Do Protein Supplementation Levels Influence the Performance of Male Nellore Calves Under a Grazing System at Pre-Weaning?
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Weather Conditions
2.2. Animals, Experimental Design, Diets and Management
2.3. Forage Samples and Nutritional Performance
2.4. Blood Samples and Measurements
2.5. Skeletal Muscle Collection and Histological Analyses
2.6. Productive Performance and Carcass Characteristics
2.7. Analytical Procedures
2.8. Statistical Analysis
3. Results
3.1. Intake, Digestibility, and Nitrogen Balance
3.2. Hormone and Metabolite Concentrations
3.3. Productive Performance and Carcass Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADG | Average daily gain |
| AP | Absorbed purines |
| apNDF | Neutral detergent fiber corrected for ash and protein residue |
| BCS | Body conditions score |
| BW | Body weight |
| CE | Daily creatinine excretion |
| CP | Crude protein |
| Cr2O3 | Chromium oxide |
| DM | Dry matter |
| DMI | Dry matter intake |
| DNDF | Digested neutral detergent fiber |
| DOM | Digested organic matter |
| EDTA | Ethylenediaminetetraacetic acid |
| EE | Ether extract |
| EFNU | Efficiency of nitrogen utilization |
| EMS | Efficiency of microbial synthesis |
| FBW | Final body weight |
| FDM | Forage dry matter |
| FE | Fecal dry matter excretion |
| FNE | Fecal nitrogen excretion |
| FP | Final pre-weaning |
| HI | High |
| IGF-1 | Insulin-like growth factor–1 |
| iNDF | Indigestible neutral detergent fiber |
| iNDFF | Concentration of iNDF in the feces |
| iNDFP | Amount of iNDF form pasture |
| iNDFS | Concentration of iNDF in the supplement |
| IP | Initial pre-weaning |
| ISI | Individual supplement intake |
| LO | Low |
| MI | Milk intake |
| MDM | Milk dry matter |
| MICNR | Microbial nitrogen to consumed nitrogen ratio |
| Milk4% | Milk production was corrected to 4% of fat |
| MM | Mineral matter |
| NB | Nitrogen balance |
| NDIA | Neutral detergent insoluble ash |
| NDIP | Neutral detergent insoluble protein |
| NFC | Non-fibrous carbohydrates |
| NI | Nitrogen intake |
| NMIC | Synthesis of nitrogenous compounds in the rumen |
| NRC | National Research Council |
| OM | Organic matter |
| PD | Purine derivatives |
| pdDM | Potentially digestible dry matter |
| SBW | Shrunk body weight |
| SEM | Standard error of means |
| SFTL | Subcutaneous fat thickness over the Longissimus muscle |
| SFTR | Subcutaneous fat thickness over the rump |
| SUN | Serum urea nitrogen |
| UEUN | Urinary excretion of urea nitrogen |
| UUN | Urinary urea nitrogen |
References
- Jammer, B.D.; Lombard, W.A.; Jordaan, H. Investigating cow-calf productive performance under early and conventional weaning practices in south african beef cattle. Vet. Anim. Sci. 2025, 29, 100472. [Google Scholar] [CrossRef]
