The Observation of Meiotic Union Behavior of Gametophytes Provides a New Basis for Ploidy of Carassius auratus gibelio
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Consent to Participate
2.2. Materials
2.3. Preparation of Chromosome Specimen of Nephrocyte
2.4. Chromosome Counting and Karyotype Analysis
2.5. The Preparation and Observation of the Meiotic Chromosomes in Egg Nuclei
2.6. Preparation and Observation of Chromosome Specimen of Meiosis in Testis
2.7. Differential Staining
2.8. Fluorescence In Situ Hybridization
2.9. Chromosomes and Hybridization Signals Observation
3. Results
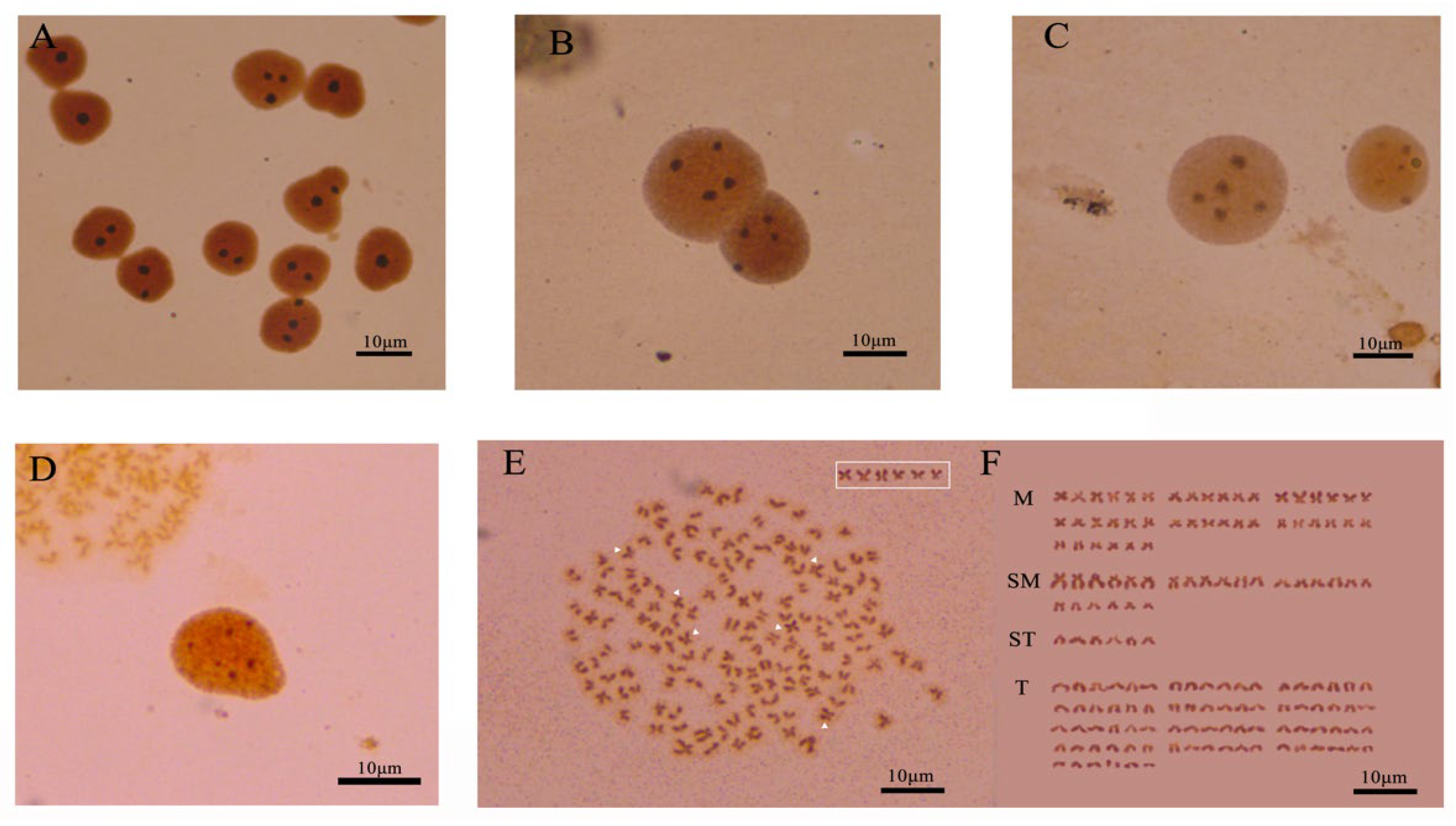
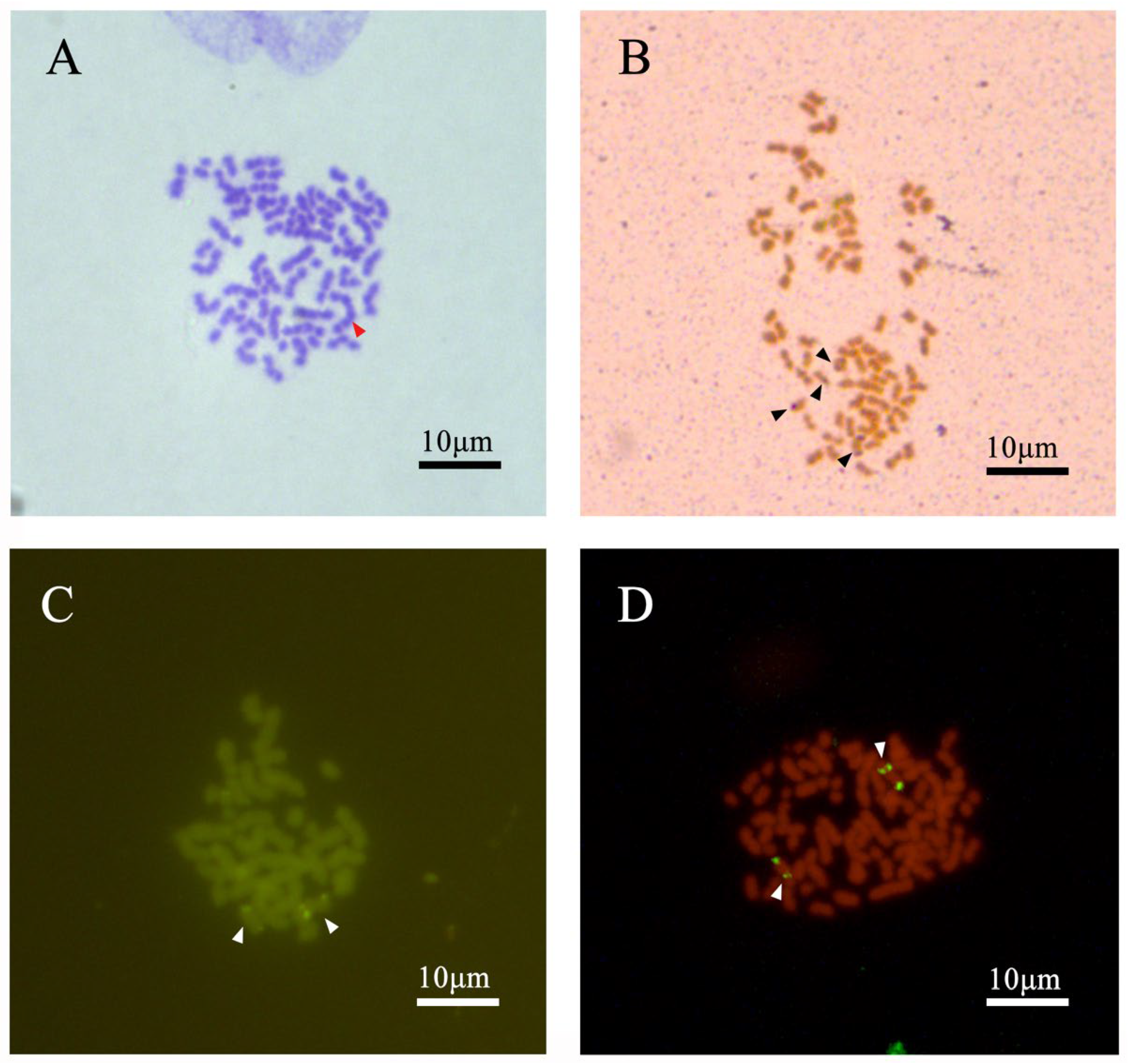
3.1. Chromosome Number and Karyotype of C. gibelio Kidney Cells
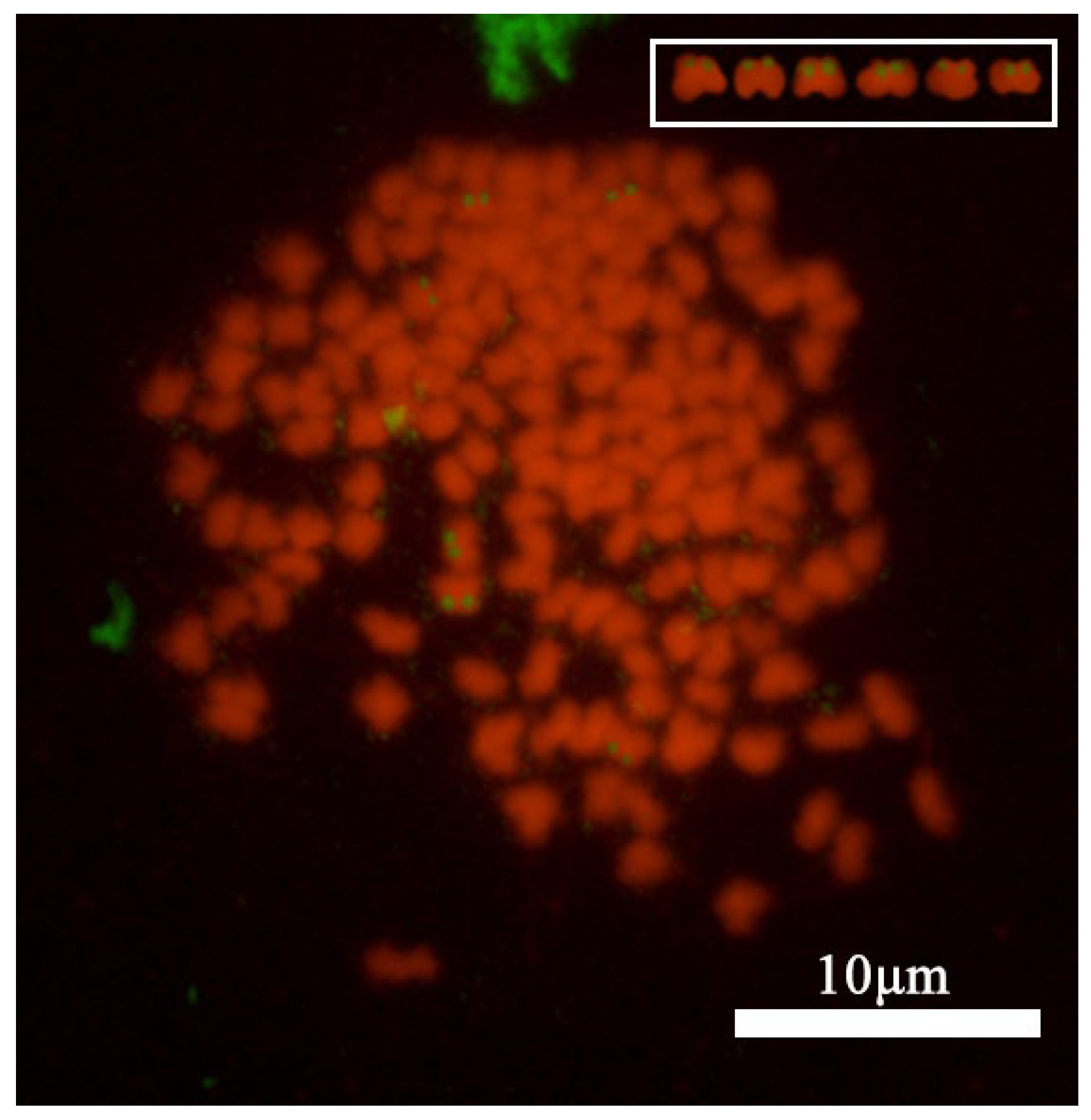
3.2. Analysis of Chromosome Banding in C. gibelio Kidney Cells
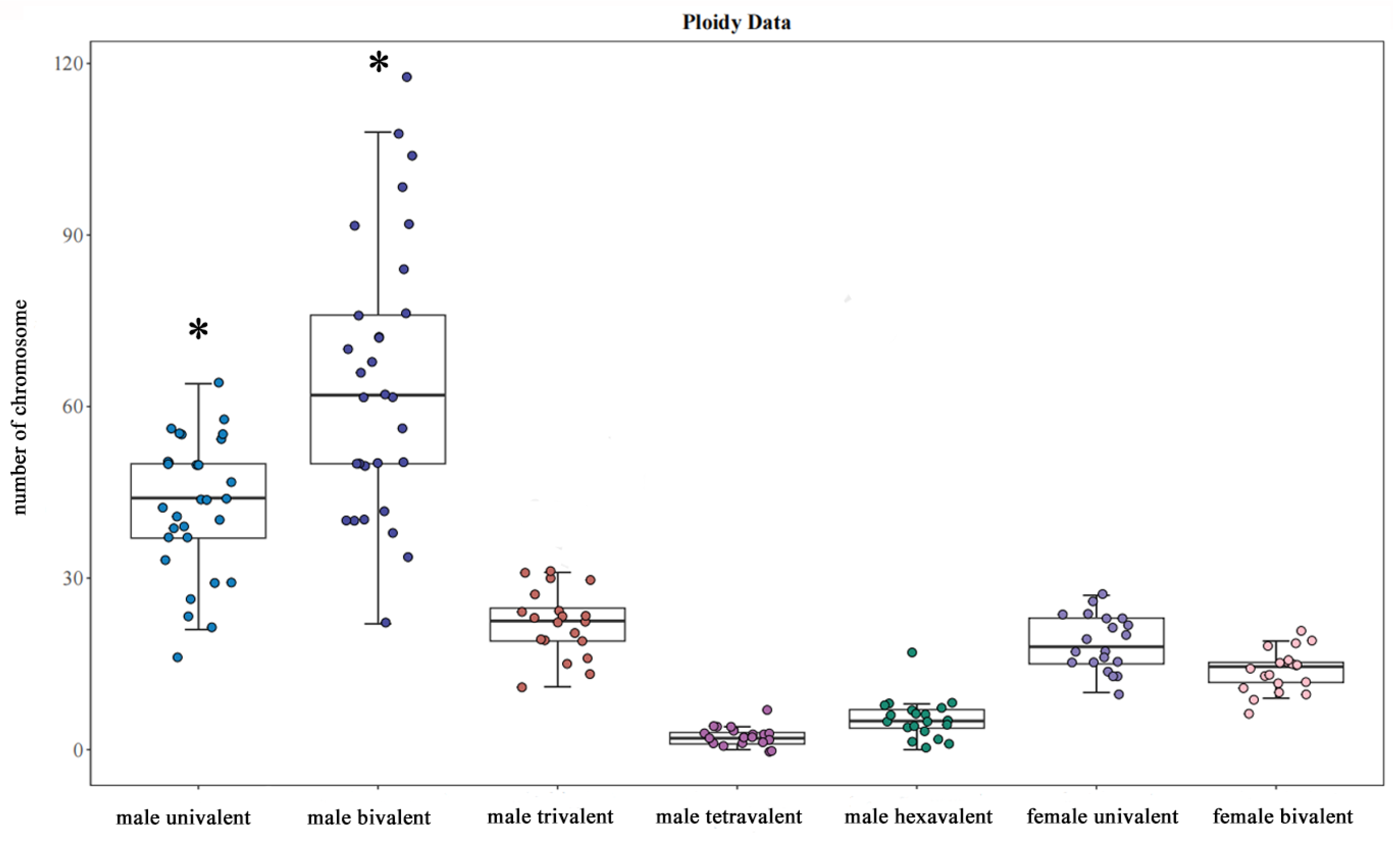
3.3. The Observation of Chromosome Behavior for Oocyte Meiosis
3.4. The Observation of Chromosome Behavior for Permatocyte Meiosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.L.; Li, X.Y.; Jiang, F.F.; Wang, Z.W.; Li, Z.; Zhang, X.J.; Zhou, L.; Gui, J.F. Numerous mitochondrial DNA haplotypes reveal multiple independent polyploidy origins of hexaploids in Carassius species complex. Ecol. Evol. 2017, 7, 10604–10615. [Google Scholar] [CrossRef]
- Zhou, L.; Gui, J. Natural and artificial polyploids in aquaculture. Aquac. Fish. 2017, 2, 103–111. [Google Scholar] [CrossRef]
