Testicular Neoplasms and Other Abnormalities in Common Carp Cyprinus carpio from the Lower Colorado River, United States
Simple Summary
Abstract
1. Introduction
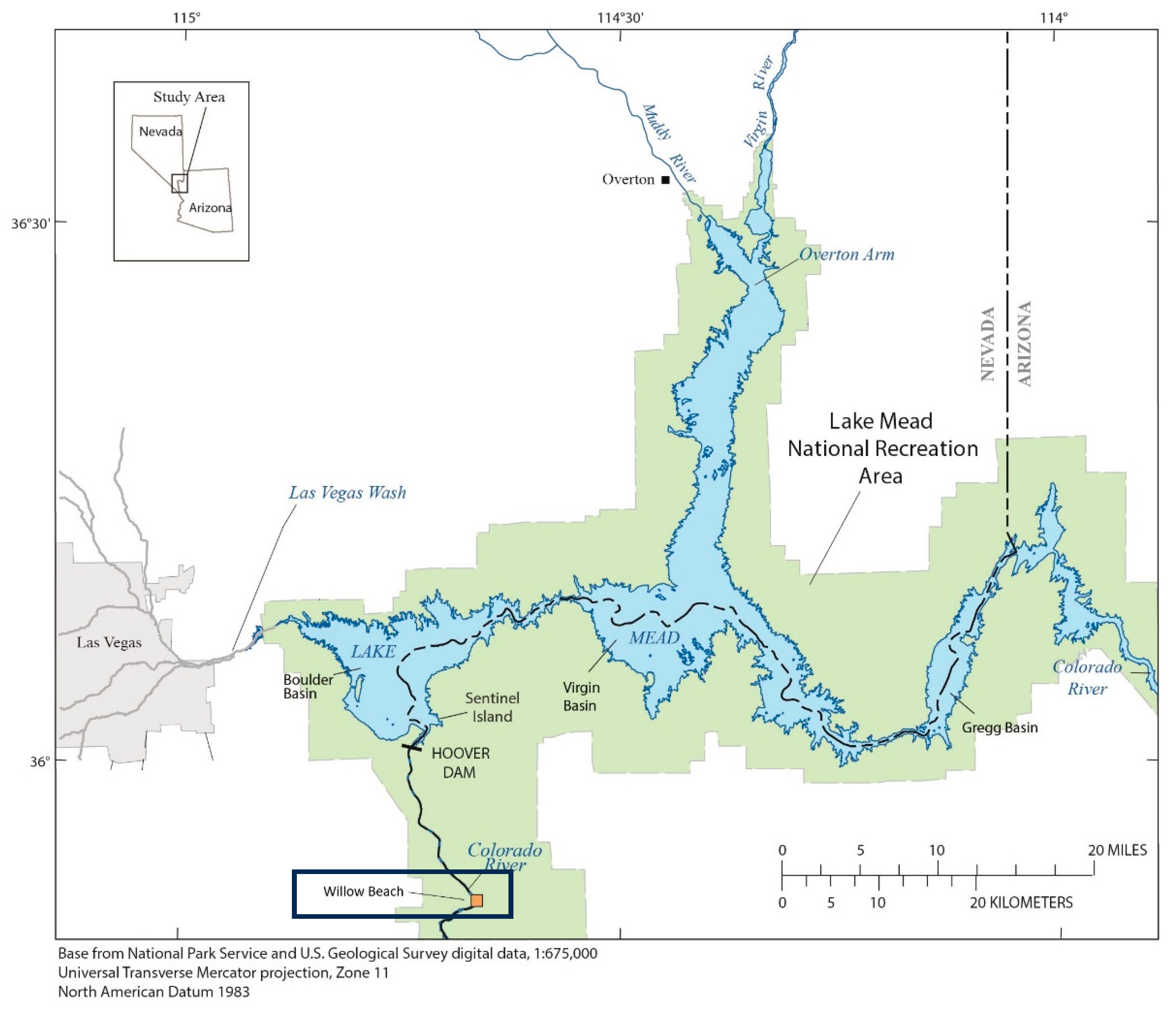
2. Methods
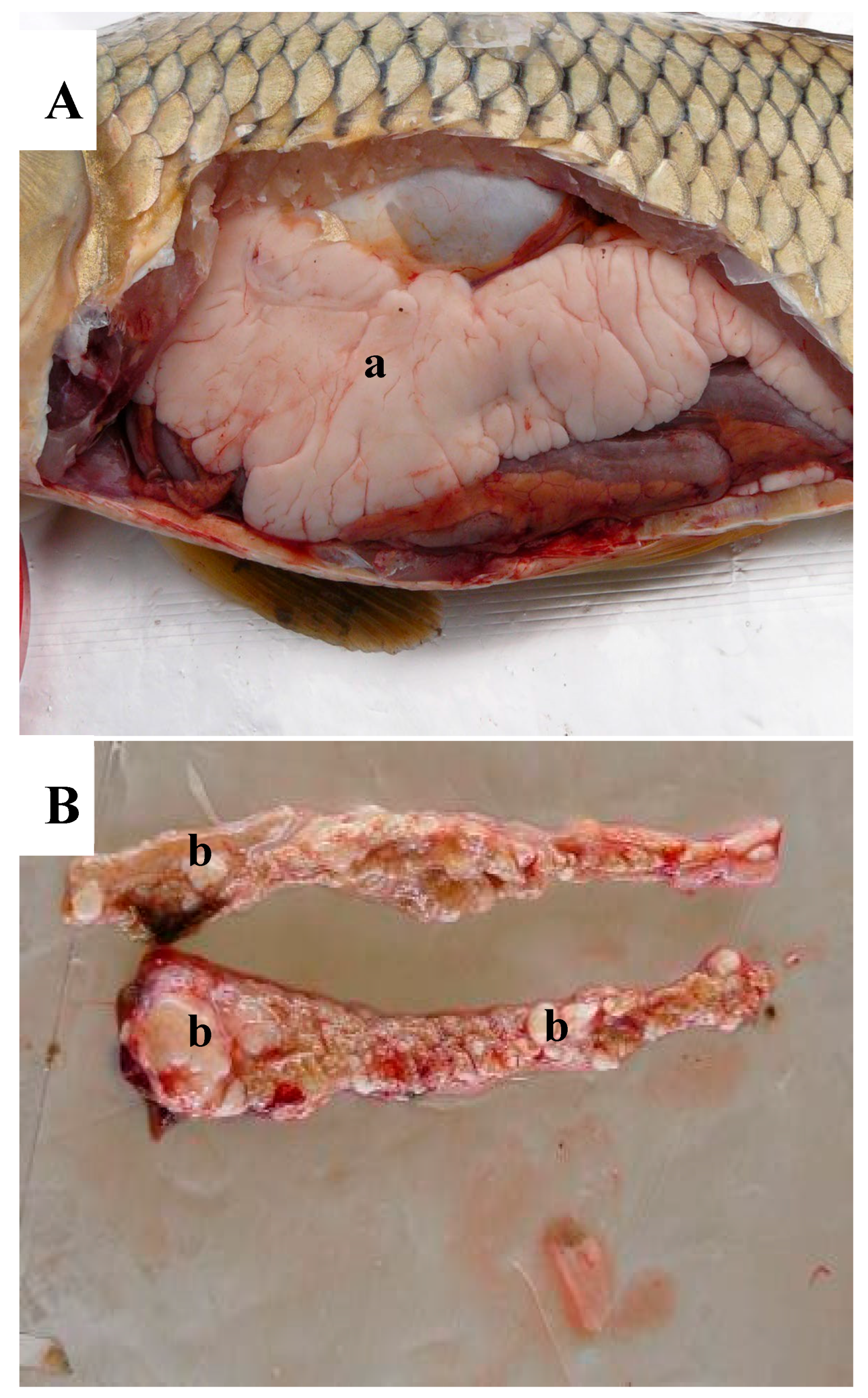
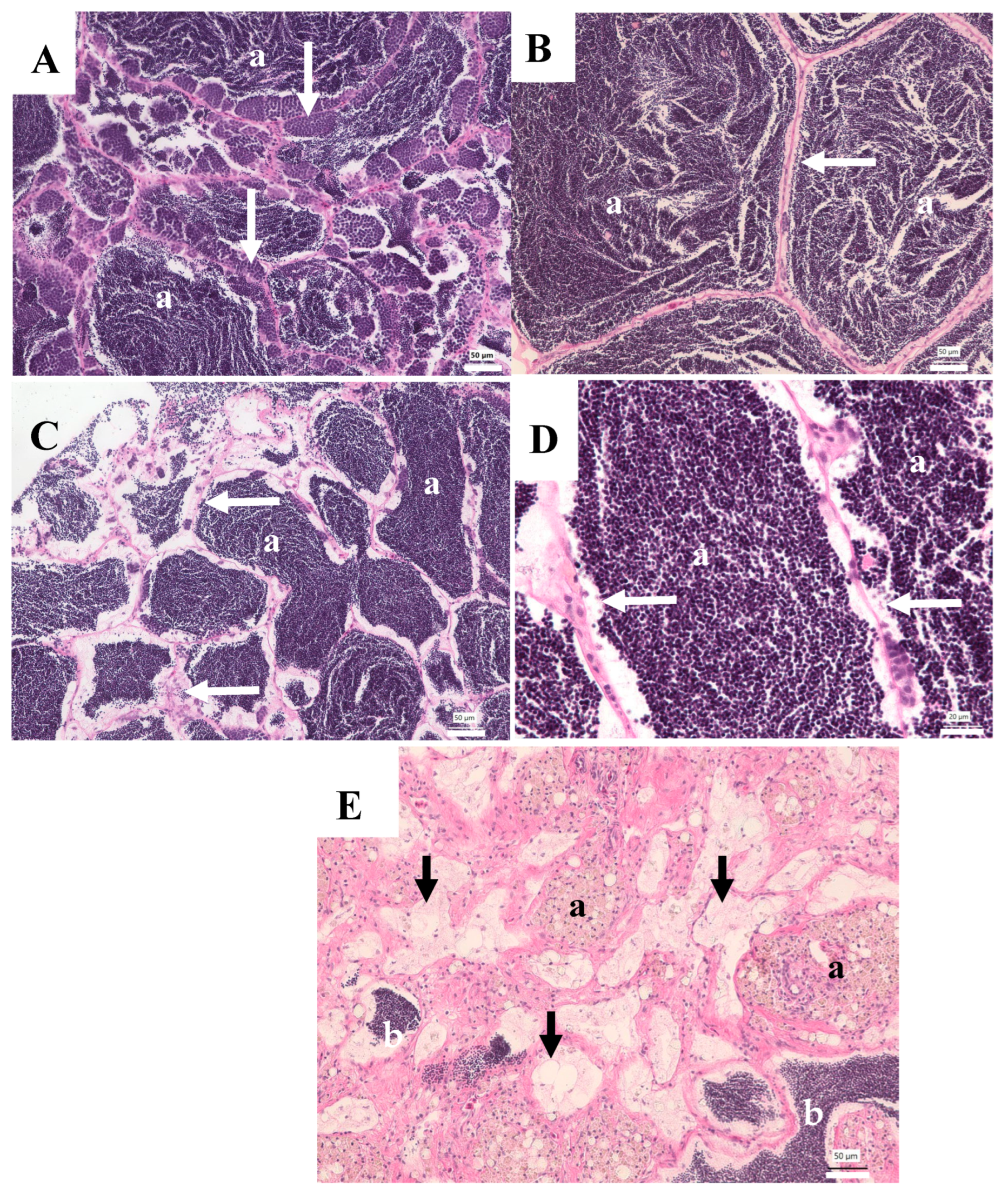
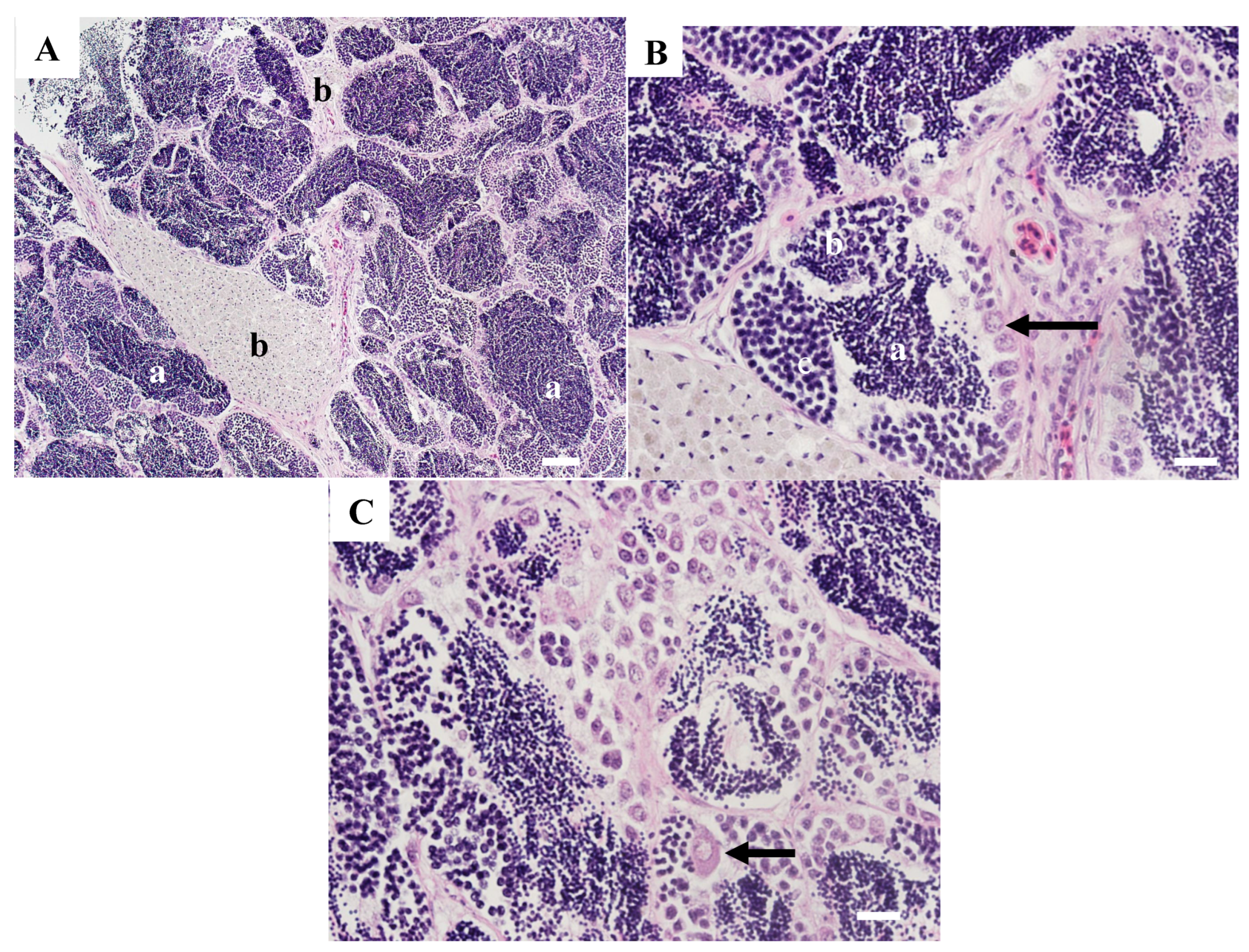
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jobling, S.; Coey, S.; Whitmore, J.G.; Kime, D.E.; Van Look, K.J.W.; McAllister, B.G.; Beresford, N.; Henshaw, C.; Brighty, G.; Tyler, C.R.; et al. Wild intersex roach (Rutilus rutilus) have reduced fertility. Biol. Reprod. 2002, 67, 515–524. [Google Scholar] [CrossRef]
- Blazer, V.S.; Iwanowicz, L.R.; Iwanowicz, D.D.; Smith, D.R.; Young, J.A.; Hedrick, J.D.; Foster, S.W.; Reeser, S.J. Intersex (testicular oocytes) in smallmouth bass from the Potomac River and selected nearby drainages. J. Aquat. Animal Health 2007, 19, 242–253. [Google Scholar] [CrossRef]
- Bahamonde, P.A.; Munkittrick, K.R.; Martyniuk, C.J. Intersex in teleost fish: Are we distinguishing endocrine disruption from natural phenomena? Gen. Comp. Endocrinol. 2013, 192, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Abdel-moneim, A.; Coulter, D.P.; Mahapatra, C.T.; Sepúlveda, M.S. Intersex in fishes and amphibians: Population implications, prevalence, mechanisms and molecular biomarkers. J. Appl. Toxicol. 2015, 35, 1228–1240. [Google Scholar] [CrossRef] [PubMed]
- Hicks, K.A.; Fuzzen, M.L.; Dhiyebi, H.A.; Bragg, L.M.; Marjan, P.; Cunningham, J.; McMaster, M.E.; Srikanthan, N.; Nikel, K.E.; Arlos, M.J.; et al. Intersex manifestation in the rainbow darter (Etheostoma caeruleum): Are adult male fish susceptible to developing and recovering from intersex after exposure to endocrine active compounds? Aquat. Toxicol. 2023, 261, 106636. [Google Scholar] [CrossRef]