- Almeida, K.C.; Bignardi, A.B.; Mercadante, M.E.Z.; El Faro, L.; Cyrillo, J.N.S.G.; Boligon, A.A.; Carvalheiro, R.; Pereira, R.J.; Santana, M.L. Longitudinal genetic dynamics of weaning index and implications for cow-calf production efficiency. Animal 2024, 18, 1751–7311. [Google Scholar] [CrossRef]
- Welk, A.; Neave, H.W.; Jensen, M.B. Invited review: The effect of weaning practices on dairy calf performance, behavior, and health—A systematic review. J. Dairy Sci. 2024, 107, 5237–5258. [Google Scholar] [CrossRef]
- Henriques, L.T.; Valadares Filho, S.C.; Fonseca, M.A.; Paulino, P.V.R.; Detmann, E.; Valadares, R.F.D. Avaliação de modelos não-lineares e da relação do consumo voluntário de vacas primíparas e de bezerros com a curva de lactação de vacas Nelore. Rev. Bras. Zootec. 2011, 40, 1287–1295. [Google Scholar] [CrossRef]
- Costa e Silva, L.F.; Engle, T.E.; Valadares Filho, S.C.; Rotta, P.P.; Villadiego, F.A.C.; Silva, F.A.S.; Martins, E.C.; Silva, L.H.R.; Paulino, M.F. Nellore cows and their calves during the lactation period: Performance, intake, milk composition, and total apparent digestibility. Trop. Anim. Health Prod. 2015, 47, 735–741. [Google Scholar] [CrossRef]
- Lins, T.O.J.D.; Silva, R.R.; Mendes, F.B.L.; da Silva, F.F.; Bastos, E.S.; Paixão, T.R.; Silva, J.W.D.; Santos, M.C.; Figueiredo, G.C.; Alba, H.D.R.; et al. Feeding behavior of post-weaned crossbred steers supplemented in the dry season of the year. Trop. Anim. Health Prod. 2022, 54, 203. [Google Scholar] [CrossRef]
- Moriel, P.; Vendramini, J.M.; Arthington, J.D.; Aguiar, A.D.; Caputti, G. Effects of crude protein level and degradability of limited creep-feeding supplements on performance of beef cow-calf pairs grazing limpograss pastures. Livest. Sci. 2017, 200, 1–5. [Google Scholar] [CrossRef]
- Poppi, D.P.; McLennan, S.R. Protein and Energy Utilization by Ruminants at pasture. J. Anim. Sci. 1995, 73, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.V.; Paulino, M.M.; Detmann, E.; Valadares Filho, S.C.; Lopes, S.A.; Rennó, L.N.; Sampaio, C.B.; Silva, A.G. A meta-analysis of the effects of creep-feeding supplementation on performance and nutritional characteristics by beef calves grazing on tropical pastures. Livest. Sci. 2019, 227, 175–182. [Google Scholar] [CrossRef]
- Aguiar, A.D.; Vendramini, J.M.B.; Arthington, J.D.; Sollenberger, L.E.; Caputti, C.; Sanchez, J.M.D.; Cunha, O.F.R.; da Silva, W.L. Limited creep-feeding supplementation effects on performance of beef cows and calves grazing limpograss pastures. Livest. Sci. 2015, 180, 129–133. [Google Scholar] [CrossRef]
