Reeve’s Muntjac (Muntiacus reevesi) Habitat Suitability Under Climate Change Scenarios in Hupingshan National Nature Reserve, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
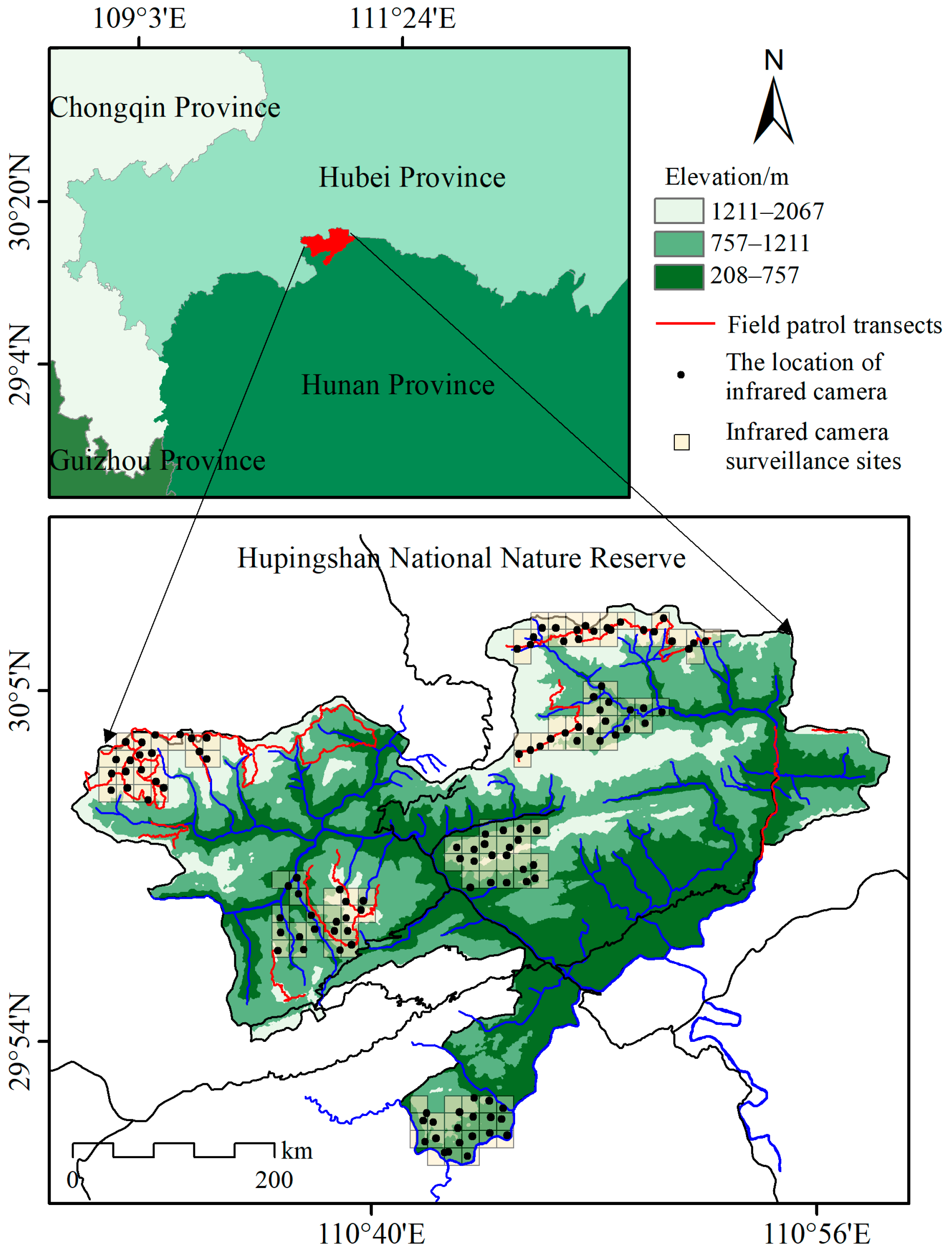
2.1. Study Area
2.2. Data and Sources
2.3. Data Preparation
2.4. Evaluation of Habitat Distribution in Current and Future Periods
2.5. Analysis of Climate Change-Induced Habitat Changes
3. Results
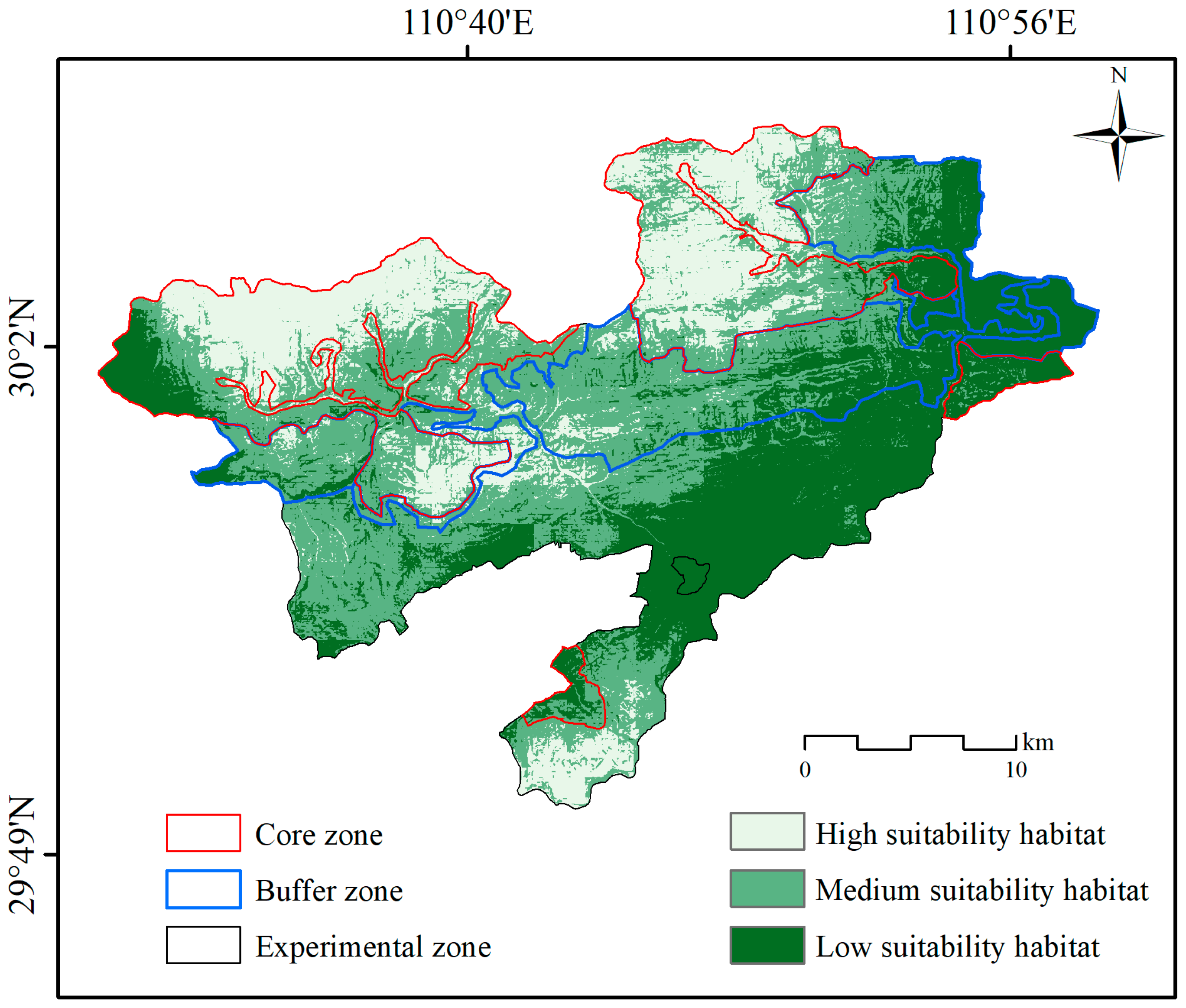
3.1. Current Habitat Distribution
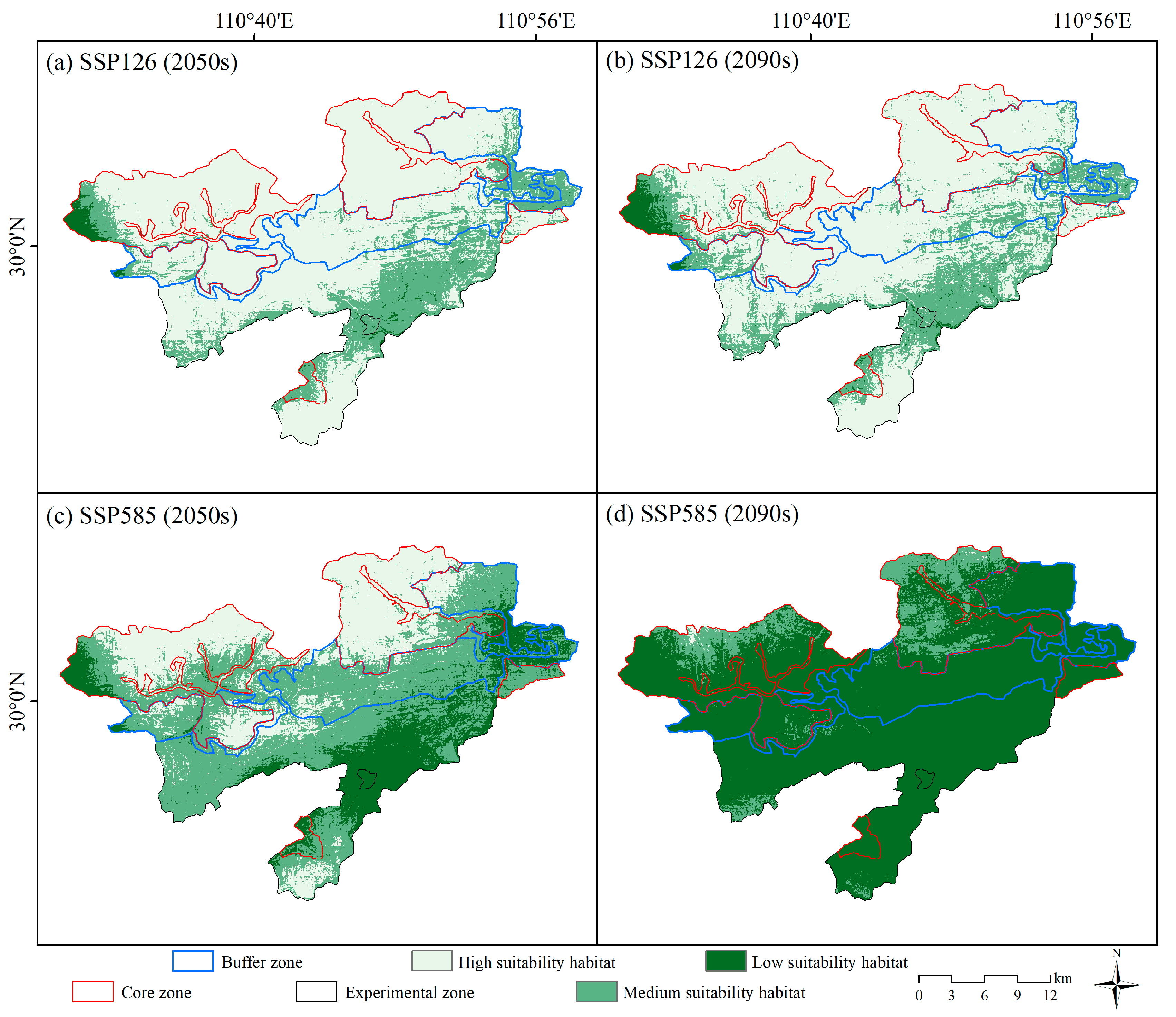
3.2. Future Habitat Distribution
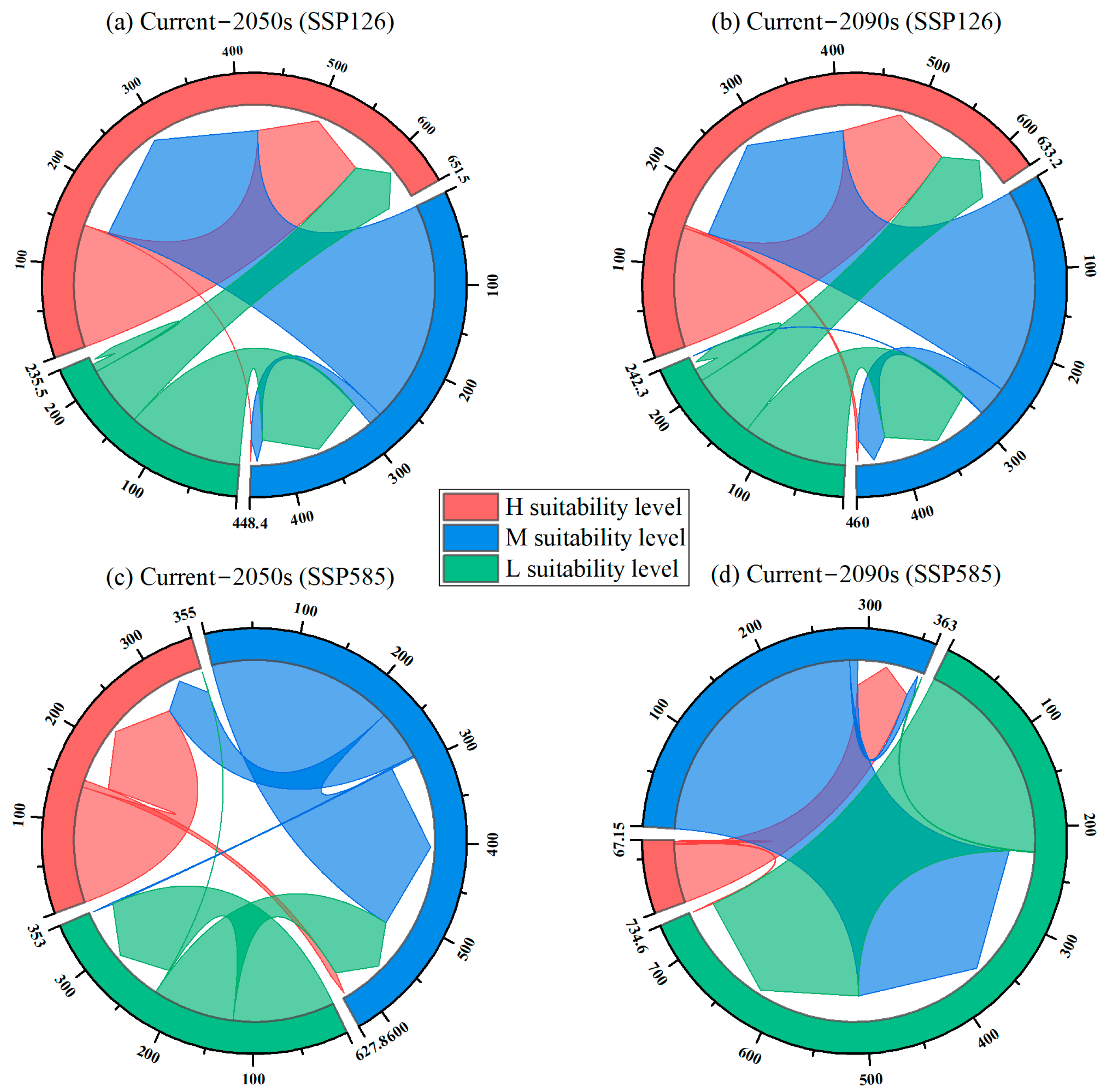
3.3. Climate Change-Induced Habitat Changes Under Different Future Periods
4. Discussion
4.1. Selection of Environmental Factors
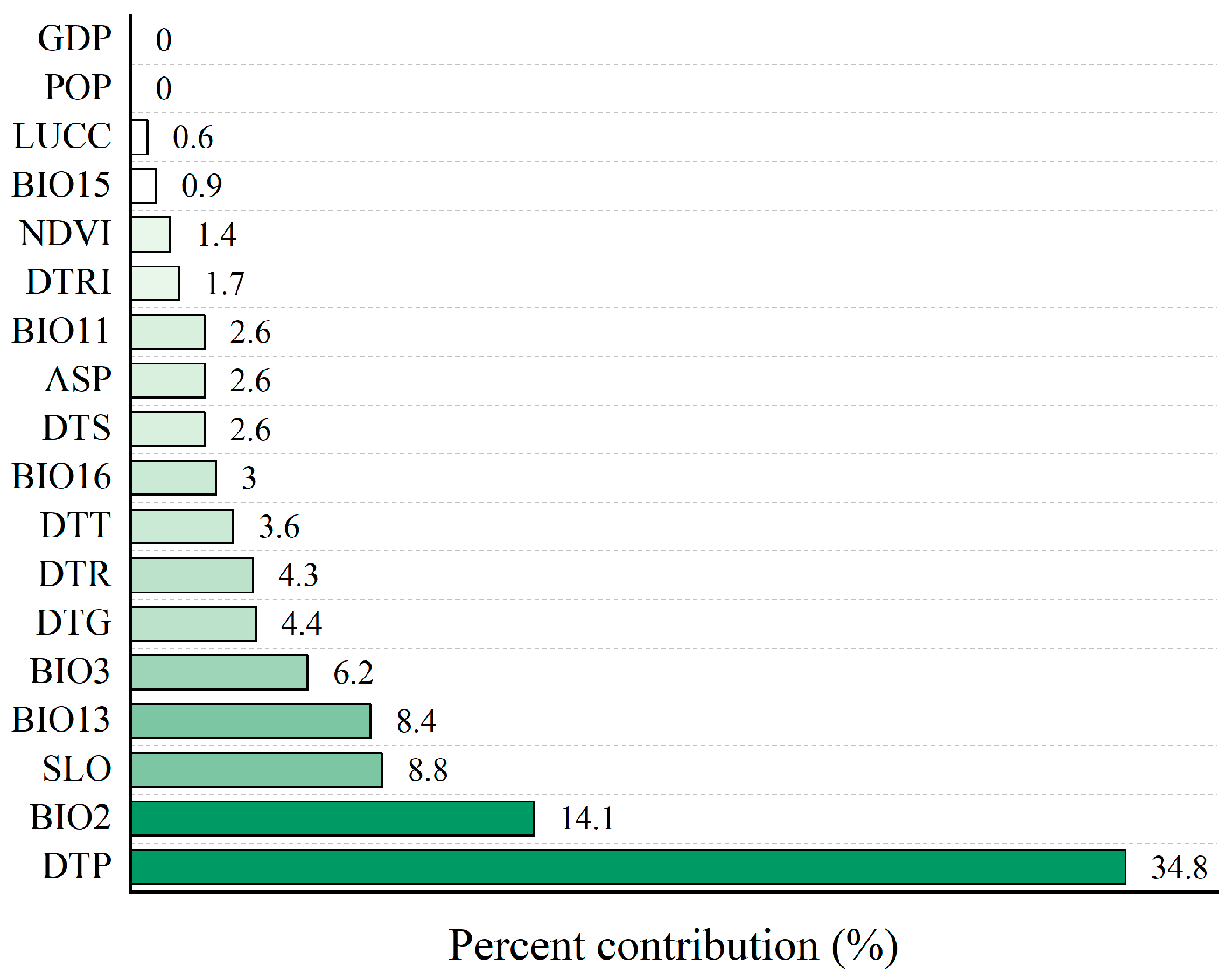
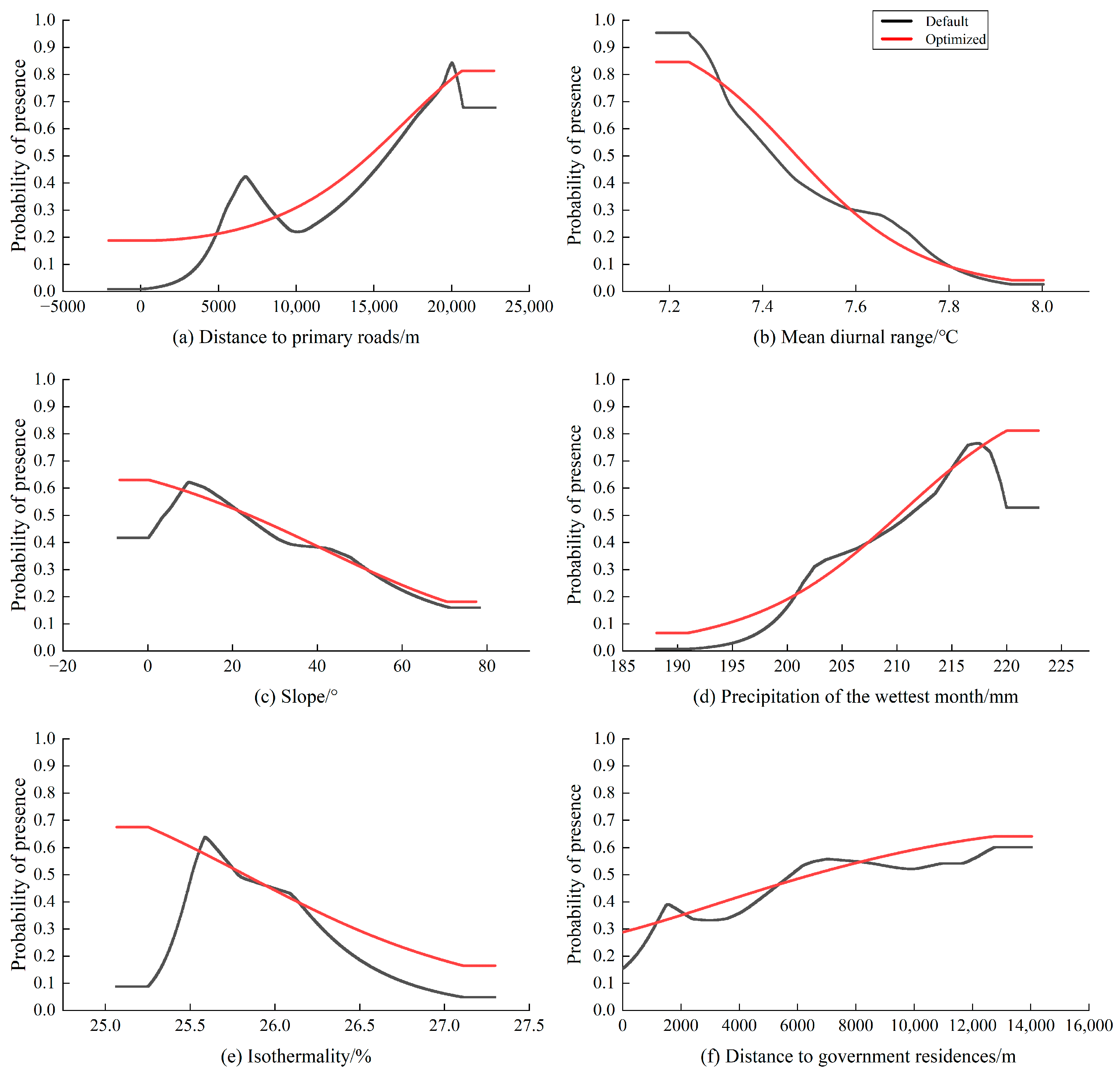
4.2. Analysis of Major Environmental Factors Affecting Habitat Selection
4.3. Impact of Climate Change
4.4. Protection of the South China Tiger
4.5. Additional Considerations and Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henry, E.H.; Burford, R.M.; Land, A.D.; Haddad, N.M. Maintaining historic disturbance regimes increases species’ resilience to catastrophic hurricanes. Glob. Chang. Biol. 2020, 26, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.; Gall, M.L.; Campbell, H.; Lamare, M.D.; Holmes, S.P. Staying in place and moving in space: Contrasting larval thermal sensitivity explains distributional changes of sympatric sea urchin species to habitat warming. Glob. Chang. Biol. 2022, 28, 3040–3053. [Google Scholar] [CrossRef]
- Johnson, T.F.; Isaac, N.J.B.; Paviolo, A.; González-Suárez, M. Socioeconomic factors predict population changes of large carnivores better than climate change or habitat loss. Nat. Commun. 2023, 14, 74. [Google Scholar] [CrossRef]
- Sun, Z.; Orozco-terWengel, P.; Chen, G.; Sun, R.; Sun, L.; Wang, H.; Shi, W.; Zhang, B. Spatial dynamics of Chinese Muntjac related to past and future climate fluctuations. Curr. Zool. 2021, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- IUCN. The IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2020. [Google Scholar]
- Tourinho, L.; Vale, M.M. Choosing among correlative, mechanistic, and hybrid models of species’ niche and distribution. Integr. Zool. 2023, 18, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Bechert, U.S.; Turner, J.W.; Baker, D.L.; Eckery, D.C.; Bruemmer, J.E.; Lyman, C.C.; Prado, T.M.; King, S.R.B.; Fraker, M.A. Fertility control options for management of free-roaming horse populations. Hum.-Wildl. Interact. 2022, 16, 179–216. [Google Scholar]
- Virkkala, R.; Leikola, N.; Kujala, H.; Kivinen, S.; Hurskainen, P.; Kuusela, S.; Valkama, J.; Heikkinen, R.K. Developing fine-grained nationwide predictions of valuable forests using biodiversity indicator bird species. Ecol. Appl. 2022, 32, e2505. [Google Scholar] [CrossRef] [PubMed]
- Linchant, J.; Lejeune, P.; Quevauvillers, S.; Vermeulen, C.; Brostaux, Y.; Lhoest, S.; Michez, A. Evaluation of an Innovative Rosette Flight Plan Design for Wildlife Aerial Surveys with UAS. Drones 2023, 7, 208. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, H.; Zhou, W.; Shi, W.; Zhu, W.; Zhang, B. How rivers and historical climate oscillations impact on genetic structure in Chinese Muntjac (Muntiacus reevesi)? Divers. Distrib. 2019, 25, 116–128. [Google Scholar] [CrossRef]