- Lu, M.; Li, Z.; Zhu, Z.Y.; Peng, F.; Wang, Y.; Li, X.Y.; Wang, Z.W.; Zhang, X.J.; Zhou, L.; Gui, J.F. Changes in ploidy drive reproduction transition and genomic diversity in a polyploid fish complex. Mol. Biol. Evol. 2022, 39, msac188. [Google Scholar] [CrossRef]
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef]
- Jacques, F.; Tichopád, T.; Demko, M.; Bystrý, V.; Křížová, K.C.; Seifertová, M.; Voříšková, K.; Fuad, M.H.F.; Vetešník, L.; Šimková, A. Reproduction-associated pathways in females of gibel carp (Carassius gibelio) shed light on the molecular mechanisms of the coexistence of asexual and sexual reproduction. BMC genomics 2024, 25, 548. [Google Scholar] [CrossRef] [PubMed]
- Fuad, M.M.H.; Vetešník, L.; Šimková, A. Is gynogenetic reproduction in gibel carp (Carassius gibelio) a major trait responsible for invasiveness? J. Vertebr. Biol. 2021, 70, 21049.1–13. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.Y.; Xu, W.J.; Wang, K.; Wu, B.; Xu, M.; Chen, Y.; Miao, L.J.; Wang, Z.W.; Li, Z.; et al. Comparative genome anatomy reveals evolutionary insights into a unique amphitriploid fish. Nat. Ecol. Evol. 2022, 6, 1354–1366. [Google Scholar] [CrossRef]
- Van de Peer, Y.; Mizrachi, E.; Marchal, K. The evolutionary significance of polyploidy. Nat. Rev. Genet. 2017, 18, 411–424. [Google Scholar]
- Doyle, J.J.; Coate, J.E. Polyploidy, the nucleotype, and novelty: The impact of genome doubling on the biology of the cell. Int. J. Plant Sci. 2019, 180, 1–52. [Google Scholar] [CrossRef]
- Bersaglieri, C.; Santoro, R. Genome organization in and around the nucleolus. Cells 2019, 8, 579. [Google Scholar] [CrossRef]
