Simple Summary
Glaesserella parasuis is an early colonizer of the upper respiratory tract of pigs and causes high morbidity and mortality. Although macrolides have gained wide clinical application for the treatment of G. parasuis infections, the susceptibility profiles of different macrolide drugs have not been extensively compared. We evaluated the comparative sensitivity of 14-, 15-, and 16-membered macrolides against G. parasuis, and investigated the associated resistance mechanisms. Comprehensive data on the macrolide susceptibility of G. parasuis were obtained. In addition, the presence of integrative and conjugative element (ICE)-borne erm(T) was also a novel observation. Our results provide valuable insights into macrolide resistance in G. parasuis and may guide empirical treatment recommendations.
Abstract
Glaesserella parasuis is the etiological agent of Glässer’s disease, which causes high morbidity and mortality in pigs worldwide. Macrolide resistance poses an urgent threat to their treatment, as macrolides are widely used for preventing and treating G. parasuis infections. Here, we determined the susceptibilities to five macrolides and characterized the genetic markers of macrolide resistance. The antimicrobial susceptibility of 117 G. parasuis isolates to erythromycin, tulathromycin, gamithromycin, tylosin, and tilmicosin was evaluated using broth microdilution method. Erythromycin-resistant isolates were sequenced using whole-genome sequencing. Further analysis of these sequences revealed the genetic basis of macrolide resistance in G. parasuis. Our results show that most G. parasuis isolates remained susceptible to the macrolide drugs. For commonly used agents (e.g., tylosin and tilmicosin), elevated minimum inhibitory concentrations (MICs) were observed, whereas for the newer macrolides (e.g., tulathromycin and gamithromycin), the MICs remained almost unchanged. The macrolide resistance gene erm(T) and the A2059G mutation in 23S rRNA were detected in the current study. To the best of our knowledge, integrative and conjugative element (ICE)-borne erm(T) in G. parasuis is reported for the first time in this study. Taken together, these results provide insights into the susceptibility of G. parasuis to macrolides. The presence of erm(T) on ICEs may facilitate its transfer, reducing the effectiveness of macrolide treatment.
1. Introduction
Glaesserella parasuis is a member of the family Pasteurellaceae and an early colonizer of the upper respiratory tract of healthy pigs, comprising virulent and non-virulent strains [1,2]. Under certain circumstances, the virulent strains can cause Glässer’s disease, characterized by polyarthritis, fibrinous polyserositis, and meningitis, resulting in economic losses and reduced pig welfare [3]. Macrolides are widely used to control such infections. However, misuse of these agents drives the emergence of antimicrobial resistance (AMR) and selects for resistant groups, making the treatment of pigs ineffective and leading to more severe infections [4].
To address the threat posed by AMR, it is important to conduct complete susceptibility tests for macrolides and detect the genetic basis of resistance to the agents [5]. Several previous studies demonstrated the susceptibility of G. parasuis to certain macrolides, such as erythromycin, tylosin, tilmicosin, and tulathromycin, whereas few have focused on gamithromycin, an azalide macrolide drug that was recently approved for the treatment and control of respiratory diseases [6,7,8,9]. G. parasuis isolates are sensitive to macrolides, and only a few resistant strains harbor macrolide resistance genes or mutations [10,11].
The macrolide resistance known to occur in Pasteurellaceae is mediated by rRNA methylases, mutations in ribosomal proteins and 23S rRNA, and active efflux or inactivation by phosphotransferases [12]. More recently, estT conferring resistance only to 16-membered macrolides was identified on a plasmid in Sphingobacterium faecium [13]. The erm(T) gene has been identified preferentially in Gram-positive genera such as Lactobacillus, Streptococcus, Staphylococcus, Enterococcus, and Erysipelothrix, as well as in Gram-negative genera such as Glaesserella, Mannheimia, and Acinetobacter [14]. In most cases, erm(T) is detected in various plasmids. Recently, integrative and conjugative element (ICE)-borne erm(T) was identified in some genera, such as Streptococcus suis and Mannheimia haemolytica [14,15]. In isolates of G. parasuis, plasmid-borne erm(T) and the A2059G mutation in 23S rRNA were reported to be responsible for macrolide resistance [10,16]. Notably, several recent studies showed that AMR genes were frequently associated with ICEs in G. parasuis, and were capable of being transferred between different bacterial species [17].