- Hu, J.; Xie, P.G.; Li, T.T.; Guo, R.; Xu, L.J.; Song, X.; Xu, A.C. Temporal and spatial niche differentiation of sympatric black muntjac and Reeves’ muntjac. Acta Theriol. Sin. 2022, 42, 641–651. [Google Scholar] [CrossRef]
- Meng, B.S.; Huang, X.L.; Xie, B.; Wang, W.X.; Huang, J.C.; Zhang, T.; Ran, J.C.; Zhang, M.M. Distribution of suitable habitat for ungulates in Fanjingshan National Nature Reserve, Guizhou Province. Acta Theriol. Sin. 2023, 43, 664–675. [Google Scholar] [CrossRef]
- Shi, F.N.; Liu, S.L.; An, Y.; Sun, Y.X.; Zhao, S.; Liu, Y.X.; Li, M.Q. Climatic factors and human disturbance influence ungulate species distribution on the Qinghai-Tibet Plateau. Sci. Total Environ. 2023, 869, 161681. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yao, W.; Ma, Y.; Shang, E.; Zhang, S.; Chen, F.; Zeng, Y. Optimizing natural boundary definition and functional zoning in protected areas: An integrated framework encompassing species, landscapes and ecosystems. Glob. Ecol. Conserv. 2024, 49, e02781. [Google Scholar] [CrossRef]
- Ahmadi, M.; Hemami, M.R.; Kaboli, M.; Shabani, F. MaxEnt brings comparable results when the input data is being completed; model parameterization and background manipulation of four species distribution models. Ecol. Evol. 2023, 13, e9827. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.S.; Dick, J.T.A.; Reid, N. Dealing with non-equilibrium bias and survey effort in presence-only invasive Species Distribution Models (iSDM); predicting the range of muntjac deer in Britain and Ireland. Ecol. Inform. 2022, 69, 101683. [Google Scholar] [CrossRef]
- Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J. Maxent is not a presence–absence method: A comment on Thibaud et al. Methods Ecol. Evol. 2014, 5, 1192–1197. [Google Scholar] [CrossRef]
- Dimson, M.; Berio Fortini, L.; Tingley, M.; Gillespie, T. Citizen science can complement professional invasive plant surveys and improve estimates of suitable habitat. Divers. Distrib. 2023, 29, 1141–1156. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Wang, G.; Zhu, P.B.D.; Liu, W.; Li, C.; Jin, K. Morphological, Phaneroptic, Habitat and Population Description of Three Muntjac Species in a Tibetan Nature Reserve. Animals 2022, 12, 2909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hughes, A.C.; Zhao, Z.H.; Li, Z.H.; Qin, Y.J. Including climate change to predict the global suitable area of an invasive pest: Bactrocera correcta (Diptera: Tephritidae). Glob. Ecol. Conserv. 2022, 34, e02021. [Google Scholar] [CrossRef]
- Qin, Y.; Nyhus, P. South China Tiger Prey Habitat Suitability Assessment in Hupingshan-Houhe National Nature Reserve Complex, China. Atlas Maine 2010, 2010, 4. [Google Scholar]
- Tian, S.R.; Wang, M.X.; Jiang, G.S.; Li, L.; Kang, Z.J.; Huang, T.F.; Gui, X.J. Population Viability Analysis on Reintroduction of South China Tiger (Panthera tigris amoyensis) at East Wuling Mountain Range. Chin. J. Wildl. 2022, 43, 5–16. [Google Scholar] [CrossRef]