- Banerjee, S.N. Nucleolar Organizer Region (NOR) Polymorphism in Some Indian Anuran Amphibian. Adv. Res. Biol. Sci. 2024, 8, 141–158. [Google Scholar]
- Mao, L.J.; Li, Y.J. Karyotype analyses of five species of marine fishes. J. Dalian Fish. Univ. 2002, 17, 108–113. [Google Scholar]
- Li, Y.J.; Mao, L.J.; Wang, Z.C. Chromosome ploidy test technique of Haliotis discus Hannai. J. Dalian Fish. Univ. 2002, 17, 297–300. [Google Scholar]
- Li, Y.J.; Tian, Y.; Zhang, M.Z.; Tian, P.P.; Yu, Z.; Abe, S.; Arai, K. Chromosome banding and FISH with rDNA probe in the diploid and tetraploid loach Misgurnus anguillicaudatus. Ichthyol. Res. 2010, 57, 358–366. [Google Scholar] [CrossRef]
- Li, Y.J.; Yu, Z.; Zhang, M.Z.; Qian, C.; Abe, S.; Arai, K. The origin of natural tetraploid loach Misgurnus anguillicaudatus (Teleostei: Cobitidae) inferred from meiotic chromosome configurations. Genetica 2011, 139, 805–811. [Google Scholar] [CrossRef]
- Howell, W.M.; Black, D.A. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: A 1-step method. experientia 1980, 36, 1014–1015. [Google Scholar] [CrossRef]
- Schweizer, D. Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosoma 1976, 58, 307–324. [Google Scholar] [CrossRef]
- Schweizer, D. Simultaneous fluorescent staining of R bands and specific heterochromatic regions (DA-DAPI bands) in human chromosomes. Cytogenet. Genome Res. 1980, 27, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, A.; Abe, S.; Yamaha, E.; Yamazaki, F.; Yoshida, M.C. Chromosomal localization and heterochromatin association of ribosomal RNA gene loci and silver-stained nucleolar organizer regions in salmonid