- Catussi, B.L.C.; Ferreira, J.R.; LoTurco, E.G.; Morgulis, S.C.F.; Baruselli, P.S. Metabolic imprinting in beef calves supplemented with creep feeding on performance, reproductive efficiency and metabolome profile. Sci. Rep. 2024, 14, 9702. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.L.; Son, G.H.; Lee, E.K.; Kim, N.O.; Choi, C.S.; Lee, K.H.; Cha, H.J.; Shin, J.-S.; Kim, M.J.; et al. Effects of Creep Feeding from Birth to Suckling Period on Hanwoo Calves’ Growth Performance and Microbiota. Animals 2025, 15, 2169. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine (NASEM). Nutrient Requirements of Beef Cattle: Eighth Revised Edition; The National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Cardenas, J.E.G.; Paulino, M.F.; Lopes, S.A.; Silva, A.G.; Barros, L.; Valente, É.L.L. Productive performance, intake and digestibility of grazing female calves supplemented with different levels of crude protein. Arch. Zootec. 2015, 64, 167–174. [Google Scholar]
- Menezes, A.; Valadares Filho, S.C.; Costa e Silva, L.F.; Pacheco, M.V.C.; Pereira, J.M.V.; Rotta, P.P.; Zanetti, D.; Detmann, E.; Silva, F.A.S.; Godoi, L.A.; et al. Does a reduction in dietary crude protein content affect performance, nutrient requirements, nitrogen losses, and methane emissions in finishing Nellore bulls? Agric. Ecosyst. Environ. 2016, 223, 239–249. [Google Scholar] [CrossRef]
- Kim, W.S.; Nejad, J.G.; Peng, D.Q.; Jo, Y.J.; Kim, J.; Lee, H.G. Effects of different protein levels on growth performance and stress parameters in beef calves under heat stress. Sci. Rep. 2022, 12, 8113. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Dairy Cattle; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Valadares Filho, S.C.; Saraiva, D.T.; Benedeti, P.B.; Silva, F.A.S.; Chizzotti, M.L. (Eds.) Nutrient Requirements of Zebu and Crossbred Cattle—BR-CORTE, 4th ed.; Suprema: Visconde de Rio Branco, Brazil, 2023. [Google Scholar]
- de Vries, M.F.W. Estimating forage intake and quality in grazing cattle: A reconsideration of the hand-plucking method. J. Range Manag. Arch. 1995, 48, 370–375. [Google Scholar] [CrossRef]
- Da Silva Júnior, J.M.; Rodrigues, J.P.P.; Valadares Filho, S.C.; Detmann, E.; Paulino, M.F.; Rennó, L.N. Estimating purine derivatives and nitrogen compound excretion using total urine collection or spot urine samples in grazing heifers. J. Anim. Physiol. Anim. Nutr. 2021, 105, 861–873. [Google Scholar] [CrossRef]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Detmann, E.; Costa e Silva, L.F.; Palma, M.N.N.; Rodrigues, J.P.P. Methods for Food Analysis—INCT—Ciência Animal, 2nd ed.; Suprema: Visconde do Rio Branco, Brazil, 2021. [Google Scholar]
- Hall, M.B. Neutral Detergent-Soluble Carbohydrates Nutritional Relevance and Analysis; University of Florida: Gainesville, FL, USA, 2000. [Google Scholar]