- Nyhus, P. Panthera tigris ssp. amoyensis (South China Tiger). In The IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2008. [Google Scholar]
- Majumder, A. Prey Selection, Food Habits and Population Structure of Sympatric Cornivores: Tiger Panthera tigris tigris (L.), Leopard Panthera pardus (L.) and Dhole Cuon alpinus (Pallas) in Pench Tiger Reserve, Madhya Pradesh (India). Ph.D. Thesis, Saurastra University, Rajkot, India, 2011. [Google Scholar]
- Tilson, R.; Defu, H.; Muntifering, J.; Nyhus, P.J. Dramatic decline of wild South China tigers Panthera tigris amoyensis: Field survey of priority tiger reserves. Oryx 2004, 38, 40–47. [Google Scholar] [CrossRef]
- Qin, Y.; Nyhus, P.J.; Larson, C.L.; Carroll, C.J.W.; Muntifering, J.; Dahmer, T.D.; Jun, L.; Tilson, R.L. An assessment of South China tiger reintroduction potential in Hupingshan and Houhe National Nature Reserves, China. Biol. Conserv. 2015, 182, 72–86. [Google Scholar] [CrossRef]
- Karanth, K.U.; Nichols, J.D.; Kumar, N.S.; Link, W.A.; Hines, J.E. Tigers and their prey: Predicting carnivore densities from prey abundance. Proc. Natl. Acad. Sci. USA 2004, 101, 4854–4858. [Google Scholar] [CrossRef] [PubMed]
- Dahmer, T.D.; Gui, X.; Tian, S. Camera-trapping for South China Tiger in Hupingshan National Nature Reserve, Hunan Province, China. Chin. J. Wildl. 2014, 35, 19–25. [Google Scholar]
- Tang, T.; Li, J.; Sun, H.; Deng, C. Priority areas identified through spatial habitat suitability index and network analysis: Wild boar populations as proxies for tigers in and around the Hupingshan and Houhe National Nature Reserves. Sci. Total Environ. 2021, 774, 145067. [Google Scholar] [CrossRef]
- Tian, S.R.; Yang, C.C.; Jiang, B.W.; Du, j.; Liao, Q.Y.; Li, D.Q. Spatial and Temporal Patterns of Amphibian and Reptile Diversity in Hupingshan National Nature Reserve. Chin. J. Zool. 2019, 54, 825–834. [Google Scholar] [CrossRef]
- Yu, G.; Kang, Z.; Liu, M.; Chen, Z.; Deng, Z. Preliminary survey using infrared camera reveals fauna and avifauna diversity at Hupingshan National Nature Reserve, Hunan, China. Acta Theriol. Sin. 2018, 38, 104–112. [Google Scholar] [CrossRef]
- Xie, J.Y.; Kang, Z.J.; Yang, J.; Yang, D.D. Length–weight relationships for 15 fish species from the Hunan Hupingshan National Nature Reserve in central China. J. Appl. Ichthyol. 2015, 31, 221–222. [Google Scholar] [CrossRef]
- Li, W.; Yan, X.H.; Zhang, Q.; Zhang, J.B.; Duan, W.W. Research Status of South China Tiger. J. Econ. Anim. 2020, 24, 115–118. [Google Scholar] [CrossRef]
- Hupingshan. Hupingshan National Nature Reserve Management Plan; Hunan Hupingshan National Nature Reserve Administration: Hupingshan, China, 2004. [Google Scholar]
- Hu, J.; Xie, P.G.; Mei, Y.Y.; Guo, R.; Song, X.; Li, T.T.; Xu, A.C. Potential suitable habitats prediction and overlap analysis of black muntjac (Muntiacus crinifrons) and small muntjac (M. reevesi) in Qingliangfeng National Nature Reserve, Zhejiang Province. Acta Ecol. Sin. 2023, 43, 2210–2219. [Google Scholar] [CrossRef]
- Tang, J.; Lu, H.; Xue, Y.; Li, J.; Li, G.; Mao, Y.; Deng, C.; Li, D. Data-driven planning adjustments of the functional zoning of Houhe National Nature Reserve. Glob. Ecol. Conserv. 2021, 29, e01708. [Google Scholar] [CrossRef]