In the present study, we determined the susceptibility of 117 clinical G. parasuis isolates to 14-, 15-, and 16-membered macrolides and characterized the genetic environment of ICE-borne erm(T). Our objective was to comprehensively understand macrolide resistance phenotypes and characterize the genetic environment of erm(T). This study provides valuable insights into macrolide resistance in G. parasuis and may guide empirical treatment recommendations.
2. Materials and Methods
2.1. Strains and Culture Conditions
All 117 G. parasuis isolates were supplied by the National Reference Laboratory of Veterinary Drug Residues (Guangzhou, China) and were isolated from pigs with polyserositis, pneumonia, or septicemia in South China between 2010 and 2017. G. parasuis isolates were cultured on tryptic soy agar supplemented with 10 μg/mL nicotinamide adenine dinucleotide and 5% (v/v) fetal calf serum. The isolates were identified using 16S diagnostic polymerase chain reaction [18].
2.2. Antimicrobial Susceptibility Testing
The antimicrobial susceptibility of the 117 isolates was determined using the broth microdilution method as described previously [19]. Briefly, the inocula were prepared from 24 h supplemented tryptic soy agar by adjusting to a 0.5 McFarland standard and further diluted to 1:200 in supplemented cation-adjusted Mueller–Hinton broth containing 25 μg/mL nicotinamide adenine dinucleotide and 1% (v/v) sterile filtered heat-inactivated chicken serum. The adjusted inoculum (50 μL) was added to each well of a microtiter plate. Microtiter plates were evaluated visually after 24 h of incubation in an ambient-air incubator.
Five macrolides were used for susceptibility testing with the following dilution ranges: erythromycin (14-membered macrolide), 0.25–128 μg/mL; tulathromycin (15-membered macrolide), 0.25–128 μg/mL; gamithromycin (15-membered macrolide), 0.25–128 μg/mL; tylosin (16-membered macrolide), 0.25–128 μg/mL; and tilmicosin (16-membered macrolide), 0.25–128 μg/mL. The quality control strains were Actinobacillus pleuropneumoniae ATCC 27090 and Staphylococcus aureus ATCC 29213. As no species-specific breakpoints were available [20], the 50% and 90% minimum inhibitory concentrations (MIC50 and MIC90, respectively) were determined for each drug. Isolates with an erythromycin MIC of >4 μg/mL were classified as “resistant” and subsequently evaluated using whole-genome sequencing.
2.3. Genomic DNA Extraction and Whole-Genome Sequencing
Five erythromycin-resistant isolates were cultured on supplemented tryptic soy agar at 37 °C for 18–24 h, and colonies were scraped from the plates for genomic DNA extraction. Genomic DNA was extracted using a TIANamp Bacteria DNA Kit (Tiangen, Beijing, China) following the manufacturer’s instructions. The extracted DNA was used for whole-genome sequencing with 250 bp reads on a MiSeq platform (Illumina, San Diego, CA, USA). The Trimmomatic output was used for de novo assembly using SPAdes v3.13.0 [21]. The G. parasuis strain H68tg with a contig that was too short (3824 bp) containing erm(T) was also selected for long-read sequencing on the Oxford Nanopore Technologies GridION Platform (Nanopore, Oxford, UK). Both short (San Diego, CA, USA) and long (Oxford, UK) reads were used for hybrid de novo whole-genome assembly using Unicycler v.0.5.0 with default settings [22].
2.4. Identification of Macrolide Resistance Genes and Mutations
Whole-genome sequences were screened for known macrolide resistance genes using ResFinder v4.6.0 http://genepi.food.dtu.dk/resfinder (accessed on 10 October 2024) with default settings, a threshold for %ID of 90%, and a minimum length of 60%. The 16-membered macrolide resistance gene estT was not available in the resistance gene database; therefore, we downloaded its reference sequence for S. faecium strain WB1 plasmid pWB1 (CP094932.1) from the NCBI database. Chromosomal mutations associated with macrolide resistance were determined in L4 and L22 ribosomal proteins, as well as in 23S rRNA, using online BLAST software v2.16.0 https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 10 October 2024). G. parasuis Nagasaki (CP018034.1) was used as the reference strain. Escherichia coli K-12 substrain MG1655 was used for the numbering of nucleotides.