- Goodbred, S.L.; Gilliom, R.J.; Gross, T.S.; Denslow, N.P.; Bryant, W.L.; Schoeb, T.R. Reconnaissance of 17beta-estradiol, 11-ketotestosterone, vitellogenin, and gonad histopathology in common carp of United States streams: Potential for contaminant-induced endocrine disruption, Sacramento, CA. In U.S Geological Survey Open-File Report OFR 96-627; U.S. Geological Survey: Reston, VA, USA, 1997; 47p. [Google Scholar]
- Hinck, J.E.; Blazer, V.S.; Schmitt, C.J.; Papoulias, D.M.; Tillitt, D.E. Widespread occurrence of intersex in black basses (Micropterus spp.) from US rivers, 1995–2004. Aquat. Toxicol. 2009, 95, 60–70. [Google Scholar] [CrossRef]
- VanMiddlesworth, M.M.; DeBoer, J.A.; Fritts, M.W.; Levengood, J.M.; Casper, A.F. Basin-scale patterns of common carp physiological condition associated with EDC exposure in a large Anthropocene river. Environ. Biol. Fishes 2021, 104, 1541–1558. [Google Scholar] [CrossRef]
- Solé, M.; Raldua, D.; Piferrer, F.; Barceló, D.; Porte, C. Feminization of wild carp, Cyprinus carpio, in a polluted environment: Plasma steroid hormones, gonadal morphology and xenobiotic metabolizing system. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 136, 145–156. [Google Scholar] [CrossRef]
- Sirri, R.; Mandrioli, L.; Grieco, V.; Bacci, B.; Brunetti, B.; Sarli, G.; Schmidt-Posthaus, H. Seminoma in a koi carp Cyprinus carpio: Histopathological and immunohistochemical findings. Dis. Aquat. Org. 2010, 92, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Knüsel, F.O.; Knüsel, R.; Doherr, M.G.; Schmidt-Posthaus, H. Frequency and histologic characterization of coelomatic neoplasms in koi Cyprinus carpio koi. Dis. Aquat. Org. 2016, 119, 219–229. [Google Scholar] [CrossRef]
