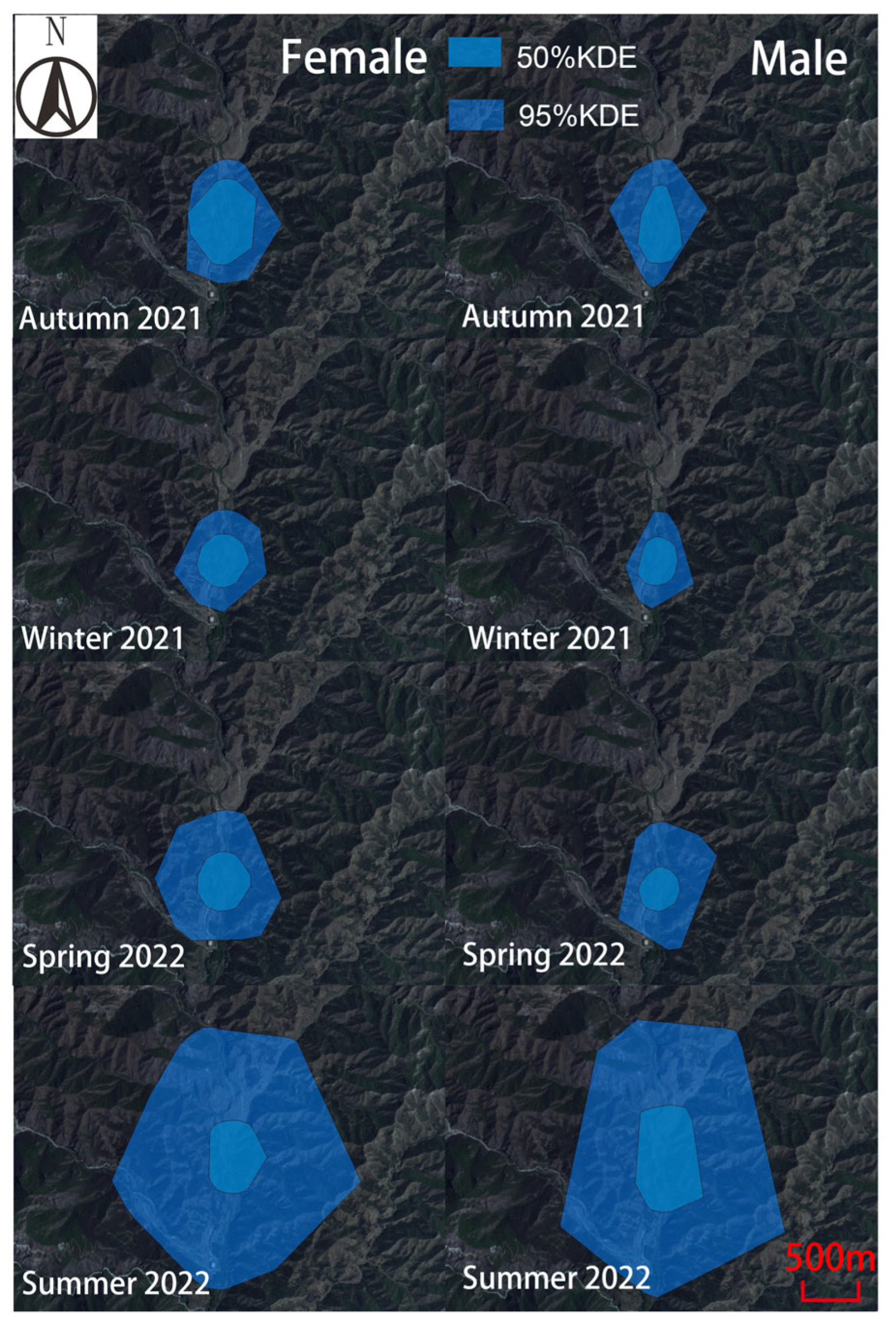
3.1. Home Range Area Analysis
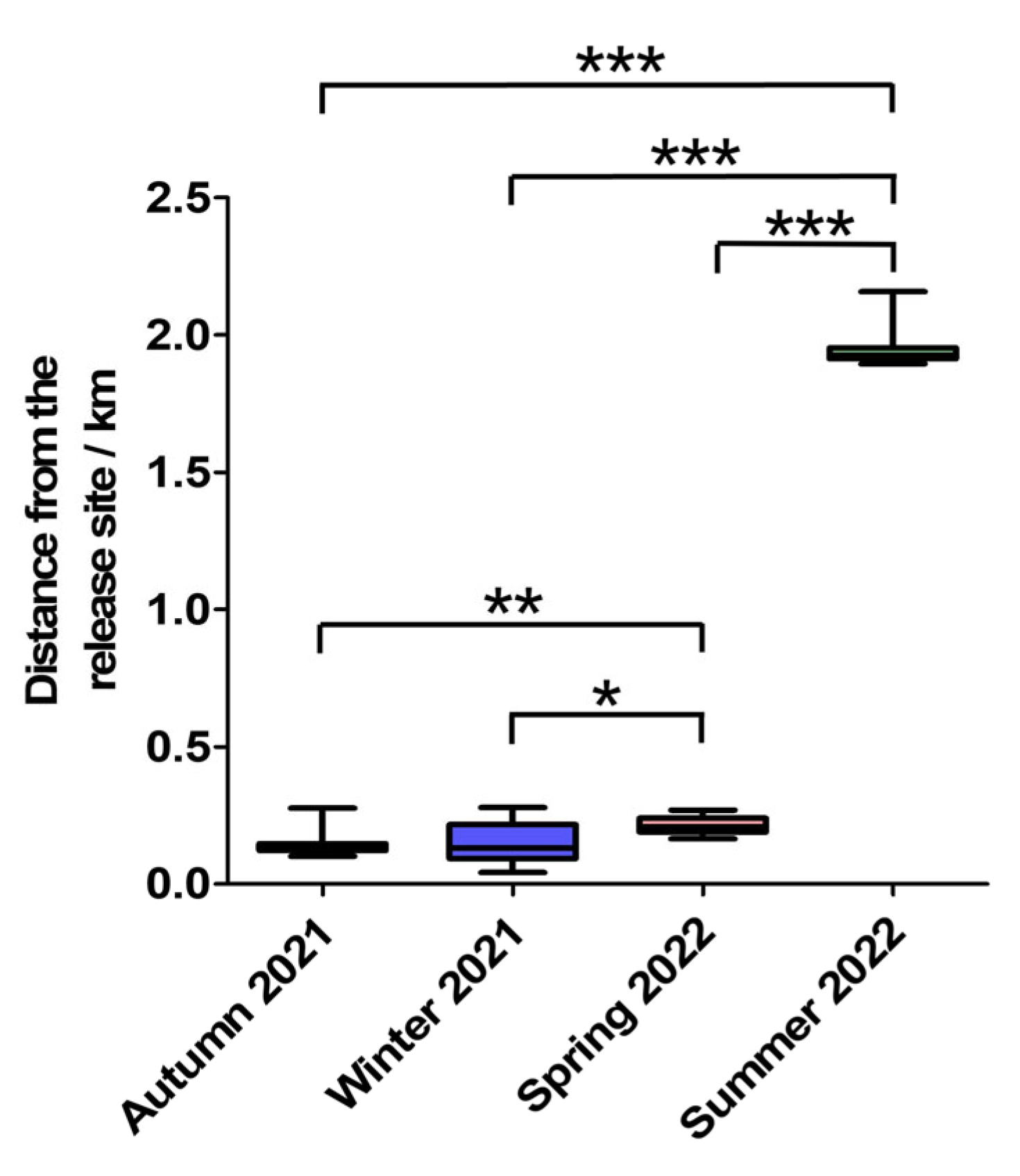
3.2. Movement Distance Analysis
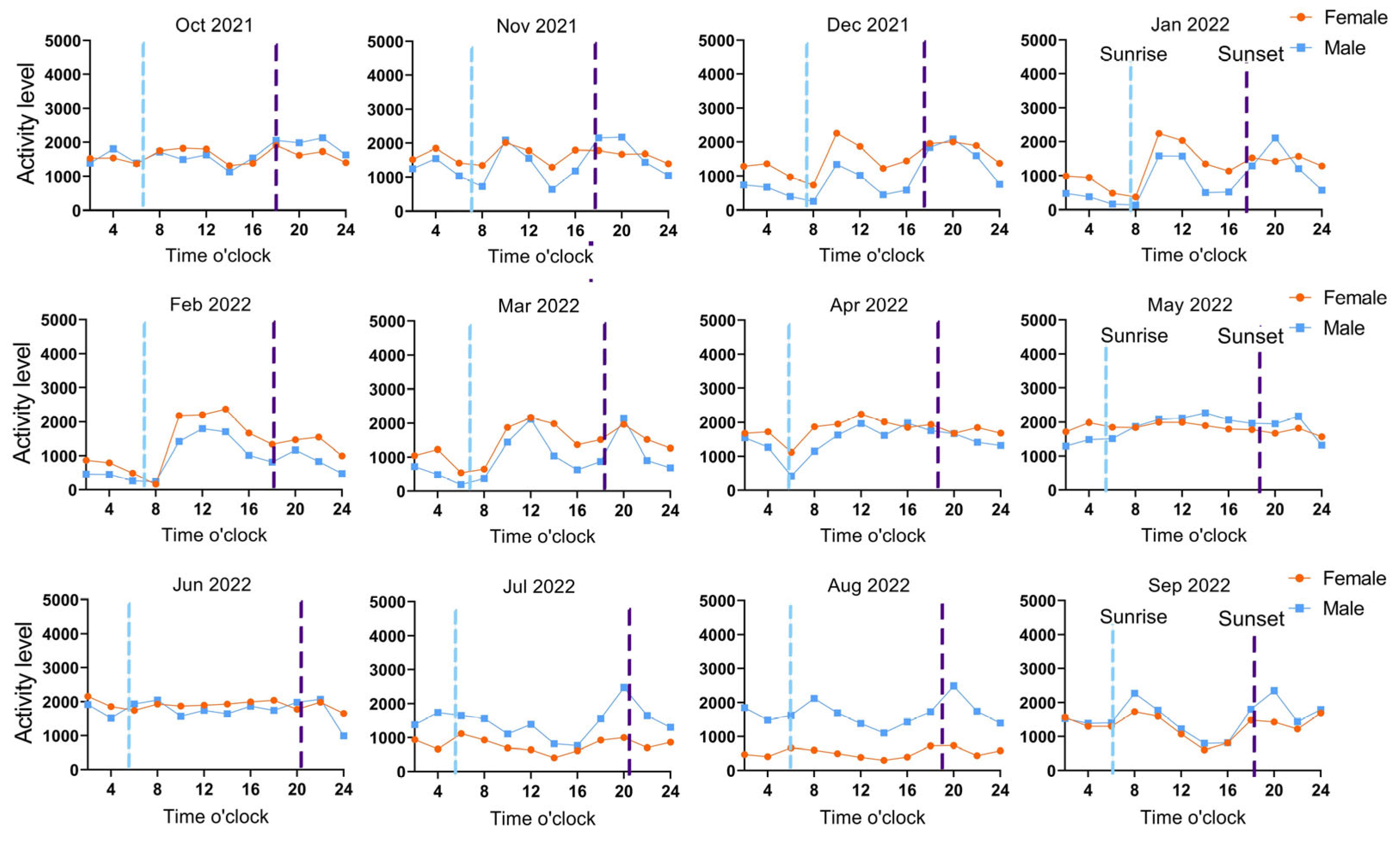
3.3. Activity Rhythms Analysis
4. Discussion
4.1. Seasonal Variation in Home Range Patterns of the Rewilding Milu
4.2. Plasticity in Movement Characteristics of the Rewilding Milu
4.3. Crepuscular Peaks and Seasonal Plasticity in Diel Activity Rhythms of the Rewilding Milu
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chapple, D.G.; Naimo, A.C.; Brand, J.; Michelangeli, M.; Martin, J.M.; Goulet, C.T.; Brunton, D.H.; Sih, A.; Wong, B.B.M.A. Biological Invasions as a Selective Filter Driving Behavioral Divergence. Nat. Commun. 2022, 13, 33755. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.C.; Beirne, C.; Gaynor, K.M.; Sun, C.; Granados, A.; Allen, M.L.; Alston, J.M.; Alvarenga, G.C.; Álvarez Calderón, F.S.; Amir, Z.; et al. Mammal Responses to Global Changes in Human Activity Vary by Trophic Group and Landscape. Nat. Ecol. Evol. 2024, 8, 924–935. [Google Scholar] [CrossRef]
- Palagi, E.; Bergman, T.J. Bridging Captive and Wild Studies: Behavioral Plasticity and Social Complexity in Theropithecus gelada. Animals 2021, 11, 3003. [Google Scholar] [CrossRef]
- Devarajan, K.; Fidino, M.; Farris, Z.J.; Adalsteinsson, S.A.; Andrade-Ponce, G.; Angstmann, J.L.; Anthonysamy, W.; Aquino, J.; Asefa, A.; Avila, B.; et al. When the Wild Things Are: Defining Mammalian Diel Activity and Plasticity. Sci. Adv. 2025, 11, eado3843. [Google Scholar] [CrossRef]
- Riddell, E.A.; Odom, J.P.; Damm, J.D.; Sears, M.W. Plasticity Reveals Hidden Resistance to Extinction under Climate Change in the Global Hotspot of Salamander Diversity. Sci. Adv. 2018, 4, eaar5471. [Google Scholar] [CrossRef]
