Early Nutrition Impacts on Growth, Skeletal Anomalies and Organ Ontogeny in Larval Atlantic Cod (Gadus morhua)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cod Larvae Husbandry
2.3. Experimental Design and Diets
2.4. Sampling Procedure
2.5. Histological Procedures
2.6. Statistical Analysis
3. Results
3.1. Growth and Survival
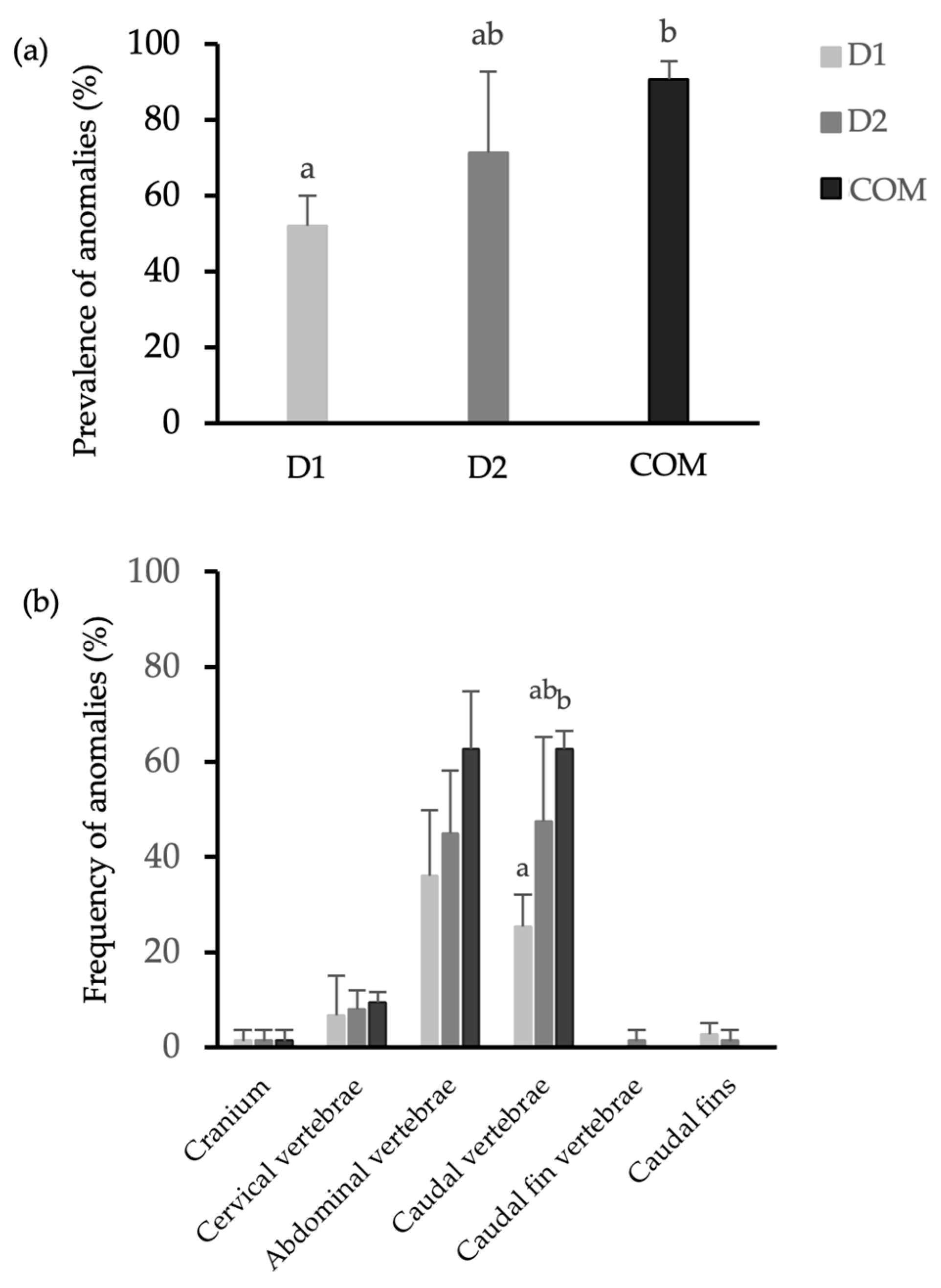
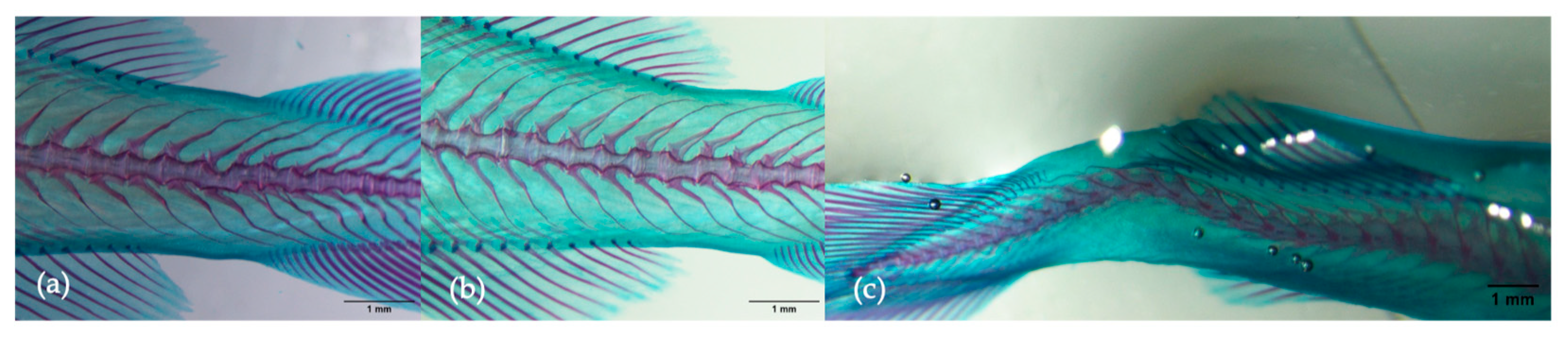
3.2. Skeletal Anomalies
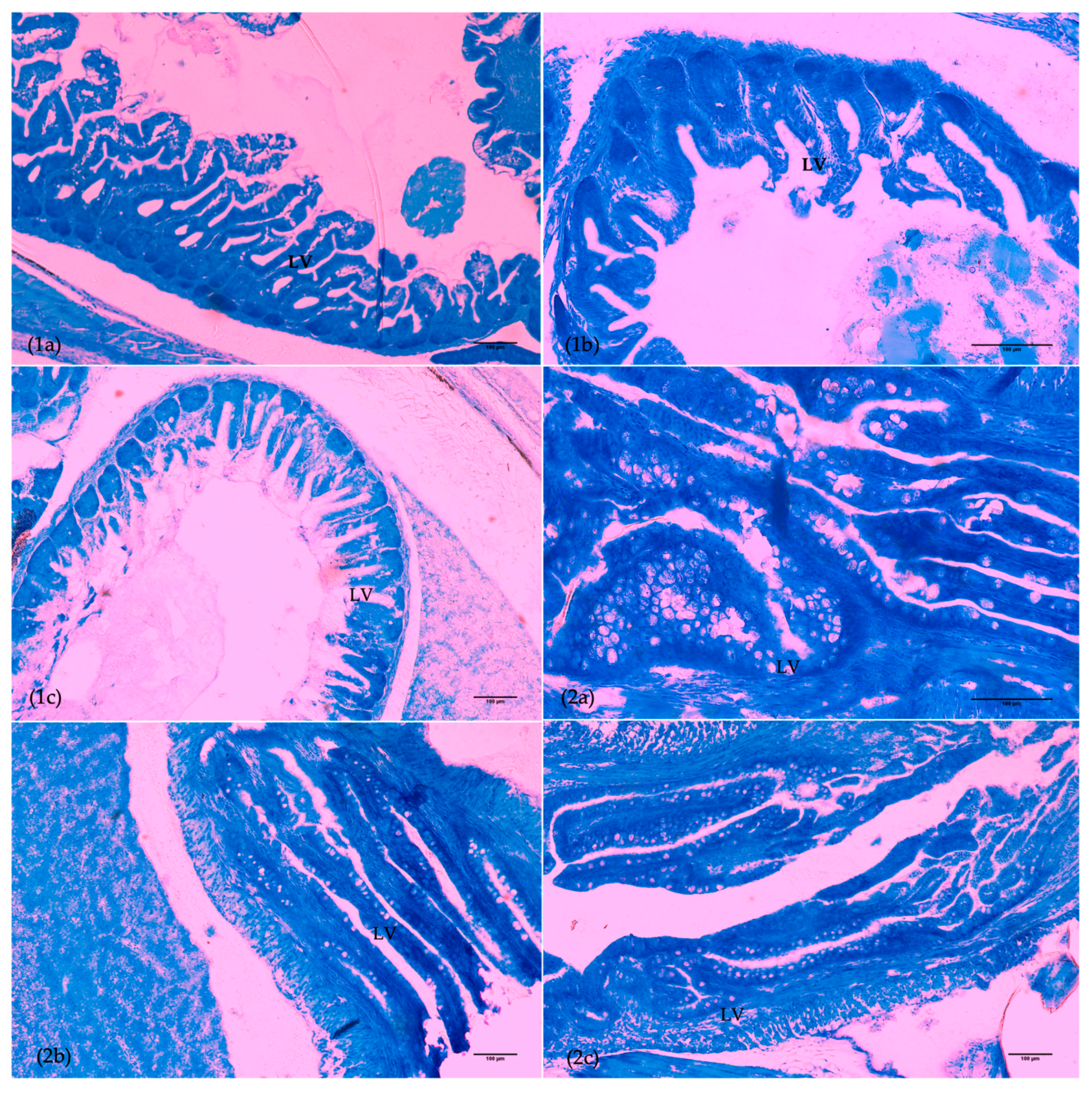
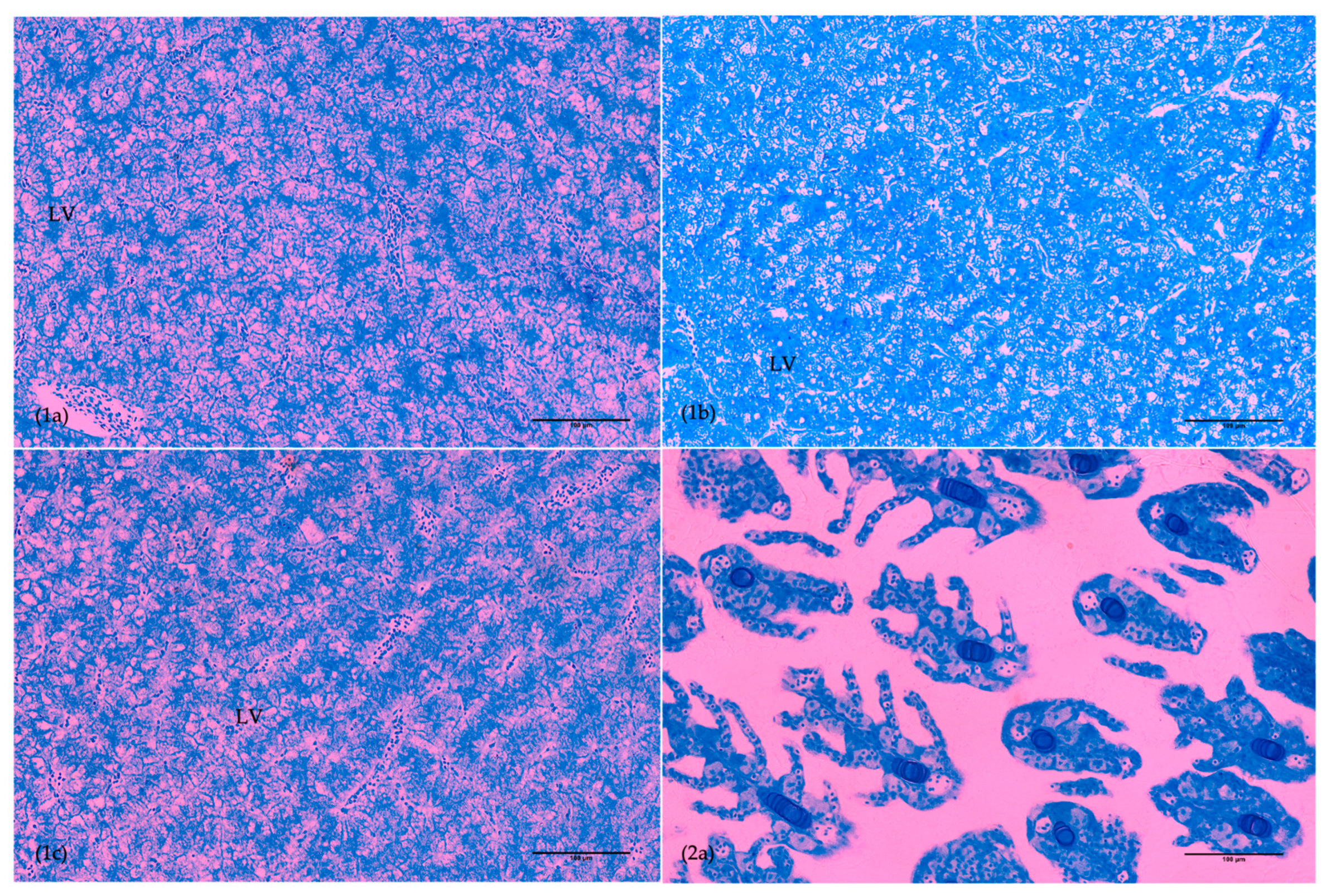
3.3. Organ Ontogeny
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| dph | Days post hatching |
| EPA | Eicosapentaenoic acid |
| ARA | Arachidonic acid |
| Cryo-S | Small barnacle nauplii |
| EFAs | Essential fatty acids |
| DHA | Docosahexaenoic acid |
| PLs | Phospholipids |
| COM | Control group |
| D1 | Experimental group 1 |
| D2 | Experimental group 2 |
| Cryo-L | Large barnacle nauplii |
| Cryo-µ | Blue mussel eggs |
| SL | Standard length |
| DW | Dry weight |
| RGR | Relative growth rate |
| FCR | Feed conversion ratio |
| ACLV | Area covered by lipid vacuoles |
| MSSSs | Multiparametric Semi-Quantitative Scoring System |
| HCC | Hypertrophic chloride cells |
References
- Puvanendran, V.; Swain, T.; Tveiten, H.; Hansen, Ø.J.; Mortensen, A. Optimizing Intensive Culture Protocols for Atlantic Cod (Gadus morhua) Larvae. Aquacult. Int. 2023, 31, 3457–3472. [Google Scholar] [CrossRef]
- ICES Advice. Report of the ICES Advisory Committee on Fishery Management and Advisory Committee on Ecosystems. Evaluation 2004, 1, 1544. [Google Scholar]
- Puvanendran, V.; Mortensen, A.; Johansen, L.; Kettunen, A.; Hansen, Ø.J.; Henriksen, E.; Heide, M. Development of Cod Farming in Norway: Past and Current Biological and Market Status and Future Prospects and Directions. Rev. Aquac. 2022, 14, 308–342. [Google Scholar] [CrossRef]
- Koedijk, R.M.; Folkvord, A.; Foss, A.; Pittman, K.; Stefansson, S.O.; Handeland, S.; Imsland, A.K. The Influence of First-feeding Diet on the Atlantic Cod Gadus morhua Phenotype: Survival, Development and Long-term Consequences for Growth. J. Fish Biol. 2010, 77, 1–19. [Google Scholar] [CrossRef]