2.5. Mobile Element Analysis
erm(T)-containing genome sequences were submitted to ICEberg 3.0 to identify putative ICEs [23]. Manual curation and comparison of putative ICEs with ICEHpa1 were performed using SnapGene v.6.0.2 and Easyfig v.2.2.5.
3. Results and Discussion
3.1. Antimicrobial Susceptibilities of Five Macrolides
As there were no species-specific breakpoints available for G. parasuis, the strains could not be defined as resistant or susceptible. The distribution of the MICs as well as the MIC50 or MIC90 values was used for comparison (Table 1).

Table 1.
MIC distribution of 117 Glaesseralla parasuis isolates for macrolides.
For erythromycin, 5 of the 117 isolates showed higher MICs than the others. The MIC50 and MIC90 values of erythromycin were 1 and 4 μg/mL, respectively, which were slightly higher than those reported by Zhou et al. (0.5 and 2 μg/mL, respectively, for strains isolated during 2007–2008), but lower than those reported by Zhang et al. (2 and 16 μg/mL, respectively, for strains isolated during 2016–2017) [7,8]. Elevated MICs were also observed for the older macrolides, tylosin and tilmicosin, with MIC90 values of 32 and 8 μg/mL [7,8], possibly because of the overuse of these two drugs in China.
For the newer macrolides (e.g., tulathromycin and gamithromycin), particularly gamithromycin, most isolates had lower MICs than those of extensively used agents (e.g., tylosin and tilmicosin). A study was previously conducted in Australia to investigat G. parasuis isolates collected between 2002 and 2013 for their susceptibility to tulathromycin [24]. The study revealed that Australian isolates of G. parasuis also had low MIC50 and MIC90 values (1 and 8 μg/mL) for tulathromycin. A study in China in which G. parasuis isolates were tested for their susceptibility to gamithromycin showed that the MIC90 value was extremely low (1 μg/mL), which was in accordance with our results [25].
3.2. Macrolide Resistance Genes and Mutations
A total of five G. parasuis isolates were “resistant” to erythromycin and were sequenced (Table 2). No acquired macrolide resistance genes or mutations were detected in strain 20. One strain, H62, exhibited high MICs for all the macrolides included in this study. Analysis of the whole-genome sequence of this isolate revealed the presence of the A2059G mutation in 23S rRNA. The mutation A2059G in G. parasuis was reported for the first time by Dayao et al. [16]. Since then, this mutation has been reported less frequently in G. parasuis. For the L4 and L22 proteins, we found mutations in isolates with both low and high macrolide MICs (data shown in another study, unpublished).

Table 2.
MIC values of five erythromycin-resistant isolates and associated resistance mechanisms.
The macrolide resistance gene erm(T) was identified in three of the five sequenced isolates. All erm(T)-containing strains (59, H44, and H68tg) showed elevated MICs for the macrolides tested. Strain H44 had higher MICs for tulathromycin and gamithromycin, but similar MICs for other macrolides, which may not be explained solely by the presence of erm(T). Since the first report of plasmid-borne erm(T) in G. parasuis, this gene has rarely been reported in this species in recent years [10]. In S. suis, erm(T) also has a low detection rate, possibly because of the fitness cost of erm(T)-carrying plasmids or ICEs [14].
3.3. Characterization of erm(T)-Carrying ICEs
One of the three erm(T)-carrying isolates (strain 59) was previously reported [10]. The other two isolates were completely sequenced, and erm(T) was identified in sufficiently long contigs (2,367,942 bp for H68tg, 231,690 bp for H44) on the chromosome. ICEfinder identified putative ICEs in both the H44 and H68tg genome sequences. Both contained the macrolide resistance gene erm(T) and shared common backbones with ICEHpa1 (CP054198.1). Compared with ICEHpa1, they had different cargo genes and were designated as ICEHpa1-like01 (H68tg) and ICEHpa1-like02 (H44) (Figure 1). ICEHpa1 was first detected in the G. parasuis strain YHP170504 [26]. Diverse ICEs from the ICEHpa1 family are prevalent in G. parasuis [17].
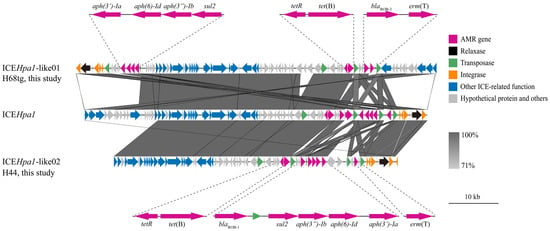
Figure 1.