- Costa e Silva, L.F.; Valadares Filho, S.C.; Chizzotti, M.L.; Rotta, P.P.; Prados, L.F.; Valadares, R.F.D.; Zanetti, D.; Braga, J.M.S. Creatinine excretion and relationship with body weight of Nellore cattle. Rev. Bras. Zootec. 2012, 41, 807–810. [Google Scholar] [CrossRef]
- Barbosa, A.M.; Valadares, R.R.D.; Valadares Filho, S.C.; Pina, D.S.; Detmann, E.; Leão, M.I. Endogenous fraction and urinary recovery of purine derivatives obtained by different methods in Nellore cattle. J. Anim. Sci. 2011, 89, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, I.; Detmann, E.; Sampaio, C.B.; Paulino, M.P.; Valadares Filho, S.C.; Souza, M.A.; Oliveira, F.A. Intake and digestibility in cattle fed low-quality tropical forage and supplemented with nitrogenous compounds. Rev. Bras. Zootec. 2009, 38, 2021–2030. [Google Scholar] [CrossRef]
- Sampaio, C.B.; Detmann, E.; Paulino, M.F.; Valadares Filho, S.C.; Souza, M.A.; Lazzarini, I.; Paulino, P.V.R.; Queiroz, A.C. Intake and digestibility in cattle fed low-quality tropical forage and supplemented with nitrogenous compounds. Trop. Anim. Health Prod. 2010, 42, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Detmann, E.; Valente, É.E.L.; Batista, E.D.; Huhtanen, P. An evaluation of the performance and efficiency of nitrogen utilization in cattle fed tropical grass pastures with supplementation. Livest. Sci. 2014, 162, 141–153. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Bougouin, A.; Hristov, A.; Zanetti, D.; Valadares Filho, S.C.; Rennó, L.N.; Menezes, A.C.B.; Silva, J.M., Jr.; Alhadas, H.M.; Mariz, L.D.S.; Prados, L.F.; et al. Nitrogen excretion from beef cattle fed a wide range of diets compiled in an intercontinental dataset: A meta-analysis. J. Anim. Sci. 2022, 100, skac150. [Google Scholar] [CrossRef]
- Wood, B.J.; Archer, J.A.; Van Der Werf, J.H.J. Response to selection in beef cattle using IGF-1 as a selection criterion for residual feed intake under different Australian breeding objectives. Livest. Prod. Sci. 2004, 91, 69–81. [Google Scholar] [CrossRef]
- Drewnoski, M.E.; Huntington, G.B.; Poore, M.H. Reduced supplementation frequency increased insulin-like growth factor 1 in beef steers fed medium quality hay and supplemented with a soybean hull and corn gluten feed blend. J. Anim. Sci. 2014, 92, 2546–2553. [Google Scholar] [CrossRef]
- Lobley, G.E. Control of the metabolic fate of amino acids in ruminants: A review. J. Anim. Sci. 1992, 70, 3264–3275. [Google Scholar] [CrossRef]
- Lawrence, T.L.J.; Fowler, V.R.; Novakofski, J.E. Growth of Farm Animals; CABI Publishing: London, UK, 2012. [Google Scholar]
- Berends, H.; Van den Borne, J.J.G.C.; Røjen, B.A.; Van Baal, J.; Gerrits, W.J.J. Urea recycling contributes to nitrogen retention in calves fed milk replacer and low-protein solid feed. J. Nutr. 2014, 144, 1043–1049. [Google Scholar] [CrossRef]