- Katan, T.; Nash, G.W.; Rise, M.L.; Hall, J.R.; Fernandes, J.M.O.; Boyce, D.; Johnsen, C.A.; Gamperl, A.K. A Little Goes a Long Way: Improved Growth in Atlantic Cod (Gadus morhua) Fed Small Amounts of Wild Zooplankton. Aquaculture 2016, 451, 271–282. [Google Scholar] [CrossRef]
- Folkvord, A.; Koedijk, R.; Grahl-Nielsen, O.; Meier, S.; Rydland Olsen, B.; Blom, G.; Otterlei, E.; Imsland, A.K. You Are What You Eat? Differences in Lipid Composition of Cod Larvae Reared on Natural Zooplankton and Enriched Rotifers. Aquacult. Nutr. 2018, 24, 224–235. [Google Scholar] [CrossRef]
- Conceição, L.; Aragão, C.; Rønnestad, I. Proteins. In Larval Fish Nutrition; Holt, G.J., Ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 83–116. [Google Scholar] [CrossRef]
- Hamre, K.; Yúfera, M.; Rønnestad, I.; Boglione, C.; Conceição, L.E.C.; Izquierdo, M. Fish Larval Nutrition and Feed Formulation: Knowledge Gaps and Bottlenecks for Advances in Larval Rearing. Rev. Aquac. 2013, 5, S26–S58. [Google Scholar] [CrossRef]
- Støttrup, J.G.; Shields, R.; Gillespie, M.; Gara, M.B.; Sargent, J.R.; Bell, J.G.; Henderson, R.J. The Production and Use of Copepods in Larval Rearing of Halibut, Turbot and Cod. Bull. Aquac. Assoc. Can. 1998, 4, 41–45. [Google Scholar]
- Imsland, A.K.; Foss, A.; Koedijk, R.; Folkvord, A.; Stefansson, S.O.; Jonassen, T.M. Short- and Long-Term Differences in Growth, Feed Conversion Efficiency and Deformities in Juvenile Atlantic Cod (Gadus morhua) Startfed on Rotifers or Zooplankton. Aquac. Res. 2006, 37, 1015–1027. [Google Scholar] [CrossRef]
- Hamre, K. Nutrition in Cod (Gadus morhua) Larvae and Juveniles. ICES J. Mar. Sci. 2006, 63, 267–274. [Google Scholar] [CrossRef]
- Conceição, L.E.C.; Yúfera, M.; Makridis, P.; Morais, S.; Dinis, M.T. Live Feeds for Early Stages of Fish Rearing. Aquac. Res. 2010, 41, 613–640. [Google Scholar] [CrossRef]
- Busch, K.E.T.; Falk-Petersen, I.-B.; Peruzzi, S.; Rist, N.A.; Hamre, K. Natural Zooplankton as Larval Feed in Intensive Rearing Systems for Juvenile Production of Atlantic Cod (Gadus morhua L.): Farmed Cod Larvae Fed Natural Zooplankton. Aquac. Res. 2010, 41, 1727–1740. [Google Scholar] [CrossRef]