- Sirri, R.; Tura, G.; Budai, J.; Beraldo, P.; Fiorentino, M.; Barbé, T.; Galeotti, M.; Sarli, G.; Mandrioli, L. Histological and immunohistochemical characterization of 17 gonadal tumours in koi carp (Cyprinus carpio koi). J. Fish Dis. 2021, 44, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Pigoli, C.; Ghisleni, G.; Armando, F.; Grieco, V.; Ghidelli, A.; Brambilla, E. Cytology of a seminoma in a koi (Cyprinus carpio): A rapid diagnostic tool. Vet. Res. Commun. 2024, 48, 2589–2593. [Google Scholar] [CrossRef] [PubMed]
- Hinck, J.E.; Schmitt, C.J.; Blazer, V.S.; Denslow, N.D.; Bartish, T.M.; Anderson, P.J.; Coyle, J.J.; Dethloff, G.M.; Tillitt, D.E. Environmental contaminants and biomarker responses in fish from the Columbia River and its tributaries: Spatial and temporal trends. Sci. Total Environ. 2006, 366, 549–578. [Google Scholar] [CrossRef] [PubMed]
- Romano, L.A.; Klosterhoff, M.; Fuhr, F.; Rodrigues, R.V.; Gusmao, E.P.; Garrido-Pereira, M.A.R.; Sampaio, L.A.; Tesser, M.B. Neoplasia of the Sertoli cells in wild carp, Cyprinus carpio: Optical, immunohistochemical and ultrastructural study. Bull. Eur. Assoc. Fish Pathol. 2013, 33, 84–90. [Google Scholar]
- Leatherland, J.F.; Sonstegard, R.A. Structure of normal testis and testicular tumors in cyprinids from Lake Ontario. Can. Res. 1978, 38, 3164–3173. [Google Scholar]
- Down, N.E.; Leatherland, J.F. Histopathology of gonadal neoplasms in cyprinid fish from the lower Great Lakes of North America. J. Fish Dis. 1989, 12, 415–437. [Google Scholar] [CrossRef]
- Dickman, M.D.; Steele, P.O. Gonadal neoplasms in wild carp-goldfish hybrids from the Welland River near Niagara Falls, Canada. Hydrobiologia 1986, 134, 257–263. [Google Scholar] [CrossRef]
- Granado-Lorencio, C.; Garcia-Novo, F.; Lopez-Campos, J. Testicular tumors in carp–funa hybrid: Annual cycle and effect on a wild population. J. Wildl. Dis. 1987, 23, 422–427. [Google Scholar] [CrossRef]