- Ahmed, N.; Atzberger, C.; Zewdie, W. Species Distribution Modelling performance and its implication for Sentinel-2-based prediction of invasive Prosopis juliflora in lower Awash River basin, Ethiopia. Ecol. Process. 2021, 10, 18. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, Z.X.; Zhang, J.M.; Zhu, B.; Tian, J. Projection of the potential distribution of suitable habitats for Siberian crane (Grus leucogeranus) in the middle and lower reaches of the Yangtze River basin. Front. Earth Sci 2023, 11, 1193677. [Google Scholar] [CrossRef]
- Try, S.; Tanaka, S.; Tanaka, K.; Sayama, T.; Khujanazarov, T.; Oeurng, C. Comparison of CMIP5 and CMIP6 GCM performance for flood projections in the Mekong River Basin. J. Hydrol. Reg. Stud. 2022, 40, 101035. [Google Scholar] [CrossRef]
- Hemami, M.R.; Watkinson, A.R.; Dolman, P.M. Habitat selection by sympatric muntjac (Muntiacus reevesi) and roe deer (Capreolus capreolus) in a lowland commercial pine forest. For. Ecol. Manag. 2004, 194, 49–60. [Google Scholar] [CrossRef]
- Kong, F.Q.; Shen, B.W.; Li, X.Y.; Zhou, Y.X.; Li, Y.K.; Li, J.Q.; Wan, Y.Q.; Zhan, J.W.; Liu, W.H.; Hu, H.J.; et al. Analysis of seasonal activity rhythm and interspecific differences in sympatric ungulates in Jiangxi Taohongling Reserve. Chin. J. Wildl. 2024, 45, 242–250. [Google Scholar]
- Lu, Q.B.; You, W.Y.; Gao, X.; Yu, A.J.; Yang, X.Y.; Zhou, Q.; Zhang, S.Y.; Weng, D.M. Evaluation on Selective Factors of Habitat of Muntiacus reevesi in Qingliangfeng National Nature Reserve. J. Zhejiang For. Sci. Technol. 2007, 27, 28–32. [Google Scholar]
- Ma, Z.Y.; LI, J.Q.; Wan, Y.Q.; Li, Y.K.; Shan, J.H.; Wang, Z.Y.; Shao, R.Q.; Zhang, C.; Li, X.Y. Regional Differences in the Spatial Distribution and Activity Rhythms of Muntjacs in Subtropical Forests. J. Ecol. Rural Environ. 2024, 40, 222–232. [Google Scholar] [CrossRef]
- Luo, Z.H.; Jiang, Z.G.; Tang, S.H. Impacts of climate change on distributions and diversity of ungulates on the Tibetan Plateau. Ecol. Appl. 2015, 25, 24–38. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Feng, X.; Park, D.S.; Liang, Y.; Pandey, R.; Papeş, M. Collinearity in ecological niche modeling: Confusions and challenges. Ecol. Evol. 2019, 9, 10365–10376. [Google Scholar] [CrossRef] [PubMed]
- Kufa, C.A.; Bekele, A.; Atickem, A. Impacts of climate change on predicted habitat suitability and distribution of Djaffa Mountains Guereza (Colobus guereza gallarum, Neumann 1902) using MaxEnt algorithm in Eastern Ethiopian Highland. Glob. Ecol. Conserv. 2022, 35, e02094. [Google Scholar] [CrossRef]
- Zhao, G.H.; Cui, X.Y.; Sun, J.J.; Li, T.T.; Wang, Q.; Ye, X.Z.; Fan, B.G. Analysis of the distribution pattern of Chinese Ziziphus jujuba under climate change based on optimized biomod2 and MaxEnt models. Ecol. Indic. 2021, 132, 108256. [Google Scholar] [CrossRef]
- Yang, X.-Q.; Kushwaha, S.P.S.; Saran, S.; Xu, J.; Roy, P.S. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia adhatoda L. in Lesser Himalayan foothills. Ecol. Eng. 2013, 51, 83–87. [Google Scholar] [CrossRef]