- Penglase, S.; Edvardsen, R.B.; Furmanek, T.; Rønnestad, I.; Karlsen, Ø.; Van Der Meeren, T.; Hamre, K. Diet Affects the Redox System in Developing Atlantic Cod (Gadus morhua) Larvae. Redox Biol. 2015, 5, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Øie, G.; Galloway, T.; Sørøy, M.; Holmvaag Hansen, M.; Norheim, I.A.; Halseth, C.K.; Almli, M.; Berg, M.; Gagnat, M.R.; Wold, P.-A.; et al. Effect of Cultivated Copepods (Acartia tonsa) in First-Feeding of Atlantic Cod (Gadus morhua) and Ballan Wrasse (Labrus bergylta) Larvae. Aquacult. Nutr. 2017, 23, 3–17. [Google Scholar] [CrossRef]
- Rise, M.L.; Hall, J.R.; Nash, G.W.; Xue, X.; Booman, M.; Katan, T.; Gamperl, A.K. Transcriptome Profiling Reveals That Feeding Wild Zooplankton to Larval Atlantic Cod (Gadus morhua) Influences Suites of Genes Involved in Oxidation-Reduction, Mitosis, and Selenium Homeostasis. BMC Genom. 2015, 16, 1016. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, Ø.; Van Der Meeren, T.; Rønnestad, I.; Mangor-Jensen, A.; Galloway, T.F.; Kjørsvik, E.; Hamre, K. Copepods Enhance Nutritional Status, Growth and Development in Atlantic Cod (Gadus morhua L.) Larvae—Can We Identify the Underlying Factors? PeerJ 2015, 3, e902. [Google Scholar] [CrossRef]
- Malzahn, A.M.; Ribičić, D.; Hansen, B.H.; Sarno, A.; Kjørsvik, E.; Aase, A.S.N.; Musialak, L.A.; García-Calvo, L.; Hagemann, A. First Feed Matters: The First Diet of Larval Fish Programmes Growth, Survival, and Metabolism of Larval Ballan Wrasse (Labrus bergylta). Aquaculture 2022, 561, 738586. [Google Scholar] [CrossRef]
- Etayo, A.; Lie, K.K.; Bjelland, R.M.; Hordvik, I.; Øvergård, A.-C.; Sæle, Ø. The Thymus and T-Cell Ontogeny in Ballan Wrasse (Labrus bergylta) Is Nutritionally Modelled. Front. Immunol. 2023, 14, 1166785. [Google Scholar] [CrossRef]
- Høeg, J.T.; Møller, O.S. When Similar Beginnings Lead to Different Ends: Constraints and Diversity in Cirripede Larval Development. Invertebr. Reprod. Dev. 2006, 49, 125–142. [Google Scholar] [CrossRef]
- Høeg, J.T.; Deutsch, J.; Chan, B.K.K.; Semmler Le, H. “Crustacea”: Cirripedia. In Evolutionary Developmental Biology of Invertebrates 4; Wanninger, A., Ed.; Springer Vienna: Vienna, Austria, 2015; pp. 153–181. [Google Scholar] [CrossRef]
- Calvo, L.G. Effects of Different Live Feeding Regimes on the Development and Lipid Composition of Ballan Wrasse (Labrus bergylta) Larvae. Master’s Thesis, NTNU, Trondheim, Norway, 2024. [Google Scholar]