- Charline, C.; Dany, G.; Maxime, A.; Clermont, J.; Réale, D. Behavioral Variation in Natural Contests: Integrating Plasticity and Personality. Behav. Ecol. 2021, 32, 277–285. [Google Scholar] [CrossRef]
- Finand, B.; Loeuille, N.; Bocquet, C.; Fédérici, P.; Ledamoisel, J.; Monnin, T. Habitat Fragmentation through Urbanization Selects for Low Dispersal in an Ant Species. Oikos 2024, 2024, e10325. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Zhao, H.; Zhao, Q.; Deng, J.; Kong, F.; Jiang, W.; Zhang, H.; Liu, H.; Kouba, A. Abiotic and Biotic Influences on the Movement of Reintroduced Chinese Giant Salamanders (Andrias davidianus) in Two Montane Rivers. Animals 2021, 11, 1480. [Google Scholar] [CrossRef]
- Xu, W.; Barker, K.; Shawler, A.; Van Scoyoc, A.; Smith, J.A.; Mueller, T.; Sawyer, H.; Andreozzi, C.; Bidder, O.R.; Karandikar, H.; et al. The plasticity of ungulate migration in a changing world. Ecology 2021, 102, e03293. [Google Scholar] [CrossRef] [PubMed]
- Wolff, C.L.; Demarais, S.; Brooks, C.P.; Barton, B.T. Behavioral Plasticity Mitigates the Effect of Warming on White-Tailed Deer. Ecol. Evol. 2020, 10, 2579–2587. [Google Scholar] [CrossRef]
- Poole, K.G.; Lamb, C.T.; Medcalf, S.; Amos, L. Migration, movements, and survival in a partially migratory elk (Cervus canadensis) population. Conserv. Sci. Pract. 2024, 6, e13128. [Google Scholar] [CrossRef]
- Olson, E.R.; Van Deelen, T.R. Competition and sex-age class alter the effects of group size on vigilance in white-tailed deer Odocoileus virginianus. Acta Ethol. 2024, 27, 39–50. [Google Scholar] [CrossRef]
- Pouchet, C.; Fernandez-Prada, C.; Dussault, C.; Leclerc, M.; Tremblay, J.; Cóté, S.D. Linking weather conditions and winter tick abundance in moose. J. Wildl. Manag. 2024, 88, e22551. [Google Scholar] [CrossRef]