- Xue, Y.; Li, J.; Zhang, Y.; Li, D.; Yuan, L.; Cheng, Y.; Liu, S.; Hacker, C.E. Assessing the vulnerability and adaptation strategies of wild camel to climate change in the Kumtag Desert of China. Glob. Ecol. Conserv. 2021, 29, e01725. [Google Scholar] [CrossRef]
- Fitzgibbon, A.; Pisut, D.; Fleisher, D. Evaluation of Maximum Entropy (Maxent) Machine Learning Model to Assess Relationships between Climate and Corn Suitability. Land 2022, 11, 1382. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Kass, J.; Muscarella, R.; Galante, P.; Bohl, C.; Pinilla-Buitrago, G.; Boria, R.; Soley-Guardia, M.; Anderson, R. ENMeval 2.0: Redesigned for customizable and reproducible modeling of species’ niches and distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Campos, J.C.; Garcia, N.; Alírio, J.; Arenas-Castro, S.; Teodoro, A.C.; Sillero, N. Ecological Niche Models using MaxEnt in Google Earth Engine: Evaluation, guidelines and recommendations. Ecol. Inform. 2023, 76, 102147. [Google Scholar] [CrossRef]
- Yalcin, M.; Sari, F.; Yildiz, A. Exploration of potential geothermal fields using MAXENT and AHP: A case study of the Büyük Menderes Graben. Geothermics 2023, 114, 102792. [Google Scholar] [CrossRef]
- Chadaeva, V.; Pshegusov, R. Identification of degradation factors in mountain semiarid rangelands using spatial distribution modelling and ecological niche theory. Geocarto Int. 2022, 37, 15235–15251. [Google Scholar] [CrossRef]
- Sánchez-Tolentino, L.A.; Pérez-Sato, J.A.; Trejo-Téllez, L.; Sánchez-Páez, R.; Contreras-Oliva, A.; Hernández-Cázares, A.S.; Gómez-Merino, F.C. Distributional analysis of the stingless bee Scaptotrigona mexicana (Apidae: Meliponini) in Mexico: Baseline information for Veracruz. Agro Product. 2019, 12, 67–72. [Google Scholar] [CrossRef]
- West, A.M.; Kumar, S.; Brown, C.S.; Stohlgren, T.J.; Bromberg, J. Field validation of an invasive species Maxent model. Ecol. Inform. 2016, 36, 126–134. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Venne, S.; Currie, D.J. Can habitat suitability estimated from MaxEnt predict colonizations and extinctions? Divers. Distrib. 2021, 27, 873–886. [Google Scholar] [CrossRef]
- Duan, X.; Li, J.; Wu, S. MaxEnt Modeling to Estimate the Impact of Climate Factors on Distribution of Pinus densiflora. Forests 2022, 13, 402. [Google Scholar] [CrossRef]
- Reagh, J.J.; Zheng, H.; Stolz, U.; Parry, B.A.; Chang, A.M.; House, S.L.; Giordano, N.J.; Cohen, J.; Singer, A.J.; Francis, S.; et al. Sex-related differences in D-dimer levels for venous thromboembolism screening. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2021, 28, 873–881. [Google Scholar] [CrossRef]
- Liu, F.; Feng, C.; Zhou, Y.; Zhang, L.; Du, J.; Huang, W.; Luo, J.; Wang, W. Effectiveness of functional zones in National Nature Reserves for the protection of forest ecosystems in China. J. Environ. Manag. 2022, 308, 114593. [Google Scholar] [CrossRef]
- Qi, W.; Hu, Y.; Linsheng, Z.; Hui, W. Optimising the relationship between ecological protection and human development through functional zoning. Biol. Conserv. 2023, 281, 110001. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Zhang, L.; He, H.L.; Ren, X.L.; Lv, Y.; Niu, Z.E.; Chang, Q.Q.; Xu, Q.; Liu, W.H. Improvement of ecosystem quality in National Key Ecological Function Zones in China during 2000–2015. J. Environ. Manag. 2022, 324, 116406. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Cao, W.; Huang, L.; Xiao, T.; Zhai, J. The Impacts of Human Activities on Ecosystems within China’s Nature Reserves. Sustainability 2019, 11, 6629. [Google Scholar] [CrossRef]