- Engrola, S.; Figueira, L.; Conceição, L.E.C.; Gavaia, P.J.; Ribeiro, L.; Dinis, M.T. Co-Feeding in Senegalese Sole Larvae with Inert Diet from Mouth Opening Promotes Growth at Weaning. Aquaculture 2009, 288, 264–272. [Google Scholar] [CrossRef]
- Fletcher, R.C.; Roy, W.; Davie, A.; Taylor, J.; Robertson, D.; Migaud, H. Evaluation of New Microparticulate Diets for Early Weaning of Atlantic Cod (Gadus morhua): Implications on Larval Performances and Tank Hygiene. Aquaculture 2007, 263, 35–51. [Google Scholar] [CrossRef]
- Estévez, A.; Papandroulakis, N.; Wille, M.; Sorgeloos, P. Early Life Stages and Weaning. In Organic Aquaculture; Lembo, G., Mente, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 79–102. [Google Scholar] [CrossRef]
- Rosenlund, G.; Stoss, J.; Talbot, C. Co-Feeding Marine Fish Larvae with Inert and Live Diets. Aquaculture 1997, 155, 183–191. [Google Scholar] [CrossRef]
- Engrola, S.; Dinis, M.T.; Conceição, L.E.C. Senegalese Sole Larvae Growth and Protein Utilization Is Depressed When Co-Fed High Levels of Inert Diet and Artemia Since First Feeding: Co-Fed High Levels of Inert Diet since First Feeding. Aquac. Nutr. 2010, 16, 457–465. [Google Scholar] [CrossRef]
- Wold, P.A.; Hoehne-Reitan, K.; Cahu, C.L.; Infante, J.Z.; Rainuzzo, J.; Kjørsvik, E. Comparison of Dietary Phospholipids and Neutral Lipids: Effects on Gut, Liver and Pancreas Histology in Atlantic Cod (Gadus morhua L.) Larvae. Aquac. Nutr. 2009, 15, 73–84. [Google Scholar] [CrossRef]
- Kjørsvik, E.; Olsen, C.; Wold, P.-A.; Hoehne-Reitan, K.; Cahu, C.L.; Rainuzzo, J.; Olsen, A.I.; Øie, G.; Olsen, Y. Comparison of Dietary Phospholipids and Neutral Lipids on Skeletal Development and Fatty Acid Composition in Atlantic Cod (Gadus morhua). Aquaculture 2009, 294, 246–255. [Google Scholar] [CrossRef]
- Hansen, Ø.J.; Puvanendran, V.; Jøstensen, J.P.; Ous, C. Effects of Dietary Levels and Ratio of Phosphatidylcholine and Phosphatidylinositol on the Growth, Survival and Deformity Levels of Atlantic Cod Larvae and Early Juveniles: Dietary PC and PI on Cod Larval Performance. Aquac. Res. 2011, 42, 1026–1033. [Google Scholar] [CrossRef]
- Aragão, C.; Conceição, L.E.C.; Martins, D.; Rønnestad, I.; Gomes, E.; Dinis, M.T. A Balanced Dietary Amino Acid Profile Improves Amino Acid Retention in Post-Larval Senegalese Sole (Solea senegalensis). Aquaculture 2004, 233, 293–304. [Google Scholar] [CrossRef]