- Bonnet, M.; Cassar, M.; Chilliard, Y.; Picard, B. Ontogenesis of muscle and adipose tissues and their interactions in ruminants and other species. Animal 2010, 4, 1093–1109. [Google Scholar] [CrossRef]
- Du, M.; Huang, Y.; Das, A.K.; Yang, Q.; Duarte, M.S.; Dodson, M.V.; Zhu, M.J. Meat Science and Muscle Biology Symposium: Manipulating mesenchymal progenitor cell differentiation to optimize performance and carcass value of beef cattle. J. Anim. Sci. 2013, 91, 1419–1427. [Google Scholar] [CrossRef]
- Duarte, M.S.; Gionbelli, M.P.; Paulino, P.V.R.; Serão, N.V.L.; Nascimento, C.S.; Botelho, M.E.; Martins, T.S.; Valadares Filho, S.C.; Dodson, M.V.; Guimarães, S.E.F.; et al. Maternal overnutrition enhances mRNA expression of adipogenic markers and collagen deposition in skeletal muscle of beef cattle fetuses. J. Anim. Sci. 2014, 92, 3846–3854. [Google Scholar] [CrossRef]
- Du, M.; Tong, J.; Zhao, J.; Underwood, K.R.; Zhu, M.; Ford, S.P.; Nathanielsz, P.W. Fetal programming of skeletal muscle development in ruminant animals. J. Anim. Sci. 2010, 88, 51–60. [Google Scholar] [CrossRef]
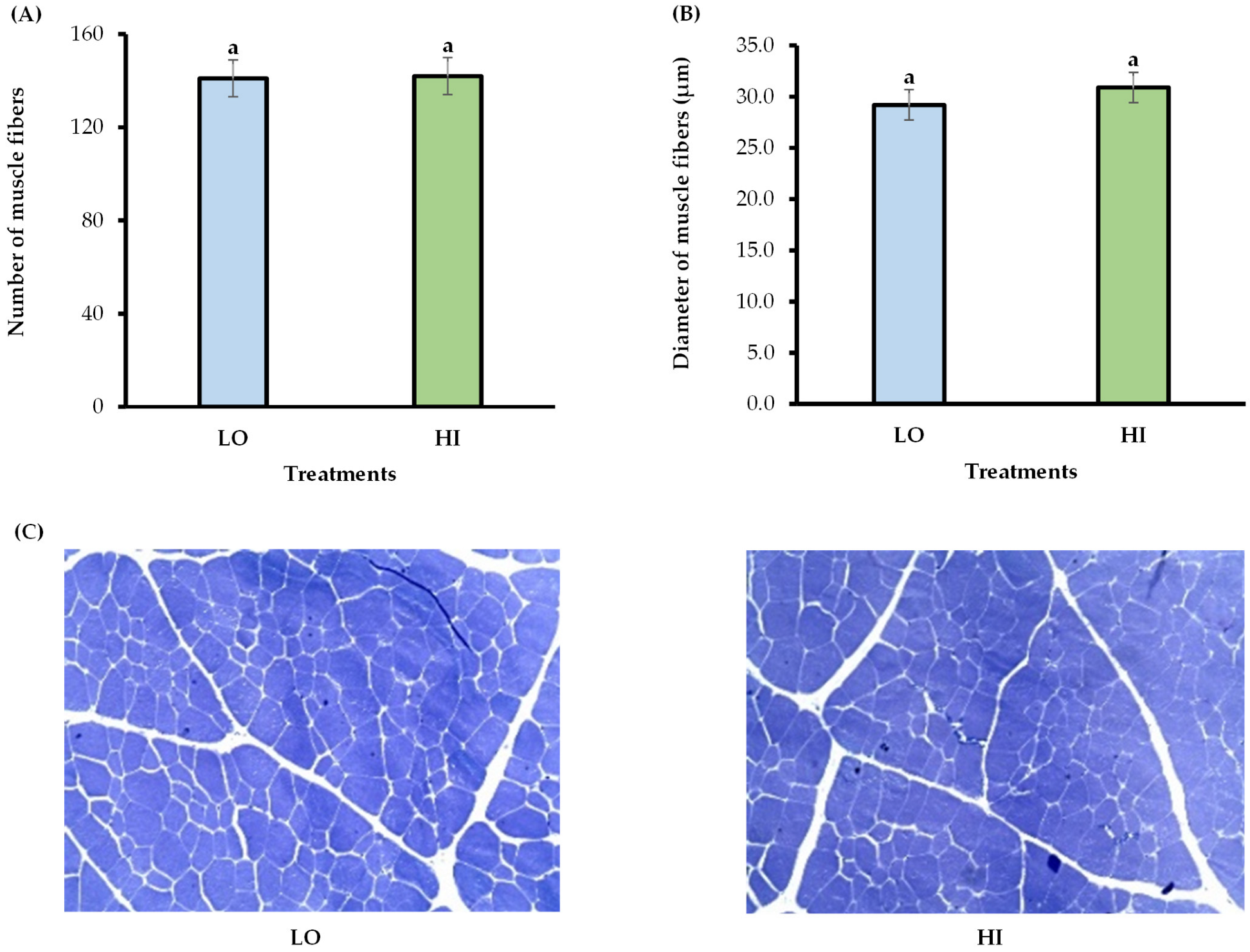
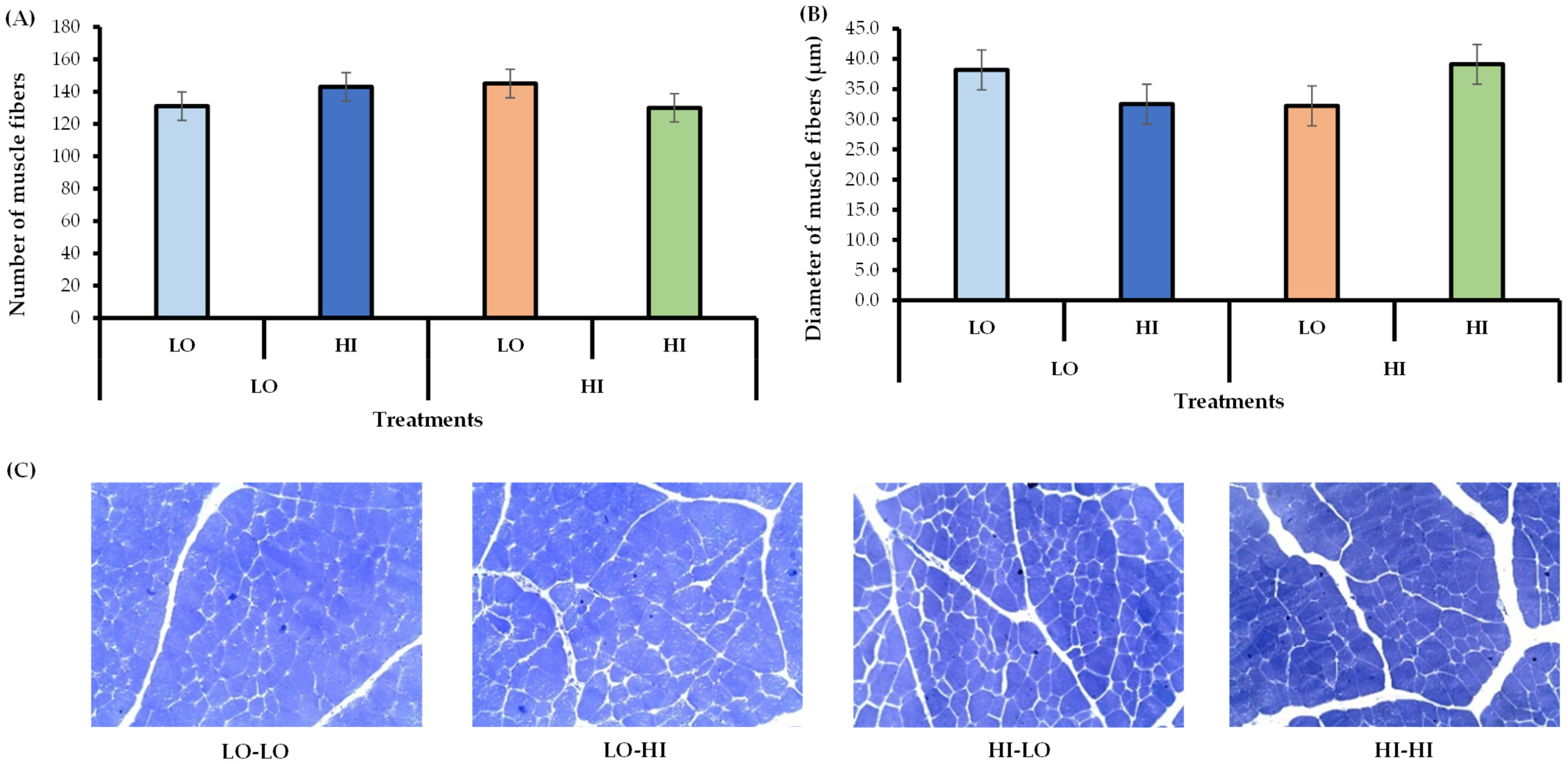
| Item | Low Protein Supplement | High Protein Supplement | Forage 4 | Forage 5 | Forage 6 |
|---|---|---|---|---|---|
| Ingredient % (as fed basis) | |||||
| Corn meal | 18.0 | 55.0 | – | – | – |
| Wheat bran | 55.0 | 18.0 | – | – | – |
| Soybean meal | 19.0 | 19.0 | – | – | – |
| Molasses | 3.0 | 3.0 | – | – | – |
| Mineral mix 1 | 5.0 | 5.0 | – | – | – |
| Chemical composition (g/kg DM) | |||||
| Dry matter | 883.0 | 881.0 | 274.0 ± 2.90 | 376.0 ± 13.80 | 314.0 ± 15.2 |
| Organic matter | 926.0 | 920.0 | 924.0 ± 3.30 | 929.0 ± 2.30 | 929.0 ± 3.00 |
| Crude protein | 167.0 | 324.0 | 88.4 ± 2.10 | 74.4 ± 1.20 | 76.0 ± 2.70 |
| Ether extract | 26.5 | 24.1 | 15.2 ± 0.90 | 10.5 ± 0.60 | 15.8 ± 0.90 |
| Non fibrous carbohydrates | 524.0 | 403.0 | 188.0 ± 5.20 | 133.0 ± 7.90 | 154.0 ± 10.40 |
| apNDF 2 | 202.0 | 175.0 | 632.0 ± 4.50 | 711.0 ± 6.30 | 684.0 ± 13.00 |
| iFDN 3 | 38.6 | 36.6 | 206.0 ± 10.60 | 330.0 ± 4.2 | 254.0 ± 16.30 |
| Item | Initial Pre-Weaning 2 | SEM 3 | IP | p-Value 4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| LO | HI | Overall | |||||||
| Final Pre-Weaning | IP | FP | IP × FP | ||||||
| LO | HI | LO | HI | ||||||
| kg/day | |||||||||
| Yield milk | 7.12 | 7.07 | 0.331 | 0.930 | – | – | – | ||
| Milk4% 1 | 8.16 | 8.12 | 0.450 | 0.942 | – | – | – | ||
| Fat | 0.36 | 0.35 | 0.023 | 0.951 | – | – | – | ||
| Protein | 0.23 | 0.24 | 0.019 | 0.851 | – | – | – | ||
| Lactose | 0.33 | 0.33 | 0.024 | 0.964 | – | – | – | ||
| Total solids | 0.99 | 0.99 | 0.053 | 0.915 | – | – | – | ||
| Yield milk | 5.41 | 5.08 | 5.56 | 5.66 | 0.444 | 0.472 | 0.820 | 0.663 | |