- Patiño, R.; Goodbred, S.L.; Draugelis-Dale, R.; Barry, C.E.; Foott, J.S.; Wainscott, M.R.; Gross, T.S.; Covay, K.J. Morphometric and histopathological parameters of gonadal development in adult common carp from contaminated and reference sites in Lake Mead, Nevada. J. Aquat. Animal Health 2003, 15, 55–68. [Google Scholar] [CrossRef]
- Hinck, J.E.; Blazer, V.S.; Denslow, N.D.; Echols, K.R.; Gross, T.S.; May, T.W.; Anderson, P.J.; Coyle, J.J.; Tillitt, D.E. Chemical contaminants, health indicators, and reproductive biomarker responses in fish from the Colorado River and its tributaries. Sci. Total Environ. 2007, 378, 376–402. [Google Scholar] [CrossRef]
- Goodbred, S.L.; Patiño, R.; Alvarez, D.A.; Johnson, D.; Hannoun, D.; Echols, K.R.; Jenkins, J.A. Fish health altered by contaminants and low water temperatures compounded by prolonged regional drought in the Lower Colorado River Basin, USA. Toxics 2024, 12, 708. [Google Scholar] [CrossRef]
- Patiño, R.; VanLandeghem, M.M.; Goodbred, S.L.; Orsak, E.; Jenkins, J.A.; Echols, K.; Rosen, M.R.; Torres, L. Novel associations between contaminant body burdens and biomarkers of reproductive condition in male Common Carp along multiple gradients of contaminant exposure in Lake Mead National Recreation Area, USA. Gen. Comp. Endocrinol. 2015, 219, 112–124. [Google Scholar] [CrossRef]
- Luna, L.G. Histopathological Methods and Color Atlas of Special Stains and Tissue Artifacts; American Histolabs, Inc.: Gaithersburg, MD, USA, 1992; 767p. [Google Scholar]
- Wolke, R.E. Piscine macrophage aggregates: A review. Ann. Rev. Fish Dis. 1992, 2, 91–108. [Google Scholar] [CrossRef]
- Fournie, J.W.; Summers, J.K.; Courtney, L.A.; Engle, V.D.; Blazer, V.S. Utility of splenic macrophage aggregates as an indicator of fish exposure to degraded environments. J. Aquat. Animal Health 2001, 13, 105–116. [Google Scholar] [CrossRef]