Putative ICEs identified in this study: ICEHpa1-like01 and ICEHpa1-like02. AMR gene, relaxase, transposase, integrase, ICE-related function sequences, and hypothetical proteins are indicated by colors according to the legend. Scale bar represents 10 kb pairs. AMR, antimicrobial resistance; ICEs, integrative and conjugative elements.
ICEHpa1-like01 was 72,249 bp in size and contained seven antimicrobial resistance genes, which conferred resistance to macrolides (erm(T)), β-lactams (blaROB-3), tetracyclines (tet(B)), sulfonamides (sul2), and aminoglycosides (aph(3″)-Ib, aph(6)-Id, and aph(3′)-Ia). Compared with ICEHpa1, ICEHpa1-like01 carried erm(T) but not aac(6′)-aph(2″). erm(T) and blaROB-3 are flanked by two ISApl1 elements. ISApl1 was originally identified in A. pleuropneumoniae from pigs [27] and has been frequently observed to flank AMR genes in the ICEHpa1 family in G. parasuis, as well as in other Pasteurellaceae of animal origin [17,28,29]. Therefore, the ISApl1-blaROB-3-erm(T)-ISApl1 structure may facilitate the transfer of erm(T) between different Pasteurellaceae species.
ICEHpa1-like02 is 57,037 bp in size and carries AMR genes similar to ICEHpa1-like01. However, the fragment IS4-like-tetR-tet(B)-ISApl1-blaROB-1-ΔISApl1-sul2-aph(3″)-Ib-aph(6)-Id-aph(3′)-Ia-ΔISApl1-erm(T)-ISApl1 differed from ICEHpa1-like01, as well as those reported previously [26,30,31]. ICEHpa1-like02 had two ISApl1 copies flanking several genes, conferring resistance to β-lactams, sulfonamides, aminoglycosides, and macrolides. Moreover, four copies of ISApl1 were identified in this structure, which may form various circular intermediates and transfer between bacterial species through a “copy-out-paste-in” mechanism [29].
3.4. Limitations
We determined the mechanisms underlying macrolide resistance and characterized the erm(T)-containing ICEs. However, we did not determine the transferability of these two putative ICEs. Further studies are needed to evaluate the transferability, fitness costs, and other molecular traits.
4. Conclusions
Most G. parasuis isolates remained susceptible to macrolide drugs. For commonly used agents (e.g., tylosin and tilmicosin), elevated MICs were observed, whereas for the newer macrolides (e.g., tulathromycin and gamithromycin), the MICs remained almost unchanged. The macrolide resistance gene erm(T) and the A2059G mutation in 23S rRNA were detected in the current study. To the best of our knowledge, ICE-borne erm(T) in G. parasuis is reported for the first time in this study. Taken together, these results provide insights into the susceptibility of G. parasuis to macrolides. The presence of erm(T) on ICEs may facilitate its transfer which will reduce the effectiveness of macrolide treatment.