| Milk4% | 6.85 | 6.13 | 6.79 | 7.24 | 0.661 | 0.493 | 0.857 | 0.451 | |
| Fat | 0.31 | 0.27 | 0.30 | 0.33 | 0.041 | 0.529 | 0.878 | 0.419 | |
| Protein | 0.19 | 0.19 | 0.21 | 0.22 | 0.020 | 0.274 | 0.881 | 0.604 | |
| Lactose | 0.23 | 0.22 | 0.25 | 0.24 | 0.026 | 0.475 | 0.765 | 0.811 | |
| Total solids | 0.77 | 0.73 | 0.81 | 0.84 | 0.075 | 0.370 | 0.957 | 0.649 | |
| Item | Initial Pre-Weaning 7 | SEM 8 | IP | p-Value 9 | |||||
|---|---|---|---|---|---|---|---|---|---|
| LO | HI | Overall | |||||||
| Final Pre-Weaning | IP | FP | IP × FP | ||||||
| LO | HI | LO | HI | ||||||
| kg/day | |||||||||
| Total DM 1 | 3.05 | 2.99 | 0.148 | 0.744 | – | – | – | ||
| Forage DM | 1.27 | 1.38 | 0.136 | 0.554 | – | – | – | ||
| Milk DM | 0.99 | 0.99 | 0.055 | 0.938 | – | – | – | ||
| OM 2 | 2.89 | 2.82 | 0.137 | 0.718 | – | – | – | ||
| Crude protein | 0.48 | 0.56 | 0.025 | 0.011 | – | – | – | ||
| Ether extract | 0.4 | 0.39 | 0.027 | 0.865 | – | – | – | ||
| apNDF 3 | 0.96 | 0.98 | 0.082 | 0.851 | – | – | – | ||
| NFC 4 | 1.06 | 0.89 | 0.058 | 0.044 | – | – | – | ||
| Indigestible NDF 5 | 0.29 | 0.31 | 0.035 | 0.697 | – | – | – | ||
| Digested NDF | 0.58 | 0.61 | 0.062 | 0.687 | – | – | – | ||
| Digested OM | 2.12 | 2.09 | 0.098 | 0.813 | – | – | – | ||
| CP: DOM (g/kg) 6 | 227.0 | 269.0 | 7.05 | 0.006 | – | – | – | ||
| Total DM | 3.71 | 3.87 | 3.92 | 4.04 | 0.214 | 0.461 | 0.583 | 0.927 | |
| Forage DM | 1.72 | 1.91 | 1.88 | 1.96 | 0.202 | 0.651 | 0.570 | 0.809 | |
| Milk DM | 0.78 | 0.74 | 0.82 | 0.85 | 0.076 | 0.370 | 0.956 | 0.650 | |
| Organic matter | 3.48 | 3.64 | 3.69 | 3.80 | 0.207 | 0.436 | 0.562 | 0.932 | |
| Crude protein | 0.52 | 0.73 | 0.55 | 0.75 | 0.025 | 0.353 | 0.001 | 0.916 | |
| Ether extract | 0.36 | 0.33 | 0.36 | 0.38 | 0.049 | 0.524 | 0.851 | 0.486 | |
| apNDF | 1.48 | 1.57 | 1.57 | 1.64 | 0.189 | 0.680 | 0.678 | 0.966 | |
| NFC | 1.12 | 1.02 | 1.21 | 1.03 | 0.045 | 0.292 | 0.027 | 0.438 | |
| Indigestible NDF | 0.62 | 0.70 | 0.66 | 0.68 | 0.068 | 0.844 | 0.499 | 0.667 | |
| Digested NDF | 0.64 | 0.61 | 0.62 | 0.68 | 0.102 | 0.816 | 0.908 | 0.669 | |
| Digested OM | 2.04 | 1.96 | 2.02 | 2.11 | 0.103 | 0.549 | 0.988 | 0.455 | |
| CP: DOM (g/kg) | 260.0 | 375.0 | 275.0 | 360.0 | 7.35 | 0.976 | <0.001 | 0.130 | |
| Item | Initial Pre-Weaning 3 | SEM 4 | IP | p-Value 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| LO | HI | Overall | |||||||
| Final Pre-Weaning | IP | FP | IP × FP | ||||||
| LO | HI | LO | HI | ||||||
| g/g | |||||||||
| Dry matter | 0.695 | 0.692 | 0.0182 | 0.912 | – | – | – | ||
| Organic matter | 0.740 | 0.745 | 0.0156 | 0.823 | – | – | – | ||
| Crude protein | 0.724 | 0.756 | 0.0240 | 0.391 | – | – | – | ||
| Ether extract | 0.877 | 0.871 | 0.0128 | 0.733 | – | – | – | ||