- Valente, L.M.P.; Moutou, K.A.; Conceição, L.E.C.; Engrola, S.; Fernandes, J.M.O.; Johnston, I.A. What Determines Growth Potential and Juvenile Quality of Farmed Fish Species? Rev. Aquac. 2013, 5, S168–S193. [Google Scholar] [CrossRef]
- Kjørsvik, E.; Galloway, T.F.; Estevez, A.; Sæle, Ø.; Moren, M. Effects of Larval Nutrition on Development. In Larval Fish Nutrition; Holt, G.J., Ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 219–248. [Google Scholar] [CrossRef]
- Cahu, C.L.; Infante, J.L.Z.; Barbosa, V. Effect of Dietary Phospholipid Level and Phospholipid:Neutral Lipid Value on the Development of Sea Bass (Dicentrarchus labrax) Larvae Fed a Compound Diet. Br. J. Nutr. 2003, 90, 21–28. [Google Scholar] [CrossRef]
- Boglione, C.; Gavaia, P.; Koumoundouros, G.; Gisbert, E.; Moren, M.; Fontagné, S.; Witten, P.E. Skeletal Anomalies in Reared European Fish Larvae and Juveniles. Part 1: Normal and Anomalous Skeletogenic Processes. Rev. Aquac. 2013, 5, S99–S120. [Google Scholar] [CrossRef]
- El Kertaoui, N.; Lund, I.; Assogba, H.; Domínguez, D.; Izquierdo, M.S.; Baekelandt, S.; Cornet, V.; Mandiki, S.N.M.; Montero, D.; Kestemont, P. Key Nutritional Factors and Interactions during Larval Development of Pikeperch (Sander lucioperca). Sci. Rep. 2019, 9, 7074. [Google Scholar] [CrossRef]
- Hamre, K.; Penglase, S.J.; Rasinger, J.D.; Skjærven, K.H.; Olsvik, P.A. Ontogeny of Redox Regulation in Atlantic Cod (Gadus morhua) Larvae. Free Radic. Biol. Med. 2014, 73, 337–348. [Google Scholar] [CrossRef]
- Sæle, Ø.; Haugen, T.; Karlsen, Ø.; Van Der Meeren, T.; Bæverfjord, G.; Hamre, K.; Rønnestad, I.; Moren, M.; Lie, K.K. Ossification of Atlantic Cod (Gadus morhua)–Developmental Stages Revisited. Aquaculture 2017, 468, 524–533. [Google Scholar] [CrossRef]
- Loufi, K.; Sfakianakis, D.G.; Karapanagiotis, S.; Tsele, N.; Makridis, P. The Effect of Copepod Acartia tonsa During the First Days of Larval Rearing in Skeleton Ontogeny and Skeletal Deformities in Greater Amberjack (Seriola dumerili Risso, 1810). Aquaculture 2024, 579, 740169. [Google Scholar] [CrossRef]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Available online: https://eur-lex.europa.eu/eli/dir/2010/63/oj/eng (accessed on 6 October 2025).