- Ivanova, L.; Rebok, K.; Jordanova, M.; Dragun, Z.; Kostov, V.; Ramani, S.; Valić, D.; Krasnići, N.; Marijić, V.F.; Kapetanović, D. The effect of different pollutants exposure on the content of pigmented macrophage aggregates in the spleen of Vardar chub (Squalius vardarensis Karaman, 1928). Microsc. Res. Tech 2020, 83, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Matsche, M.A.; Blazer, V.S.; Pulster, E.; Mazik, P.M. Biological and anthropogenic influences on macrophage aggregates in white perch Morone americana from Chesapeake Bay, USA. Dis. Aquat. Org. 2021, 143, 79–100. [Google Scholar] [CrossRef]
- Micale, V.; Perdichizzi, A.; Muglia, U.; Rinelli, P.; Cosenza, A.; Mita, D.G. Gonadal macrophage aggregates in fish: A preliminary quantitative study in red mullet. J. Exp. Zool. Part A Ecol. Integ. Physiol. 2019, 331, 357–361. [Google Scholar] [CrossRef]
- Martin-Skilton, R.; Lavado, R.; Thibaut, R.; Minier, C.; Porte, C. Evidence of endocrine alteration in the red mullet, Mullus barbatus from the NW Mediterranean. Environ. Pollut. 2006, 141, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Lavado, R.; Thibaut, R.; Raldúa, D.; Martín, R.; Porte, C. First evidence of endocrine disruption in feral carp from the Ebro River. Toxicol. Appl. Pharmacol. 2004, 196, 247–257. [Google Scholar] [CrossRef]
- Kaptaner, B.; Kankaya, E.; Dogan, A.; Durmuş, A. Alterations in histology and antioxidant defense system in the testes of the lake Van fish (Alburnus tarichi Güldenstädt, 1814). Environ. Monitor. Assess. 2016, 188, 474. [Google Scholar] [CrossRef]
- Louiz, I.; Ben-Attia, M.; Ben-Hassine, O.K. Gonadosomatic index and gonad histopathology of Gobius niger (Gobiidea, Teleost) from Bizerta lagoon (Tunisia): Evidence of reproduction disturbance. Fish. Res. 2009, 100, 266–273. [Google Scholar] [CrossRef]