| apNDF 1 | 0.603 | 0.622 | 0.0275 | 0.634 | – | – | – | ||
| NFC 2 | 0.800 | 0.800 | 0.0126 | 0.803 | – | – | – | ||
| Dry matter | 0.545 | 0.496 | 0.503 | 0.510 | 0.0251 | 0.613 | 0.474 | 0.342 | |
| Organic matter | 0.592 | 0.547 | 0.554 | 0.568 | 0.0227 | 0.757 | 0.551 | 0.287 | |
| Crude protein | 0.647 | 0.702 | 0.606 | 0.700 | 0.0245 | 0.454 | 0.045 | 0.496 | |
| Ether extract | 0.747 | 0.666 | 0.726 | 0.706 | 0.0406 | 0.817 | 0.298 | 0.515 | |
| apNDF | 0.434 | 0.394 | 0.400 | 0.415 | 0.0140 | 0.696 | 0.456 | 0.141 | |
| NFC | 0.699 | 0.611 | 0.659 | 0.619 | 0.0456 | 0.746 | 0.242 | 0.642 | |
| Item 1 | Initial Pre-Weaning 2 | SEM 3 | IP | p-Value 4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| LO | HI | Overall | |||||||
| Final Pre-Weaning | IP | FP | IP × FP | ||||||
| LO | HI | LO | HI | ||||||
| NI (g/day) | 76.10 | 89.10 | 2.517 | 0.011 | – | – | – | ||
| NMIC (g/day) | 16.10 | 15.90 | 2.506 | 0.957 | – | – | – | ||
| MICNR (g/g) | 0.22 | 0.18 | 0.039 | 0.359 | – | – | – | ||
| EMS (g/kg DOM) | 49.20 | 47.70 | 0.934 | 0.883 | – | – | – | ||
| SUN (mg/dL) | 10.20 | 15.70 | 1.773 | 0.073 | – | – | – | ||
| UUN (g/day) | 16.30 | 26.40 | 1.159 | <0.001 | – | – | – | ||
| UEUN (g/day) | 24.40 | 34.70 | 1.753 | 0.006 | – | – | – | ||
| FNE (g/day) | 21.20 | 21.80 | 2.164 | 0.837 | – | – | – | ||
| NB (g/day) | 30.50 | 32.50 | 2.448 | 0.594 | – | – | – | ||
| EFNU (g/g) | 0.40 | 0.36 | 0.034 | 0.336 | – | – | – | ||
| NI (g/day) | 83.60 | 117.00 | 88.40 | 121.00 | – | – | – | 0.001 | 0.912 |
| NMIC (g/day) | 38.10 | 28.50 | 32.20 | 31.60 | 3.109 | 0.803 | 0.167 | 0.202 | |
| MICNR (g/g) | 0.46 | 0.24 | 0.38 | 0.27 | 0.048 | 0.595 | 0.022 | 0.255 | |
| EMS (g/kg DOM) | 117.00 | 88.40 | 102.00 | 97.60 | 12.507 | 0.824 | 0.272 | 0.398 | |
| SUN (mg/dL) | 12.00 | 16.90 | 9.77 | 16.60 | 1.253 | 0.360 | 0.010 | 0.496 | |
| UUN (g/day) | 24.00 | 46.40 | 19.70 | 49.80 | 2.072 | 0.860 | <0.001 | 0.158 | |
| UEUN (g/day) | 37.30 | 61.40 | 32.40 | 58.30 | 2.164 | 0.153 | <0.001 | 0.713 | |
| FNE (g/day) | 29.50 | 35.00 | 34.80 | 36.80 | 2.953 | 0.315 | 0.293 | 0.597 | |
| NB (g/day) | 16.90 | 20.20 | 21.20 | 25.40 | 3.566 | 0.253 | 0.415 | 0.871 | |
| EFNU (g/g) | 0.19 | 0.17 | 0.23 | 0.21 | 0.034 | 0.265 | 0.507 | 0.972 | |
| Item | Initial Pre-Weaning 2 | SEM 3 | IP | p-Value 4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| LO | HI | Overall | |||||||
| Final Pre-Weaning | IP | FP | IP × FP | ||||||
| LO | HI | LO | HI | ||||||
| Glucose (mg/dL) | 77.60 | 81.60 | 3.803 | 0.476 | – | – | – | ||
| Triglycerides (mg/dL) | 32.80 | 39.20 | 3.781 | 0.261 | – | – | – | ||
| Total proteins (g/dL) | 6.52 | 6.67 | 0.117 | 0.388 | – | – | – | ||
| Albumin (mg/dL) | 3.24 | 3.42 | 0.004 | 0.015 | – | – | – | ||