- Hernández-Carrasco, D.; Tylianakis, J.M.; Lytle, D.A.; Tonkin, J.D. Ecological and Evolutionary Consequences of Changing Seasonality. Science 2025, 388, eads4880. [Google Scholar] [CrossRef]
- Geremia, C.; Hamilton, E.W.; Merkle, J.A. Yellowstone’s free-moving large bison herds provide a glimpse of their past ecosystem function. Science 2025, 389, 904–908. [Google Scholar] [CrossRef]
- Seddon, P.J.; Griffiths, C.J.; Soorae, P.S.; Armstrong, D.P. Reversing Defaunation: Restoring Species in a Changing World. Science 2014, 345, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Cartledge, E.L.; Bellis, J.; White, I.; Hurst, J.L.; Stockley, P.; Dalrymple, S. Current and Future Climate Suitability for the Hazel Dormouse in the UK and the Impact on Reintroduced Populations. Conserv. Sci. Pract. 2024, 6, e13254. [Google Scholar] [CrossRef]
- Shaw, R.E.; Farquharson, K.A.; Bruford, M.W.; Coates, D.J.; Elliott, C.P.; Mergeay, J.; Ottewell, K.M.; Segelbacher, G.; Hoban, S.; Hvilsom, C.; et al. Global Meta-Analysis Shows Action Is Needed to Halt Genetic Diversity Loss. Nature 2025, 638, 704–710. [Google Scholar] [CrossRef]
- Banes, G.L.; Galdikas, B.M.F.; Vigilant, L. Reintroduction of Confiscated and Displaced Mammals Risks Outbreeding and Introgression in Natural Populations, as Evidenced by Orang-Utans of Divergent Subspecies. Sci. Rep. 2016, 6, 22026. [Google Scholar] [CrossRef] [PubMed]
- Molloy, S.W.; Burbidge, A.H.; Comer, S.; Davis, R.A. Using Climate Change Models to Inform the Recovery of the Western Ground Parrot Pezoporus flaviventris. Oryx 2020, 54, 52–61. [Google Scholar] [CrossRef]