- ISO 6496:1999; Animal Feeding Stuffs. Determination of Moisture and Other Volatile Matter Content. International Organization for Standardization: Geneva, Switzerland, 1999.
- Gavaia, P.J.; Dinis, M.T.; Cancela, M.L. Osteological Development and Abnormalities of the Vertebral Column and Caudal Skeleton in Larval and Juvenile Stages of Hatchery-Reared Senegal Sole (Solea senegalensis). Aquaculture 2002, 211, 305–323. [Google Scholar] [CrossRef]
- Deschamps, M.H.; Kacem, A.; Ventura, R.; Courty, G.; Haffray, P.; Meunier, F.J.; Sire, J.-Y. Assessment of “Discreet” Vertebral Abnormalities, Bone Mineralization and Bone Compactness in Farmed Rainbow Trout. Aquaculture 2008, 279, 11–17. [Google Scholar] [CrossRef]
- Bennett, H.S.; Wyrick, A.D.; Lee, S.W.; McNeil, J.H. Science and Art in Preparing Tissues Embedded in Plastic for Light Microscopy, with Special Reference to Glycol Methacrylate, Glass Knives and Simple Stains. Stain Technol. 1976, 51, 71–97. [Google Scholar] [CrossRef] [PubMed]
- Escaffre, A.-M.; Kaushik, S.; Mambrini, M. Morphometric Evaluation of Changes in the Digestive Tract of Rainbow Trout (Oncorhynchus mykiss) Due to Fish Meal Replacement with Soy Protein Concentrate. Aquaculture 2007, 273, 127–138. [Google Scholar] [CrossRef]
- Papadakis, I.E.; Zaiss, M.M.; Kyriakou, Y.; Georgiou, G.; Divanach, P.; Mylonas, C.C. Histological Evaluation of the Elimination of Artemia Nauplii from Larval Rearing Protocols on the Digestive System Ontogeny of Shi Drum (Umbrina cirrosa L.). Aquaculture 2009, 286, 45–52. [Google Scholar] [CrossRef]
- Pacorig, V.; Galeotti, M.; Beraldo, P. Multiparametric Semi-Quantitative Scoring System for the Histological Evaluation of Marine Fish Larval and Juvenile Quality. Aquac. Rep. 2022, 26, 101285. [Google Scholar] [CrossRef]
- Vo, T.A.; Galloway, T.F.; Arukwe, A.; Edvardsen, R.B.; Hamre, K.; Karlsen, Ø.; Rønnestad, I.; Kjørsvik, E. Effect of Diet on Molecular Relationships between Atlantic Cod Larval Muscle Growth Dynamics, Metabolism, and Antioxidant Defense System. Front. Mar. Sci. 2022, 9, 814022. [Google Scholar] [CrossRef]
- Wold, P.-A.; Hoehne-Reitan, K.; Cahu, C.L.; Infante, J.Z.; Rainuzzo, J.; Kjørsvik, E. Phospholipids vs. Neutral Lipids: Effects on Digestive Enzymes in Atlantic Cod (Gadus morhua) Larvae. Aquaculture 2007, 272, 502–513. [Google Scholar] [CrossRef]
- Rocha, G.S.; Katan, T.; Parrish, C.C.; Kurt Gamperl, A. Effects of Wild Zooplankton Versus Enriched Rotifers and Artemia on the Biochemical Composition of Atlantic Cod (Gadus Morhua) Larvae. Aquaculture 2017, 479, 100–113. [Google Scholar] [CrossRef]
- Wiborg, K.F. Investigations on Cod Larvae in the Coastal Waters of Northern Norway. Fisk. Skr. Ser. Havundersoekelser 1948, 9, 1–27. [Google Scholar]
- Last, J.M. The Food of Three Species of Gadoid Larvae in the Eastern English Channel and Southern North Sea. Mar. Biol. 1978, 48, 377–386. [Google Scholar] [CrossRef]
- Fortier, L.; Harris, R. Optimal Foraging and Density-Dependent Competition in Marine Fish Larvae. Mar. Ecol. Prog. Ser. 1989, 51, 19–33. [Google Scholar] [CrossRef]
- Pedersen, T.; Falk-Petersen, I.B. Morphological Changes During Metamorphosis in Cod (Gadus morhua L.), with Particular Reference to the Development of the Stomach and Pyloric Caeca. J. Fish Biol. 1992, 41, 449–461. [Google Scholar] [CrossRef]
- Morrison, C.M. Histology of the Atlantic Cod, Gadus Morhua: An Atlas; Eleutheroembryo and Larva = Atlas d’histologie de La Morue Franche, Gadus Morhua; Éleuthéro-Embryon et Larve; Canadian Special Publications of Fisheries and Aquatic Sciences; NRC Research Press: Ottawa, ON, Canada, 1993. [Google Scholar]