fishes. Chromosome Res. 1998, 6, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Emiroğlu, Ö.; Bayramoğlu, G.; Öztürk, D.; Yaylacı, Ö.K. Determination of the gynogenetic reproduction character of Carassius gibelio in Uluabat Lake. Asian J. Anim. Vet. Adv. 2011, 6, 648–653. [Google Scholar] [CrossRef]
- Mao, H.L.; Ge, X.Q.; Liu, J.L.; Shu, H.D.; Chen, T. Karyotype Analysis of Pelteobagrus fulvidraco in Poyang Lake and Discussion of Its Evolutionary Position. Acta Agric. Univ. Jiangxiensis 2012, 34, 1222–1225. [Google Scholar]
- Ling, J.X. Studies on the karyotypes of eight species of fish. Wuhan Univ. J. Nat. Sci. 1982, 6, 111–114+123–124. [Google Scholar]
- Yang, R.J.; Li, B.X.; Feng, H.; Yang, C.; Sun, Y.; Zhang, X.; Luo, C. Cytogenetic analysis of chromosome number and ploidy of Carassius auratus variety pengze. Acta Zool. Sin. 2003, 49, 104–109. [Google Scholar]
- Wang, J.N.; Li, X.H.; An, M.; Zhou, Q.C. Chromosome Karyotype Analysis of Crucian carp in Caohai. Guizhou Agric. Sci. 2013, 41, 134–137. [Google Scholar]
- Shen, J.B.; Fan, Z.T.; Wang, G.R. Karyotype Studies of Male Triploid Crucian Carp (Fangzheng Crucian Carp) in Heilongjiang. Acta Genetica Sinica 1983, 10, 133–136+171–172. [Google Scholar]
- Li, X.J.; Wang, Y.W.; Gao, L.X.; Qiao, Q.Z. The morphometrical and chromosome differences in Crucian Carp Carassius auratus in Qihe River and in other waters. Fish. Sci. 2010, 29, 674–676. [Google Scholar]
- Sun, X.W. Biological characteristics of Qihe Crucian Carp. Freshw. Fish. 1986, 2, 5–8. [Google Scholar]