- Warne, R.K.; Chaber, A. Assessing Disease Risks in Wildlife Translocation Projects: A Comprehensive Review of Disease Incidents. Animals 2023, 13, 3379. [Google Scholar] [CrossRef] [PubMed]
- Mesochina, P.; Bedin, E.; Ostrowski, S. Reintroducing Antelopes into Arid Areas: Lessons Learnt from the Oryx in Saudi Arabia. C. R. Biol. 2003, 326 (Suppl. 1), S158–S165. [Google Scholar] [CrossRef]
- Jung, T.S.; Larter, N.C.; Lewis, C.J.; Thacker, C.; Taylor, S.D. Wolf (Canis lupus) Predation and Scavenging of Reintroduced Bison (Bison bison): A Hallmark of Ecological Restoration to Boreal Food Webs. Eur. J. Wildl. Res. 2023, 69, 16. [Google Scholar] [CrossRef]
- Thomas-Walters, L.; McCallum, J.; Montgomery, R.; Petros, C.; Wan, A.K.Y.; Veríssimo, D. Systematic Review of Conservation Interventions to Promote Voluntary Behavior Change. Conserv. Biol. 2022, 37, e14000. [Google Scholar] [CrossRef]
- Canessa, S.; Ottonello, D.; Rosa, G.; Salvidio, S.; Grasselli, E.; Oneto, F. Adaptive Management of Species Recovery Programs: A Real-World Application for an Endangered Amphibian. Biol. Conserv. 2019, 236, 202–210. [Google Scholar] [CrossRef]
- Frietsch, M.; Loos, J.; Löhr, K.; Sieber, S.; Fischer, J. Future-Proofing Ecosystem Restoration through Enhancing Adaptive Capacity. Commun. Biol. 2023, 6, 4736. [Google Scholar] [CrossRef] [PubMed]
- Muths, E.; Dreitz, V. Monitoring Programs to Assess Reintroduction Efforts: A Critical Component in Recovery. Anim. Biodivers. Conserv. 2008, 31, 47–56. [Google Scholar] [CrossRef]
- Ohtaishi, N.; Gao, Y. A review of the distribution of all the species of deer (Tragulidae, Moschidae and Cervidae) in China. Mammal Rev. 1990, 20, 125–144. [Google Scholar] [CrossRef]