- Zhao, L.; Du, M.X.; Zhang, W.; Li, C.J.; Liu, Q.Y.; Kang, X.; Zhou, D. Functional zoning in national parks under multifactor trade-off guidance: A case study of Qinghai Lake National Park in China. J. Geogr. Sci. 2022, 32, 1969–1997. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, L.; Zhou, X.; Chen, R.; Zhao, G.; Zhang, F. Prediction of the potentially suitable areas of Leonurus japonicus in China based on future climate change using the optimized MaxEnt model. Ecol. Evol. 2023, 13, e10597. [Google Scholar] [CrossRef] [PubMed]
- Underwood, A.J.; Chapman, M.G.; Crowe, T.P. Identifying and understanding ecological preferences for habitat or prey. J. Exp. Mar. Biol. Ecol. 2004, 300, 161–187. [Google Scholar] [CrossRef]
- Tian, S.R.; LI, Z.J.; Kang, Z.J.; Liao, Q.Y.; Li, D.Q. Study on readjustment of the area and function zones of Hunan Hupingshan National Nature Reserve and its impacts. For. Resour. Manag. 2019, 48, 21–29. [Google Scholar] [CrossRef]
- Yin, L.H.; Du, H.M.; Yu, T.; Yao, Z.Y.; Wan, M. Effects of High-speed Railway on the Spatial Distribution of Wildlife and Habitat Suitability Evaluation: A Case Study of Wuhan Wulongquan Section of Beijing-Guangzhou High-speed Railway. LA Ecol. 2024, 40, 97–103. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Zhao, Z.H.; Wang, Y.F.; Wei, L.; Ding, G.H.; Lin, Z.H. Analysis of activity rhythm and prediction of potential suitable distribution of black muntjac (Muntiacus crinifrons) based on the ITCT and MaxEnt model. Acta Ecol. Sin. 2021, 41, 3535–3547. [Google Scholar]
- Setoguchi, M. Food Habits of Red-Bellied Tree Squirrels on a Small Island in Japan. J. Mammal. 1990, 71, 570–578. [Google Scholar] [CrossRef]
- Gerson, A.R.; Smith, E.K.; Smit, B.; McKechnie, A.E.; Wolf, B.O. The impact of humidity on evaporative cooling in small desert birds exposed to high air temperatures. Physiol. Biochem. Zool. 2014, 87, 782–795. [Google Scholar] [CrossRef]
- Liu, X.; Qi, X.H.; Cheng, Y.; Zhang, H.G.; Cai, B. Activity Rhythm of Lophura nycthemera and Muntiacus reevesi in Wuyishan National Park and Its Influencing Factors. J. Hainan Norm. Univ. (Nat. Sci.) 2023, 36, 65–72. [Google Scholar]
- Chen, X. Study on the Activity Rhythm and Time Budget of Ungulates on the Southern Slope of the Qinling Mountains. Master’s Thesis, Shaanxi University of Technology, Hanzhong, China, 2023. [Google Scholar]
- Hull, V.; Zhang, J.; Zhou, S.; Huang, J.; Viña, A.; Liu, W.; Tuanmu, M.-N.; Li, R.; Liu, D.; Xu, W.; et al. Impact of livestock on giant pandas and their habitat. J. Nat. Conserv. 2014, 22, 256–264. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Z.; Tang, Z.; Zhao, S.; Fang, J. Geographic patterns and environmental correlates of terrestrial mammal species richness in China. Biodivers. Sci. 2009, 17, 652. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, J.; Lin, Y.; Chen, G.; Cao, Q.; Feng, Y. Land Change Simulation and Forest Carbon Storage of Central Yunnan Urban Agglomeration, China Based on SSP-RCP Scenarios. Forests 2022, 13, 2030. [Google Scholar] [CrossRef]