- Blazer, V.S.; Wolke, R.E.; Brown, J.; Powell, C.A. Piscine macrophage aggregate parameters as health monitors: Effect of age, sex, relative weight, season and site quality in largemouth bass (Micropterus salmoides). Aquat. Toxicol. 1987, 10, 199–215. [Google Scholar] [CrossRef]
- Lara, N.L.M.; Costa, G.M.J.; Figueiredo, A.F.A.; de Franca, L.R. The Sertoli cell: What can we learn from different vertebrate models? Anim. Reprod. 2019, 16, 81–92. [Google Scholar] [CrossRef]
- Schulz, R.W.; Menting, S.; Bogerd, J.; França, L.R.; Vilela, D.A.; Godinho, H.P. Sertoli cell proliferation in the adult testis—Evidence from two fish species belonging to different orders. Biol. Reprod. 2005, 73, 891–898. [Google Scholar] [CrossRef] [PubMed]
- França, L.R.; Hess, R.A.; Dufour, J.M.; Hofmann, M.C.; Griswold, M.D. The Sertoli cell: One hundred fifty years of beauty and plasticity. Andrology 2016, 4, 189–212. [Google Scholar] [CrossRef]
- Mahmud, R.; Purser, J.; Patil, J.G. A novel testicular degenerative condition in a wild population of the common carp Cyprinus carpio (L). J. Fish Dis. 2020, 43, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Miura, C.; Ito, A.; Agusa, T.; Iwata, H.; Tanabe, S.; Tuyen, B.C.; Miura, T. Effects of lead, molybdenum, rubidium, arsenic and organochlorines on spermatogenesis in fish: Monitoring at Mekong Delta area and in vitro experiment. Aquat. Toxicol. 2007, 83, 43–51. [Google Scholar] [CrossRef]
- Berney, D.M.; Cree, I.; Rao, V.; Moch, H.; Srigley, J.R.; Tsuzuki, T.; Amin, M.B.; Comperat, E.M.; Hartmann, A.; Menon, S.; et al. An introduction to the WHO 5th edition 2022 classification of testicular tumours. Histopathology 2022, 81, 459–466. [Google Scholar] [CrossRef]
- Mikuz, G. Update on the pathology of testicular tumors. Anal. Quant. Cytopathol. Histopathol. 2015, 37, 75–85. [Google Scholar]
- Aschim, E.L.; Haugen, T.B.; Tretli, S.; Daltveit, A.K.; Grotmol, T. Risk factors for testicular cancer–differences between pure non-seminoma and mixed seminoma/non-seminoma? Inter. J. Androl. 2006, 29, 458–467. [Google Scholar] [CrossRef]