- Cheng, Z.; Tian, X.; Zhong, Z.; Li, P.; Sun, D.; Bai, J.; Meng, Y.; Zhang, S.; Zhang, Y.; Wang, L.; et al. Reintroduction, Distribution, Population Dynamics and Conservation of a Species Formerly Extinct in the Wild: A Review of Thirty-Five Years of Successful Milu (Elaphurus davidianus) Reintroduction in China. Glob. Ecol. Conserv. 2021, 31, e01860. [Google Scholar] [CrossRef]
- Jiang, Z.; Harris, R.B. Elaphurus davidianus. The IUCN Red List of Threatened Species; IUCN Red List: Gland, Switzerland, 2016; p. e.T7121A22159785. [Google Scholar] [CrossRef]
- Ding, J.; Chang, Q.; Ding, Y.; Zhu, L.; Liu, H.; Jiang, Z.; Li, C. Seasonal Home Range Patterns of the Reintroduced and Rewild Female Père David’s Deer Elaphurus davidianus. Biol. Rhythm Res. 2017, 48, 485–497. [Google Scholar] [CrossRef]
- Yang, D.; Jiang, Z.; Ma, J.; Hu, H.; Li, P. Causes of Endangerment or Extinction of Some Mammals and Its Relevance to the Reintroduction of Père David’s Deer in the Dongting Lake Drainage Area. Biodivers. Sci. 2005, 13, 451–461. Available online: https://www.biodiversity-science.net/CN/Y2005/V13/I5/451 (accessed on 3 April 2025). (In Chinese). [CrossRef]
- Li, Y.; Wang, H.; Jiang, Z.; Song, Y.; Yang, D.; Li, L. Seasonal Differences of the Milu’s Home Range at the Early Rewilding Stage in Dongting Lake Area, China. Glob. Ecol. Conserv. 2022, 35, e02057. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Xu, Z.; Li, L.; Wu, L.; Duan, C.; Peng, J. Behavioural Rhythms during the Adaptive Phase of Introduced Milu/Père David’s Deer (Elaphurus davidianus) in the Dongting Lake Wetland, China. Pak. J. Zool. 2017, 49, 1657–1664. [Google Scholar] [CrossRef]
- Beck, B.B.; Wemmer, C.M. The Biology and Management of an Extinct Species: Père David’s Deer; Noyes Publications: Park Ridge, NJ, USA, 1983; pp. 1–193. Available online: https://lccn.loc.gov/83002364 (accessed on 4 April 2025).