| Globulins (mg/dL) | 3.28 | 3.25 | 0.010 | 0.824 | – | – | – | ||
| IGF-1 (ng/mL) 1 | 424.0 | 441.0 | 1.978 | 0.549 | – | – | – | ||
| Glucose (mg/dL) | 82.70 | 77.00 | 82.10 | 85.30 | 3.975 | 0.394 | 0.773 | 0.333 | |
| Triglycerides (mg/dL) | 25.50 | 31.50 | 26.40 | 28.70 | 2.876 | 0.718 | 0.243 | 0.532 | |
| Total proteins (g/dL) | 6.83 | 6.67 | 6.91 | 6.93 | 0.082 | 0.122 | 0.437 | 0.338 | |
| Albumin (mg/dL) | 3.28 | 3.28 | 3.34 | 3.43 | 0.071 | 0.231 | 0.603 | 0.606 | |
| Globulins (mg/dL) | 3.55 | 3.38 | 3.57 | 3.50 | 0.084 | 0.864 | 0.538 | 0.591 | |
| IGF-1 (ng/mL) | 338.0 | 383.0 | 349.0 | 356.0 | 25.90 | 0.772 | 0.377 | 0.520 | |
| Item | Initial Pre-Weaning 4 | SEM 5 | IP | p-Value 6 | |||||
|---|---|---|---|---|---|---|---|---|---|
| LO | HI | Overall | |||||||
| Final Pre-Weaning | IP | FP | IP × FP | ||||||
| LO | HI | LO | HI | ||||||
| Final BW (kg) 1 | 191.0 | 193.0 | 1.41 | 0.471 | – | – | – | ||
| Average daily gain (kg/day) | 0.98 | 1.00 | 0.023 | 0.471 | – | – | – | ||
| SFTL (mm) 2 | 1.38 | 1.54 | 0.124 | 0.659 | – | – | – | ||
| SFTR (mm) 3 | 2.06 | 2.30 | 0.127 | 0.194 | – | – | – | ||
| Final BW (kg) | 249.0 | 250.0 | 256.0 | 253.0 | 5.29 | 0.409 | 0.912 | 0.697 | |
| Average daily gain (kg/day) | 0.87 | 0.88 | 0.91 | 0.89 | 0.032 | 0.409 | 0.911 | 0.698 | |
| SFTL (mm) | 1.54 | 1.66 | 1.62 | 1.90 | 0.123 | 0.265 | 0.183 | 0.578 | |
| SFTR (mm) | 2.36 | 2.41 | 2.49 | 2.70 | 0.123 | 0.190 | 0.369 | 0.570 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manso, M.R.; Rennó, L.N.; Detmann, E.; Paulino, M.F.; Lopes, S.A.; Lima, N.S.d.A.; Moreno, D.P.S.; Ortega, R.M. Do Protein Supplementation Levels Influence the Performance of Male Nellore Calves Under a Grazing System at Pre-Weaning? Animals 2025, 15, 2913. https://doi.org/10.3390/ani15192913
Manso MR, Rennó LN, Detmann E, Paulino MF, Lopes SA, Lima NSdA, Moreno DPS, Ortega RM. Do Protein Supplementation Levels Influence the Performance of Male Nellore Calves Under a Grazing System at Pre-Weaning? Animals. 2025; 15(19):2913. https://doi.org/10.3390/ani15192913
Chicago/Turabian StyleManso, Marcos Rocha, Luciana Navajas Rennó, Edenio Detmann, Mário Fonseca Paulino, Sidnei Antônio Lopes, Nicole Stephane de Abreu Lima, Deilen Paff Sotelo Moreno, and Román Maza Ortega. 2025. "Do Protein Supplementation Levels Influence the Performance of Male Nellore Calves Under a Grazing System at Pre-Weaning?" Animals 15, no. 19: 2913. https://doi.org/10.3390/ani15192913
APA StyleManso, M. R., Rennó, L. N., Detmann, E., Paulino, M. F., Lopes, S. A., Lima, N. S. d. A., Moreno, D. P. S., & Ortega, R. M. (2025). Do Protein Supplementation Levels Influence the Performance of Male Nellore Calves Under a Grazing System at Pre-Weaning? Animals, 15(19), 2913. https://doi.org/10.3390/ani15192913