Author Contributions
Conceptualization, P.Z. and Y.Y.; methodology, P.Z., Q.G. and C.L.; validation, P.Z., L.F. and Y.Y.; investigation, P.Z. and S.S.; resources, P.Z., T.H. and Y.Y.; data curation, P.Z., Q.G. and J.L.; writing—original draft preparation, P.Z., C.L. and S.S.; writing—review and editing, X.L., L.F. and Y.Y.; visualization, P.Z. and Q.G.; supervision, X.L., L.F. and Y.Y.; funding acquisition, P.Z., T.H. and Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2022YFE0103200), the Guangdong Basic and Applied Basic Research Foundation (SL2022A04J01333), the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2019BT02N054), the National Natural Science Foundation of China (32002337), the Specific University Discipline Construction Project (2023B10564003), the Natural Science Foundation of Guangdong Province, China (2022A1515011194), and the China Postdoctoral Science Foundation (2020M682738).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The assembled genome sequences were deposited in NCBI under BioProject accession numbers SAMN44656869, SAMN44656860, SAMN44656893, SAMN44656902, and SAMN44731223.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dickerman, A.; Bandara, A.B.; Inzana, T.J. Phylogenomic analysis of Haemophilus parasuis and proposed reclassification to Glaesserella parasuis, gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Aragon, V.; Cerdà-Cuéllar, M.; Fraile, L.; Mombarg, M.; Nofrarías, M.; Olvera, A.; Sibila, M.; Solanes, D.; Segalés, J. Correlation between clinico-pathological outcome and typing of Haemophilus parasuis field strains. Vet. Microbiol. 2010, 142, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Costa-Hurtado, M.; Barba-Vidal, E.; Maldonado, J.; Aragon, V. Update on Glässer’s disease: How to control the disease under restrictive use of antimicrobials. Vet. Microbiol. 2020, 242, 108595. [Google Scholar] [CrossRef] [PubMed]
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Marston, H.D.; Dixon, D.M.; Knisely, J.M.; Palmore, T.N.; Fauci, A.S. Antimicrobial resistance. J. Am. Med. Assoc. 2016, 316, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, A.J.M.; Tucker, A.W.; Navas, J.; Blanco, M.; Morris, S.J.; Gutiérrez-Martín, C.B. Antimicrobial susceptibility patterns of Haemophilus parasuis from pigs in the United Kingdom and Spain. Vet. Microbiol. 2007, 120, 184–191. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, X.; Zhao, Y.; Chen, P.; Zhang, X.; Chen, H.; Cai, X. Distribution of antimicrobial resistance among different serovars of Haemophilus parasuis isolates. Vet. Microbiol. 2010, 141, 168–173. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, C.; Aragon, V.; Zhou, X.; Zou, M.; Wu, C.; Shen, Z. Investigation of Haemophilus parasuis from healthy pigs in China. Vet. Microbiol. 2019, 231, 40–44. [Google Scholar] [CrossRef] [PubMed]
- de Jong, A.; Morrissey, I.; Rose, M.; Temmerman, R.; Klein, U.; Simjee, S.; El Garch, F. Antimicrobial susceptibility among respiratory tract pathogens isolated from diseased cattle and pigs from different parts of Europe. J. Appl. Microbiol. 2023, 134, lxad132. [Google Scholar] [CrossRef]
- Yang, S.S.; Sun, J.; Liao, X.P.; Liu, B.T.; Li, L.L.; Li, L.; Fang, L.X.; Huang, T.; Liu, Y.H. Co-location of the erm(T) gene and blaROB-1 gene on a small plasmid in Haemophilus parasuis of pig origin. J. Antimicrob. Chemother. 2013, 68, 1930–1932. [Google Scholar] [CrossRef]
- Gong, X.; Cui, Q.; Zhang, W.; Shi, Y.; Zhang, P.; Zhang, C.; Hu, G.; Sahin, O.; Wang, L.; Shen, Z. Genomic insight into the diversity of Glaesserella parasuis isolates from 19 countries. mSphere 2024, 9, e00231-24. [Google Scholar] [CrossRef]
- Michael, G.B.; Bossé, J.T.; Schwarz, S. Antimicrobial resistance in Pasteurellaceae of veterinary origin. Antimicrob. Resist. Bact. Livest. Companion Anim. 2018, 331–363. [Google Scholar] [CrossRef]
- Dhindwal, P.; Thompson, C.; Kos, D.; Planedin, K.; Jain, R.; Jelinski, M.; Ruzzini, A. A neglected and emerging antimicrobial resistance gene encodes for a serine-dependent macrolide esterase. Proc. Natl. Acad. Sci. USA 2023, 120, e2219827120. [Google Scholar] [CrossRef]