- Cao, K.; Qiu, L.; Chen, B.; Miu, B. Chinese Milu Deer; Xue Lin Press: Shanghai, China, 1990; pp. 24–48. (In Chinese) [Google Scholar]
- Stiegler, J.; Gallagher, C.A.; Hering, R.; Müller, T.; Tucker, M.; Apollonio, M.; Arnold, J.; Barker, N.A.; Barthel, L.; Bassano, B.; et al. Mammals Show Faster Recovery from Capture and Tagging in Human-Disturbed Landscapes. Nat. Commun. 2024, 15, 52381. [Google Scholar] [CrossRef]
- Sarout, B.N.M.; Waterhouse, A.; Duthie, C.A.; Poli, C.H.E.C.; Haskell, M.J.; Berger, A.; Umstatter, C. Assessment of Circadian Rhythm of Activity Combined with Random Regression Model as a Novel Approach to Monitoring Sheep in an Extensive System. Appl. Anim. Behav. Sci. 2018, 207, 26–38. [Google Scholar] [CrossRef]
- Berger-Tal, O.; Saltz, D. Using the movement patterns of reintroduced animals to improve reintroduction success. Curr. Zool. 2014, 60, 515–526. [Google Scholar] [CrossRef]
- Carranza, J.; Mattioli, S.; Putman, R.; Pérez-Barbería, F.J.; Gordon, I.J. Red Deer Cervus elaphus Linnaeus, 1758. In Deer of the World: Ecology, Conservation and Management; Melletti, M., Focardi, S., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 187–208. [Google Scholar] [CrossRef]
- Stopher, K.V.; Nussey, D.H.; Clutton-Brock, T.H.; Guinness, F.; Morris, A.; Pemberton, J.M. The red deer rut revisited: Female excursions but no evidence females move to mate with preferred males. Behav. Ecol. 2011, 22, 808–818. [Google Scholar] [CrossRef]
- Kropil, R.; Smolko, P.; Garaj, P. Home range and migration patterns of male red deer Cervus elaphus in Western Carpathians. Eur. J. Wildl. Res. 2015, 61, 63–72. [Google Scholar] [CrossRef]
- Tierson, W.C.; Mattfeld, G.F.; Sage, R.W., Jr.; Behrend, D.F. Seasonal Movements and Home Ranges of White-Tailed Deer in the Adirondacks. J. Wildl. Manag. 1985, 49, 760–769. [Google Scholar] [CrossRef]
- de la Peña, E.; Pérez-González, J.; Martín, J.; Vedel, G.; Carranza, J. The dark-ventral-patch of male red deer, a sexual signal that conveys the degree of involvement in rutting behavior. BMC Zool. 2021, 6, 18. [Google Scholar] [CrossRef]
- Focardi, S.; Pecchioli, E. Social cohesion and foraging decrease with group size in fallow deer (Dama dama). Behav. Ecol. Sociobiol. 2005, 59, 84–91. [Google Scholar] [CrossRef]
- Takii, A.; Izumiyama, S.; Mochizuki, T.; Okumura, T.; Sato, S. Seasonal Migration of Sika Deer in the Oku-Chichibu Mountains, Central Japan. Mamm. Study 2012, 37, 127–137. [Google Scholar] [CrossRef]
- McGreer, M.T.; Mallon, E.E.; Vander Vennen, L.M.; Wiebe, P.A.; Baker, J.A.; Brown, G.S.; Avgar, T.; Hagens, J.; Kittle, A.M.; Mosser, A.; et al. Selection for forage and avoidance of risk by woodland caribou (Rangifer tarandus caribou) at coarse and local scales. Ecosphere 2016, 6, art288. [Google Scholar] [CrossRef]
- Walter, W.D.; Evans, T.S.; Stainbrook, D.; Wallingford, B.D.; Rosenberry, C.S.; Diefenbach, D.R. Heterogeneity of a landscape influences size of home range in a North American cervid. Sci. Rep. 2018, 8, 14667. [Google Scholar] [CrossRef]
- Dussault, C.; Courtois, R.; Ouellet, J.-P.; Girard, I. Space use of moose in relation to food availability. Can. J. Zool. 2005, 83, 1431–1437. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Xu, Z.; Chen, J.; Yang, G.; Wang, S.; Jiang, K. Home Range Characteristics of the Released Female Milu (Père David’s Deer, Elaphurus davidianus) Population during Different Periods and Effects of Water Submersion in Dongting Lake, China. Pak. J. Zool. 2022, 54, 1133–1142. [Google Scholar] [CrossRef]
- Saldo, E.A.; Jensen, A.J.; Muthersbaugh, M.S.; Ruth, C.; Cantrell, J.; Butfiloski, J.W.; Yarrow, G.K.; Kilgo, J.C.; Jachowski, D.S. Unintended Consequences of Wildlife Feeders on Spatiotemporal Activity of White—Tailed Deer, Coyotes, and Wild Pigs. J. Wildl. Manag. 2024, e22644. [Google Scholar] [CrossRef]