- Srikulnath, K.; Ahmad, S.F.; Singchat, W.; Panthum, T. Why do some vertebrates have microchromosomes? Cells 2021, 10, 2182. [Google Scholar] [CrossRef]
- Zan, R.G. Studies of sex chromosomes and C-banding karyotypes of two forms of Carassius auratus in Kunming Lake. Acta Genet. Sin. 1982, 9, 32–39. [Google Scholar]
- Wang, R.F.; Shi, L.M.; He, W.S. A comparative study of the Ag-NORs of Carassius auratus from different geographic districts. Zool. Res. 1988, 9, 165–169+208–210. [Google Scholar]
- Galetti, J.P.M. Chromosome diversity in neotropical fishes: NOR studies. Ital. J. Zool. 1998, 65, 53–56. [Google Scholar] [CrossRef]
- Foresti, F.; Almeida Toledo, L.F.; Toledo, F.S.A. Polymorphic nature of nucleolus organizer regions in fishes. Cytogenet. Genome Res. 1981, 31, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.F.; Zhou, L. Genetic basis and breeding application of clonal diversity and dual reproduction modes in polyploid Carassius auratus gibelio. Sci. China Life Sci. 2010, 53, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Sember, A.; Bohlen, J.; Šlechtová, V.; Altmanova, M.; Pelikánová, Š.; Rab, P. Dynamics of tandemly repeated DNA sequences during evolution of diploid and tetraploid botiid loaches (Teleostei: Cobitoidea: Botiidae). PLoS ONE 2018, 13, e0195054. [Google Scholar] [CrossRef] [PubMed]
- Balzano, E.; Giunta, S. Centromeres under pressure: Evolutionary innovation in conflict with conserved function. Genes 2020, 11, 912. [Google Scholar] [CrossRef] [PubMed]
- Sember, A.; Bohlen, J.; Šlechtová, V.; Altmanova, M.; Pelikánová, Š.; Rab, P. Evidence for DNA-mediated nuclear compartmentalization distinct from phase separation. Elife 2019, 8, e47098. [Google Scholar]