- Wu, J.G.; Lv, J.J.; Ai, L. The impacts of climate change on the biodiversity: Vulnerability and Adaptation. Ecol. Environ. Sci. 2009, 18, 693–703. [Google Scholar] [CrossRef]
- Li, D. Impacts of Climate Change on Suitable Habitat for Giant Pandas and Their Sympatric Mammals. Master’s Thesis, West Normal University, Nanchong, China, 2022. [Google Scholar]
- Ward, A.I.; Richardson, S.; Mergeay, J. Reeves’ muntjac populations continue to grow and spread across Great Britain and are invading continental Europe. Eur. J. Wildl. Res. 2021, 67, 34. [Google Scholar] [CrossRef]
- Karanth, K.; Sunquist, M. Behavioural correlates of predation by tiger (Panthera tigris), leopard (Panthera pardus) and dhole (Cuon alpinus) in Nagarahole, India. J. Zool. 2000, 250, 255–265. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Bell, L.E.; Donham, E.M.; Hoshijima, U.; Lummis, S.; Toy, J.A.; Willis-Norton, E. Ecological change in dynamic environments: Accounting for temporal environmental variability in studies of ocean change biology. Glob. Chang. Biol. 2020, 26, 54–67. [Google Scholar] [CrossRef]
| Category | Variable | Description | References |
|---|---|---|---|
| Vegetation factor | NDVI | Normalized difference vegetation index | [11,12,13,14,16,36,40,41,42,43,44] |
| LUCC | Land use/cover data | ||
| Topographic factor | ELE | Elevation | [4,11,12,13,14,16,36,41,42,43,44] |
| ASP | Aspect | ||
| SLO | Slope | ||
| Human activity factor | DTP | Distance to primary roads | [11,13,14,35,36,41,42,43,44] |
| DTS | Distance to secondary roads | ||
| DTT | Distance to tertial roads | ||
| DTR | Distance to residential roads | ||
| LUCC | Land use/cover data | ||
| GDP | Gross domestic product | ||
| POP | Population density | ||
| DTG | Distance to government residences | ||
| Hydrological factor | DTRI | Distance to rivers | [11,12,13,35,36] |
| DTWB | Distance to water bodies | ||
| Climatic factor | BIO1 | Annual mean temperature | [4,12,13,14,16,36,44] |
| BIO2 | Mean diurnal air temperature range | ||
| BIO3 | Isothermality | ||
| BIO4 | Temperature seasonality | ||
| BIO5 | Maximum temperature of the warmest month | ||
| BIO6 | Minimum temperature of the coldest month | ||
| BIO7 | Temperature annual range | ||
| BIO8 | Mean temperature of the wettest quarter | ||
| BIO9 | Mean temperature of the driest quarter | ||
| BIO10 | Mean temperature of the warmest quarter | ||
| BIO11 | Mean temperature of the coldest quarter | ||
| BIO12 | Annual precipitation | ||
| BIO13 | Precipitation of the wettest month | ||
| BIO14 | Precipitation of the driest month | ||
| BIO15 | Precipitation seasonality | ||
| BIO16 | Precipitation of the wettest quarter | ||
| BIO17 | Precipitation of the driest quarter | ||
| BIO18 | Precipitation of the warmest quarter | ||
| BIO19 | Precipitation of the coldest quarter |
| Number | K-Value | Significance | Changes in Future Habitat Suitability Levels Compared to Current Levels |
|---|---|---|---|
| 1 | 20 | Area is highly suitable in the current period but less suitable in the future | Descend (↓) |