- Skakkebaek, N.E.; Rajpert-De Meyts, E.; Main, K.M. Testicular dysgenesis syndrome: An increasingly common developmental disorder with environmental aspects: Opinion. Human Reprod. 2001, 16, 972–978. [Google Scholar] [CrossRef]
- Auriemma, R.S.; Menafra, D.; de Angelis, C.; Pivonello, C.; Garifalos, F.; Verde, N.; Galdiero, G.; Piscopo, M.; Colao, A.; Pivonello, R. The role of the environment in testicular dysgenesis syndrome. In Environmental Endocrinology and Endocrine Disruptors: Endocrine and Endocrine-Targeted Actions and Related Human Diseases; Pivonello, R., Diamanti-Kandarakis, E., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2023; pp. 271–308. [Google Scholar] [CrossRef]
- Baumann, P.C. Methodological considerations for conducting tumor surveys of fishes. J. Aquat. Ecosyst. Health 1992, 1, 127–133. [Google Scholar] [CrossRef]
- Rafferty, S.D.; Blazer, V.S.; Pinkney, A.E.; Grazio, J.L.; Obert, E.C.; Boughton, L. A historical perspective on the “fish tumors or other deformities” beneficial use impairment at Great Lakes Areas of Concern. J. Great Lakes Res. 2009, 35, 496–506. [Google Scholar] [CrossRef]
- Hoffman, J.C.; Blazer, V.S.; Walsh, H.L.; Shaw, C.H.; Braham, R.; Mazik, P.M. Influence of demographics, exposure, and habitat use in an urban, coastal river on tumor prevalence in a demersal fish. Sci. Total Environ. 2020, 712, 136512. [Google Scholar] [CrossRef]
- Rhodes, L.D.; Myers, M.S.; Gronlund, W.D.; McCain, B.B. Epizootic characteristics of hepatic and renal lesions in English sole, Parophrys vetulus, from Puget Sound. J. Fish Biol. 1987, 31, 395–407. [Google Scholar] [CrossRef]
- Panek, F.M. Biology and ecology of carp. In Carp in North America; Cooper, E.L., Ed.; American Fisheries Society: Bethesda, MD, USA, 1987; 15p. [Google Scholar]