- Britton, D.J.; Cazaux, B.; Catalan, J. Chromosomal dynamics of nucleolar organizer regions (NORs) in the house mouse: Micro-evolutionary insights. Heredity 2012, 108, 68–74. [Google Scholar] [CrossRef]



| The Number of Ag-NORs Site | Number of Metaphases | The Number of Interphase Nuclei in a Single Cell | Number of Nucleus |
|---|---|---|---|
| 1 | 2 | 1 | 57 |
| 2 | 14 | 2 | 82 |
| 3 | 13 | 3 | 71 |
| 4 | 26 | 4 | 48 |
| 5 | 16 | 5 | 28 |
| 6 | 29 | 6 | 14 |
| Conformation | Number | Frequency |
|---|---|---|
| 118I + 16II | 1 | 3.3% |
| 108I + 21II | 1 | 3.3% |
| 104I + 23II | 1 | 3.3% |
| 98I + 26II | 1 | 3.3% |
| 92I + 29II | 2 | 6.7% |
| 84I + 33II | 1 | 3.3% |
| 76I + 37II | 2 | 6.7% |
| 72I + 39II | 2 | 6.7% |
| 70I + 40II | 1 | 3.3% |
| 68I + 41II | 1 | 3.3% |
| 66I + 42II | 1 | 3.3% |
| 62I + 44II | 3 | 10.0% |
| 56I + 47II | 1 | 3.3% |
| 50I + 50II | 5 | 16.7% |
| 42I + 54II | 1 | 3.3% |
| 40I + 55II | 3 | 10.0% |
| 38I + 56II | 1 | 3.3% |
| 34I + 58II | 1 | 3.3% |
| 22I + 64II | 1 | 3.3% |
| Conformation | Number | Frequency |
|---|---|---|
| 6I + 11II + 10III + 17IV + 4VI | 1 | 5% |
| 9I + 24II + 17III + 6IV + 3VI | 1 | 5% |
| 10I + 24II + 20III + 8IV | 1 | 5% |
| 10I + 30II + 14III + 8IV + 1VI | 1 | 5% |
| 11I + 30II + 17III + 4IV + 2VI | 1 | 5% |
| 12I + 23II + 16III + 5IV + 4VI | 1 | 5% |
| 12I + 13II + 26III + 5IV + 3VI | 1 | 5% |
| 13I + 19II + 27III + 3VI | 1 | 5% |
| 13I + 27II + 15III + 8IV + 1VI | 1 | 5% |
| 14I + 19II + 22III + 5IV + 2VI | 1 | 5% |