- Borowik, T.; Kowalczyk, R.; Maślanko, W.; Duda, N.; Ratkiewicz, M. Annual Movement Strategy Predicts Within-Season Space Use by Moose. Behav. Ecol. Sociobiol. 2021, 75, 119. [Google Scholar] [CrossRef]
- Ma, Y.; Bao, H.; Bencini, R.; Raubenheimer, D.; Dou, H.; Liu, H.; Wang, S.; Jiang, G. Macro-Nutritional Adaptive Strategies of Moose (Alces alces) Related to Population Density. Animals 2019, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Banjade, M.; Ahn, J.; Song, D.; Son, J.; Park, Y. Seasonal Variations and Sexual Differences in Home Range Sizes and Activity Patterns of Endangered Long-Tailed Gorals in South Korea. Animals 2025, 15, 27. [Google Scholar] [CrossRef]
- Calenge, C.; Maillard, D.; Invernia, N.; Gaudin, J.-C. Reintroduction of roe deer Capreolus capreolus into a Mediterranean habitat: Female mortality and dispersion. Wildl. Biol. 2005, 11, 153–161. [Google Scholar] [CrossRef]
- Resop, J.P.; Hendrix, C.; Wynn-Thompson, T.; Hession, W.C. Channel Morphology Change after Restoration: Drone Laser Scanning versus Traditional Surveying Techniques. Hydrology 2024, 11, 54. [Google Scholar] [CrossRef]
- Song, Y.; Yang, D.; Zou, S.; Li, P.; Zhang, H.; Wen, H.; Jiang, Z. Sex-biased dispersal in naturally re-wild Milu in the Dongting Lake Region, China. Acta Ecol. Sin. 2015, 35, 4416–4424. (In Chinese) [Google Scholar] [CrossRef]
- Smith, J.B.; Keiter, D.A.; Sweeney, S.J.; Miller, R.S.; Schlichting, P.E.; Beasley, J.C. Habitat quality influences trade-offs in animal movement along the exploration–exploitation continuum. Sci. Rep. 2023, 13, 4814. [Google Scholar] [CrossRef]
- Frair, J.L.; Merrill, E.H.; Allen, J.R.; Boyce, M.S. Know thy enemy: Experience affects elk translocation success in risky landscapes. J. Wildl. Manag. 2007, 71, 541–554. [Google Scholar] [CrossRef]
- Standen, M.P.; Ditmer, M.A.; Stoner, D.C.; Hersey, K.R.; Carter, N.H. Data from: Differential Effects of Environmental Predictability on Ungulate Movement Behavior in Disparate Ecosystems [Dataset]. Dryad Digital Repository. 2025. Available online: https://datadryad.org/dataset/doi:10.5061/dryad.gtht76hxg (accessed on 4 April 2025).
- Coulon, A.; Cosson, J.F.; Morellet, N.; Angibault, J.M.; Cargnelutti, B.; Galan, M.; Aulagnier, S.; Hewison, A.J.M. Dispersal is Not Female Biased in a Resource-Defence Mating Ungulate, the European Roe Deer. Proc. R. Soc. B Biol. Sci. 2005, 273, 341–348. [Google Scholar] [CrossRef]
- Mccance, E.C.; Campbell, M.M.; Baydack, R.K. Identifying How Human Behavior Influences Urban White-Tailed Deer Movement Patterns in a Canadian Metropolitan Area. Hum. Dimens. Wildl. 2015, 20, 471–483. [Google Scholar] [CrossRef]
- Killeen, J.; Thurfjell, H.; Ciuti, S.; Paton, D.; Musiani, M.; Boyce, M.S. Habitat Selection During Ungulate Dispersal and Exploratory Movement at Broad and Fine Scale with Implications for Conservation Management. Mov. Ecol. 2014, 2, 13. [Google Scholar] [CrossRef]
- La Morgia, V.; Malenotti, E.; Badino, G.; Bona, F. Where do we go from here? Dispersal simulations shed light on the role of landscape structure in determining animal redistribution after reintroduction. Landsc. Ecol. 2011, 26, 969–981. [Google Scholar] [CrossRef]
- Cueva-Hurtado, L.; Jara-Guerrero, A.; Cisneros, R.; Espinosa, C.I. Activity patterns of the white-tailed deer (Odocoileus virginianus) in a neotropical dry forest: Changes according to age, sex, and climatic season. Therya 2024, 15, 242–252. [Google Scholar] [CrossRef]
- Thel, L.; Garel, M.; Marchand, P.; Bourgoin, G.; Loison, A. Too hot or too disturbed? Temperatures more than hikers affect circadian activity of females in northern chamois. Anim. Behav. 2024, 210, 347–367. [Google Scholar] [CrossRef]
- Silva, I.I.S. Activity Patterns of Red and Roe Deer: Differences Between Sexes. Master’s Thesis, Universidade de Coimbra, Coimbra, Portugal, 2021. Available online: https://hdl.handle.net/10316/98139 (accessed on 4 April 2025).