- Niu, J.; Liu, Y.J.; Tian, L.X.; Mai, K.S.; Yang, H.J.; Ye, C.X.; Zhu, Y. Effects of Dietary Phospholipid Level in Cobia (Rachycentron canadum) Larvae: Growth, Survival, Plasma Lipids and Enzymes of Lipid Metabolism. Fish Physiol Biochem 2008, 34, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Marthinsen, J.L.; Reitan, K.I.; Olsen, R.E.; Li, K.; Nunes, B.; Bjørklund, R.H.; Kjørsvik, E. Influence of Dietary Phospholipid Level and Bile Salt Supplementation on Larval Performance of Atlantic Cod (Gadus morhua L.). Aquacult. Int. 2025, 33, 560. [Google Scholar] [CrossRef]
- Kanazawa, A.; Teshima, S.; Inamori, S. Effects of Dietary Phospholipids on Growth of The Larval Red Sea Bream and Knife Jaw. Mem. Fac. Fish. 1983, 32, 109–114. [Google Scholar]
- Kanazawa, A.; Teshima, S.; Skamoto, M. Effects of Dietary Lipids, Fatty Acids, and Phospholipids on Growth and Survival of Prawn (Penaeus japonicus) larvae. Aquaculture 1985, 50, 39–49. [Google Scholar] [CrossRef]
- Leifson, R.M.; Homme, J.M.; Lie, Ø.; Myklebust, R.; Strøm, T. Three Different Lipid Sources in Formulated Start-Feeds for Turbot (Scophthalmus maximus L.) Larvae-Effect on Growth and Mitochondrial Alteration in Enterocytes: Lipids in Formulated Start-Feeds for Turbot Larvae. Aquac. Nutr. 2003, 9, 33–42. [Google Scholar] [CrossRef]
- Taylor, J.F.; Martinez-Rubio, L.; Del Pozo, J.; Walton, J.M.; Tinch, A.E.; Migaud, H.; Tocher, D.R. Influence of Dietary Phospholipid on Early Development and Performance of Atlantic Salmon (Salmo salar). Aquaculture 2015, 448, 262–272. [Google Scholar] [CrossRef]
- Hansen, Ø.J.; Puvanendran, V.; Jøstensen, J.P.; Falk-Petersen, I.-B. Early Introduction of an Inert Diet and Unenriched Artemia Enhances Growth and Quality of Atlantic Cod (Gadus morhua) Larvae. Aquacult. Nutr. 2018, 24, 102–111. [Google Scholar] [CrossRef]
- Cahu, C.; Zambonino Infante, J. Substitution of Live Food by Formulated Diets in Marine Fish Larvae. Aquaculture 2001, 200, 161–180. [Google Scholar] [CrossRef]
- Azarm, H.M.; Kenari, A.A.; Hedayati, M. Effect of Dietary Phospholipid Sources and Levels on Growth Performance, Enzymes Activity, Cholecystokinin and Lipoprotein Fractions of Rainbow Trout (Oncorhynchus mykiss) Fry. Aquac. Res. 2013, 44, 634–644. [Google Scholar] [CrossRef]
- Baskerville-Bridges, B.; Kling, L.J. Early Weaning of Atlantic Cod (Gadus morhua) Larvae onto a Microparticulate Diet. Aquaculture 2000, 189, 109–117. [Google Scholar] [CrossRef]
- Cahu, C.L.; Gisbert, E.; Villeneuve, L.A.N.; Morais, S.; Hamza, N.; Wold, P.-A.; Zambonino Infante, J.L. Influence of Dietary Phospholipids on Early Ontogenesis of Fish. Aquac. Res. 2009, 40, 989–999. [Google Scholar] [CrossRef]
| dph | 3 | 10 | 20 | 25 | 27 | 45 | 66 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COM | ||||||||||||
| Enriched rotifers | ||||||||||||
| Cryo-L | ||||||||||||
| Commercial microfeed | ||||||||||||
| D1 and D2 groups | ||||||||||||
| Enriched rotifers | ||||||||||||
| Cryo-μ | ||||||||||||
| Cryo-S | ||||||||||||
| Cryo-L | ||||||||||||
| D1/D2 microfeed |
| D1 | D2 | COM | |
|---|---|---|---|
| Protein (%DM) | 63.0 | 62.5 | 58.3 |
| Fat (%DM) | 18.6 | 18.9 | 17.6 |
| Ash (%DM) | 10.4 | 10.1 | 13.4 |
| Phosphorous (%DM) | 1.4 | 1.4 | 1.6 |
| EPA (%DM) | 1.1 | 1.3 | 1 |
| DHA (%DM) | 1.4 | 1.3 | 1.3 |
| Total PLs (%DM) | 9.0 | 8.8 | 10.0 |
| Marine PLs (% total PL) * | 46 | 74 | NA |
| Vegetable PLs (% total PL) * | 54 | 26 | NA |
| Regions Affected |
|---|
| A. Cranium B. Cervical vertebra (vertebrae 1–2; short vertebra centra, prominent neural spines and absence of articulations with ribs) C. Abdominal vertebrae (vertebrae 3–19; vertebrae with wing-shaped transverse processes (parapophyses) that all articulate with a rib) D. Caudal vertebra (V20–V40; vertebra centra have haemal arches with prominent haemal spines E. Caudal fin vertebrae (V41 to the last vertebra; characterized by broad neural and haemal spines, providing sites of origin for muscles inserting on the fin rays—lepidotrichs—of the tail fin) F. Anal fin G. Caudal fin H. Dorsal fin |