| 15I + 22II + 15III + 7IV + 3VI | 1 | 5% |
| 15I + 23II + 19III + 5IV + 2VI | 1 | 5% |
| 15I + 31II + 13III + 7IV + 1VI | 1 | 5% |
| 15I + 15II + 13III + 6IV + 7VI | 1 | 5% |
| 16I + 16II + 21III + 4IV + 4VI | 1 | 5% |
| 16I + 19II + 24III + 6IV | 1 | 5% |
| 17I + 31II + 15III + 3IV + 2VI | 1 | 5% |
| 19I + 20II + 23III + 1IV + 3VI | 1 | 5% |
| 19I + 31II + 15III + 3IV + 2VI | 1 | 5% |
| 21I + 22II + 23III + 1IV + 2VI | 1 | 5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, K.; Yang, Y.; Li, Y.; Li, C.; Li, T.; Ma, H.; Jiang, Z.; Zhou, H.; Wang, W. The Observation of Meiotic Union Behavior of Gametophytes Provides a New Basis for Ploidy of Carassius auratus gibelio. Animals 2025, 15, 140. https://doi.org/10.3390/ani15020140
Ma K, Yang Y, Li Y, Li C, Li T, Ma H, Jiang Z, Zhou H, Wang W. The Observation of Meiotic Union Behavior of Gametophytes Provides a New Basis for Ploidy of Carassius auratus gibelio. Animals. 2025; 15(2):140. https://doi.org/10.3390/ani15020140
Chicago/Turabian StyleMa, Kexin, Yueyao Yang, Yifan Li, Chuan Li, Taicheng Li, Haiyan Ma, Zibin Jiang, He Zhou, and Wei Wang. 2025. "The Observation of Meiotic Union Behavior of Gametophytes Provides a New Basis for Ploidy of Carassius auratus gibelio" Animals 15, no. 2: 140. https://doi.org/10.3390/ani15020140
APA StyleMa, K., Yang, Y., Li, Y., Li, C., Li, T., Ma, H., Jiang, Z., Zhou, H., & Wang, W. (2025). The Observation of Meiotic Union Behavior of Gametophytes Provides a New Basis for Ploidy of Carassius auratus gibelio. Animals, 15(2), 140. https://doi.org/10.3390/ani15020140