| 2 | 19 | Area is highly suitable in the current period but moderately suitable level in the future | Descend (↓) |
| 3 | 18 | Area remains highly suitable in both current and future periods | Unchanged (―) |
| 4 | 10 | Area is moderately suitable in the current period but less suitable in the future | Descend (↓) |
| 5 | 9 | Area remains moderately suitable in both current and future periods | Unchanged (―) |
| 6 | 8 | Area is moderately suitable in the current period but highly suitable in the future | Ascend (↑) |
| 7 | 0 | Area remains less suitable in both current and future periods | Unchanged (―) |
| 8 | −1 | Area is less suitable in the current period but moderately suitable in the future | Ascend (↑) |
| 9 | −2 | Area is less suitable in the current period but highly suitable in the future | Ascend (↑) |
| Core Zone | Buffer Zone | Experimental Zone | Total Area/km2 | Proportion/% | ||||
|---|---|---|---|---|---|---|---|---|
| Area/km2 | Proportion/% | Area/km2 | Proportion/% | Area/km2 | Proportion/% | |||
| High suitability area | 114.5 | 47.0 | 11.5 | 6.7 | 24.7 | 9.9 | 150.9 | 22.6 |
| Medium suitability area | 95.0 | 39.0 | 95.3 | 55.0 | 100.2 | 40.0 | 290.9 | 43.6 |
| Low suitability area | 34.0 | 14.0 | 66.3 | 38.3 | 125.4 | 50.1 | 225.9 | 33.8 |
| SSP126 | SSP585 | |||
|---|---|---|---|---|
| 2050s | 2090s | 2050s | 2090s | |
| Area/km2 | Area/km2 | Area/km2 | Area/km2 | |
| High suitability habitat | 500.5 | 482.2 | 203.8 | 1.5 |
| Medium suitability habitat | 157.5 | 169.1 | 336.9 | 72.1 |
| Low suitability habitat | 9.7 | 16.4 | 127.0 | 594.1 |
| K-Value | SSP126 | SSP585 | ||
|---|---|---|---|---|
| 2050s | 2090s | 2050s | 2090s | |
| Area/km2 | Area/km2 | Area/km2 (2050s) | Area/km2 (2090s) | |
| 20 | 0 | 0 | 0 | 86.9 |
| 19 | 0 | 3.5 | 8.3 | 62.5 |
| 18 | 150.9 | 147.4 | 142.6 | 1.5 |
| 10 | 0 | 0.7 | 1.9 | 282.4 |
| 9 | 16.0 | 37.2 | 227.8 | 8.5 |
| 8 | 274.9 | 253.0 | 61.2 | 0 |
| 0 | 9.7 | 15.7 | 125.1 | 224.8 |
| −1 | 141.5 | 128.4 | 100.7 | 1.1 |
| −2 | 74.7 | 81.8 | 0.1 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Ye, J.; Kang, Z.; Yu, G.; Yang, C.; Li, J.; Tang, T. Reeve’s Muntjac (Muntiacus reevesi) Habitat Suitability Under Climate Change Scenarios in Hupingshan National Nature Reserve, China. Animals 2025, 15, 160. https://doi.org/10.3390/ani15020160
Liu Q, Ye J, Kang Z, Yu G, Yang C, Li J, Tang T. Reeve’s Muntjac (Muntiacus reevesi) Habitat Suitability Under Climate Change Scenarios in Hupingshan National Nature Reserve, China. Animals. 2025; 15(2):160. https://doi.org/10.3390/ani15020160
Chicago/Turabian StyleLiu, Qi, Jianyang Ye, Zujie Kang, Guiqing Yu, Cuncun Yang, Jianjun Li, and Tao Tang. 2025. "Reeve’s Muntjac (Muntiacus reevesi) Habitat Suitability Under Climate Change Scenarios in Hupingshan National Nature Reserve, China" Animals 15, no. 2: 160. https://doi.org/10.3390/ani15020160
APA StyleLiu, Q., Ye, J., Kang, Z., Yu, G., Yang, C., Li, J., & Tang, T. (2025). Reeve’s Muntjac (Muntiacus reevesi) Habitat Suitability Under Climate Change Scenarios in Hupingshan National Nature Reserve, China. Animals, 15(2), 160. https://doi.org/10.3390/ani15020160