| Type of Anomaly |
| * Scoliosis * Lordosis * Kyphosis ** Vertebral fusion Vertebral body malformation Malformed neural arch and/or spine Malformed haemal arch and/or spine and/or rib Malformed ray (deformed, absent, fused) Malformed pterygiophores (deformed, absent, fused) Malformed hypural (deformed, absent, fused) Malformed epural (deformed, absent, fused) * Jaw deformities Reduced dental/malformed pre-maxillary and/or maxillary Vertebral slipping * Deformed or reduced operculum |
| dph | Group | Standard Length (cm) | Dry Weight (mg) | RGR (%/day) 1 | FCR | Survival (%) |
|---|---|---|---|---|---|---|
| 3 | D1 | 0.45 ± 0.03 | 0.06 ± 0.01 | - | - | - |
| D2 | 0.45 ± 0.03 | 0.06 ± 0.01 | - | - | - | |
| COM | 0.44 ± 0.03 | 0.06 ± 0.01 | - | - | - | |
| 30 | D1 | 0.86 ± 0.05 ab | 0.54 ± 0.39 ab | 7.47 ± 0.99 | - | - |
| D2 | 0.85 ± 0.04 a | 0.47 ± 0.17 a | 7.57 ±1.20 | - | - | |
| COM | 0.88 ± 0.05 b | 0.54 ± 0.21 b | 6.78 ± 0.86 | - | - | |
| 50 | D1 | 1.38 ± 0.06 a | 3.42 ± 1.58 a | 8.36 ± 1.07 | - | - |
| D2 | 1.37 ± 0.05 a | 3.13 ± 1.61 a | 8.56 ± 0.99 | - | - | |
| COM | 1.57 ± 0.04 b | 4.79 ± 2.23 b | 8.64 ± 0.67 | - | - | |
| 66 | D1 | 2.62 ± 0.08 a | 25.6 ± 11.7 a | 9.61 ± 0.17 | 7.12 ± 0.44 | 21.4 ± 3.7 |
| D2 | 2.64 ± 0.08 ab | 27.3 ± 14.2 a | 10.0 ± 0.03 | 6.86 ± 0.80 | 17.7 ± 1.2 | |
| COM | 2.79 ± 0.07 b | 34.9 ± 16.4 b | 9.75 ± 0.08 | 5.40 ± 0.70 | 24.8 ± 0.6 |
| dph | Group | Anterior Intestine | Posterior Intestine | Liver | Eye | ||
|---|---|---|---|---|---|---|---|
| Villi Length | Goblet Cells | ACLV | ACLV | Ontogeny | |||
| 15 | D1 | 3 | - | 4 | 2 | 3 | |
| D2 | 2 | - | 3 | 2 | 3 | ||
| COM | 2 | - | 4 | 2 | 3 | ||
| 30 | D1 | 4 | - | 4 | 4 | 4 | |
| D2 | 3 | - | 4 | 4 | 3 | ||
| COM | 4 | - | 2 | 2 | 3 | ||
| 66 | D1 | 5 | 5 | 4 | 4 | 3 | |
| D2 | 4 | 4 | 3 | 3 | 3 | ||
| COM | 4 | 4 | 4 | 4 | 3 | ||
| Gills | |||||||
| Goblet cells | Erythrocytes | Edema | Fusion | Clubbing | HCC | ||
| 15 | D1 | 3 | 2 | 1 | 1 | 1 | 1 |
| D2 | 3 | 2 | 1 | 1 | 1 | 1 | |
| COM | 1 | 1 | 2 | 2 | 1 | 2 | |
| 30 | D1 | 3 | 2 | 1 | 1 | 1 | 1 |
| D2 | 2 | 2 | 1 | 2 | 1 | 1 | |
| COM | 3 | 2 | 1 | 2 | 1 | 2 | |
| 66 | D1 | 3 | 3 | 1 | 1 | 1 | 1 |
| D2 | 2 | 2 | 1 | 3 | 1 | 1 | |
| COM | 3 | 2 | 1 | 2 | 1 | 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedro, J.; Henriques, J.; Bergvik, M.; Tzakris, K.; Viegas, M.; Loufi, K.; Fernandes, J.M.O.; Costas, B.; Tokle, N.; Conceição, L.E.C. Early Nutrition Impacts on Growth, Skeletal Anomalies and Organ Ontogeny in Larval Atlantic Cod (Gadus morhua). Animals 2025, 15, 2985. https://doi.org/10.3390/ani15202985
Pedro J, Henriques J, Bergvik M, Tzakris K, Viegas M, Loufi K, Fernandes JMO, Costas B, Tokle N, Conceição LEC. Early Nutrition Impacts on Growth, Skeletal Anomalies and Organ Ontogeny in Larval Atlantic Cod (Gadus morhua). Animals. 2025; 15(20):2985. https://doi.org/10.3390/ani15202985
Chicago/Turabian StylePedro, Joana, João Henriques, Maria Bergvik, Konstantinos Tzakris, Michael Viegas, Katerina Loufi, Jorge M. O. Fernandes, Benjamín Costas, Nils Tokle, and Luís E. C. Conceição. 2025. "Early Nutrition Impacts on Growth, Skeletal Anomalies and Organ Ontogeny in Larval Atlantic Cod (Gadus morhua)" Animals 15, no. 20: 2985. https://doi.org/10.3390/ani15202985
APA StylePedro, J., Henriques, J., Bergvik, M., Tzakris, K., Viegas, M., Loufi, K., Fernandes, J. M. O., Costas, B., Tokle, N., & Conceição, L. E. C. (2025). Early Nutrition Impacts on Growth, Skeletal Anomalies and Organ Ontogeny in Larval Atlantic Cod (Gadus morhua). Animals, 15(20), 2985. https://doi.org/10.3390/ani15202985