- Loe, L.E.; Bonenfant, C.; Mysterud, A.; Severinsen, T.; Øritsland, N.A.; Langvatn, R.; Stien, A.; Irvine, R.J.; Stenseth, N.C. Activity pattern of arctic reindeer in a predator-free environment: No need to keep a daily rhythm. Oecologia 2007, 152, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Takahashi, H.; Igota, H.; Matsuura, Y.; Azumaya, M.; Yoshida, T.; Kaji, K. Effects of Culling Intensity on Diel and Seasonal Activity Patterns of Sika Deer (Cervus nippon). Sci. Rep. 2019, 9, 17205. [Google Scholar] [CrossRef]
- Neumann, W.; Göran, E.; Holger, D. The impact of human recreational activities: Moose as a case study. Alces 2011, 47, 17–25. Available online: https://publications.slu.se/?file=publ/show&id=34662 (accessed on 4 April 2025).
- Vallejo-Vargas, A.F.; Sheil, D.; Semper-Pascual, A.; Beaudrot, L.; Ahumada, J.A.; Akampurira, E.; Bitariho, R.; Espinosa, S.; Estienne, V.; Jansen, P.A.; et al. Consistent Diel Activity Patterns of Forest Mammals among Tropical Regions. Nat. Commun. 2022, 13, 7102. [Google Scholar] [CrossRef]
- Eom, T.K.; Lee, J.K.; Lee, D.H.; Ko, H.; Rhim, S.J. Adaptive Response of Siberian Roe Deer Capreolus Pygargus to Climate and Altitude in the Temperate Forests of South Korea. Wildl. Biol. 2023, 2023, e01138. [Google Scholar] [CrossRef]

| No. | Age (Years) | Sex | Monitoring Period | Valid Fixes |
|---|---|---|---|---|
| F1 | ≥5 | ♀ | 1 October 2021–30 September 2022 | 9831 |
| F2 | ≥5 | ♀ | 1 October 2021–30 September 2022 | 9805 |
| M3 | 3.5 | ♂ | 1 October 2021–30 September 2022 | 6880 |
| F4 | 3.5 | ♀ | 1 October 2021–30 September 2022 | 8827 |
| F5 | 4.5 | ♀ | 1 October 2021–30 September 2022 | 10,257 |
| F6 | 2.5 | ♀ | 1 October 2021–30 September 2022 | 8724 |
| F7 | 3.5 | ♀ | 1 October 2021–28 February 2025 | 17,228 |
| F8 | 4.5 | ♀ | 1 October 2021–30 September 2022 | 8528 |
| F9 | 3.5 | ♀ | 1 October 2021–30 September 2022 | 9775 |
| M10 | 4.5 | ♂ | 1 October 2021–28 February 2025 | 16,122 |
| M11 | 3.5 | ♂ | 1 October 2021–30 September 2022 | 4796 |
| Total | 110,733 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Bai, J.; Su, R.; Ma, H.; Feng, C.; Zhong, Z.; Guo, Q.; Zhang, Q.; Cheng, Z.; Cheng, K. Behavioral Plasticity of Rewilding Milu in Mountainous Region of Northern China. Animals 2025, 15, 2993. https://doi.org/10.3390/ani15202993
Ma J, Bai J, Su R, Ma H, Feng C, Zhong Z, Guo Q, Zhang Q, Cheng Z, Cheng K. Behavioral Plasticity of Rewilding Milu in Mountainous Region of Northern China. Animals. 2025; 15(20):2993. https://doi.org/10.3390/ani15202993
Chicago/Turabian StyleMa, Jialiang, Jiade Bai, Ritu Su, Haibo Ma, Chenmiao Feng, Zhenyu Zhong, Qingyun Guo, Qingxun Zhang, Zhibin Cheng, and Kun Cheng. 2025. "Behavioral Plasticity of Rewilding Milu in Mountainous Region of Northern China" Animals 15, no. 20: 2993. https://doi.org/10.3390/ani15202993
APA StyleMa, J., Bai, J., Su, R., Ma, H., Feng, C., Zhong, Z., Guo, Q., Zhang, Q., Cheng, Z., & Cheng, K. (2025). Behavioral Plasticity of Rewilding Milu in Mountainous Region of Northern China. Animals, 15(20), 2993. https://doi.org/10.3390/ani15202993