- Yu, R.; Xu, Y.; Schwarz, S.; Shang, Y.; Yuan, X.; Zhang, Y.; Li, D.; Du, X.D. erm(T)-mediated macrolide-lincosamide resistance in Streptococcus suis. Microbiol. Spectrum 2022, 10, e01657-21. [Google Scholar] [CrossRef] [PubMed]
- Kostova, V.; Hanke, D.; Schink, A.K.; Kaspar, H.; Schwarz, S.; Krüger-Haker, H. ICE-borne erm(T)-mediated macrolide resistance in Mannheimia haemolytica. J. Antimicrob. Chemother. 2023, 78, 2379–2381. [Google Scholar] [CrossRef]
- Dayao, D.A.E.; Seddon, J.M.; Gibson, J.S.; Blackall, P.J.; Turni, C. Whole genome sequence analysis of pig respiratory bacterial pathogens with elevated minimum inhibitory concentrations for macrolides. Microb. Drug Resist. 2016, 22, 531–537. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, J.; Miao, Q.; Zhai, Y.; Pan, Y.; Yuan, L.; Yan, F.; Wu, H.; Hu, G. Genomic insight into the integrative conjugative elements from ICEHpa1 family. Front. Vet. Sci. 2022, 9, 986824. [Google Scholar] [CrossRef]
- Oliveira, S.; Galina, L.; Pijoan, C. Development of a PCR test to diagnose Haemophilus parasuis infections. J. Vet. Diagn. Investig. 2001, 13, 495–501. [Google Scholar] [CrossRef]
- Prüller, S.; Turni, C.; Blackall, P.J.; Beyerbach, M.; Klein, G.; Kreienbrock, L.; Strutzberg-Minder, K.; Kaspar, H.; Meemken, D.; Kehrenberg, C. Towards a standardized method for broth microdilution susceptibility testing of Haemophilus parasuis. J. Clin. Microbiol. 2017, 55, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Brogden, S.; Pavlović, A.; Tegeler, R.; Kaspar, H.; De Vaan, N.; Kehrenberg, C. Antimicrobial susceptibility of Haemophilus parasuis isolates from Germany by use of a proposed standard method for harmonized testing. Vet. Microbiol. 2018, 217, 32–35. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, G.; Liu, M.; Tai, C.; Deng, Z.; Song, J.; Ou, H.Y. ICEberg 3.0: Functional categorization and analysis of the integrative and conjugative elements in bacteria. Nucleic Acids Res. 2024, 52, D732–D737. [Google Scholar] [CrossRef]
- Dayao, D.A.E.; Kienzle, M.; Gibson, J.S.; Blackall, P.J.; Turni, C. Use of a proposed antimicrobial susceptibility testing method for Haemophilus parasuis. Vet. Microbiol. 2014, 172, 586–589. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Bu, M.X.; Liu, P.; Sun, J.; Liu, Y.H.; Liao, X.P. Epidemiological and PK/PD cutoff values determination and PK/PD-based dose assessment of gamithromycin against Haemophilus parasuis in piglets. BMC Vet. Res. 2020, 16, 81. [Google Scholar] [CrossRef]
- Sun, H.R.; Cui, X.D.; Liu, X.K.; Li, S.H.; Yi, K.F.; Pan, Y.S.; Wu, H.; Yuan, L.; Hu, G.Z.; He, D.D. Molecular characterization of a novel integrative conjugative element ICEHpa1 in Haemophilus parasuis. Front. Microbiol. 2020, 11, 1884. [Google Scholar] [CrossRef]
- Liu, J.; Tan, C.; Li, J.; Chen, H.; Xu, P.; He, Q.; Bei, W.; Chen, H. Characterization of ISApl1, an insertion element identified from Actinobacillus pleuropneumoniae field isolate in China. Vet. Microbiol. 2008, 132, 348–354. [Google Scholar] [CrossRef]
- Roy Chowdhury, P.; Alhamami, T.; Venter, H.; Veltman, T.; Carr, M.; Mollinger, J.; Trott, D.J.; Djordjevic, S.P. Identification and evolution of ICE-Pmu ST394: A novel integrative conjugative element in Pasteurella multocida ST394. J. Antimicrob. Chemother. 2024, 79, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xia, L.; Pan, R.; Xuan, H.; Guo, H.; Song, Q.; Wei, J.; Shao, D.; Liu, K.; Li, Z. Identification of mcr-1 and a novel chloramphenicol resistance gene catT on an integrative and conjugative element in an Actinobacillus strain of swine origin. Vet. Microbiol. 2021, 254, 108983. [Google Scholar] [CrossRef]
- An, J.; Guo, G.; Yu, D.; Zhu, K.; Zhang, C.; Li, Y. ICEHpsaHPS7, a novel multiple drug resistance integrative conjugative element in Glaesserella parasuis. Antimicrob. Agents Chemother. 2021, 65, e01716-20. [Google Scholar] [CrossRef]
- Sun, H.; Yang, Y.; Yi, K.; Zhang, M.; Luo, X.; He, D.; Hu, G.; Wu, H. ICEGpa1804, a novel integrative and conjugative element carrying eight resistance genes, identified in Glaesserella parasuis. Int. J. Antimicrob. Agents 2023, 61, 106740. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).