- Resseguie, T.; Kelsch, S. Influence of temperature and discharge on reproductive timing of common carp in a northern Great Plains River. Prairie Nat. 2008, 40, 23–36. [Google Scholar]
- James, M.O.; Kleinow, K.M. Seasonal influences on PCB retention and biotransformation in fish. Environ. Sci. Pollut. Res. 2014, 21, 6324–6333. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Chen, Y.; Zhang, D.; Pan, J.; Xie, Z.; Xu, C.; Li, S.; Zhang, X.; Gao, Y.; Hou, J.; et al. Estimates of over-time trends in incidence and mortality of testicular cancer from 1990 to 2030. Transl. Androl. Urol. 2020, 9, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Znaor, A.; Skakkebaek, N.E.; Rajpert-De Meyts, E.; Kuliš, T.; Laversanne, M.; Gurney, J.; Sarfati, D.; McGlynn, K.A.; Bray, F. Global patterns in testicular cancer incidence and mortality in 2020. Int. J. Cancer 2020, 151, 692–698. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, X.; Bassig, B.; Hauser, R.; Holford, T.R.; Zheng, E.; Shi, D.; Zhu, Y.; Schwartz, S.M.; Chen, C.; et al. Serum polychlorinated biphenyl (PCB) levels and risk of testicular germ cell tumors: A population-based case-control study in Connecticut and Massachusetts. Environ. Pollut. 2021, 273, 116458. [Google Scholar] [CrossRef]
- Macedo, S.; Teixeira, E.; Gaspar, T.B.; Boaventura, P.; Soares, M.A.; Miranda-Alves, L.; Soares, P. Endocrine-disrupting chemicals and endocrine neoplasia: A forty-year systematic review. Environ. Res. 2023, 218, 114869. [Google Scholar] [CrossRef] [PubMed]
- Modica, R.; Benevento, E.; Colao, A. Endocrine-disrupting chemicals (EDCs) and cancer: New perspectives on an old relationship. J. Endocrinol. Invest. 2023, 46, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Jarošová, B.; Bláha, L.; Giesy, J.P.; Hilscherová, K. What level of estrogenic activity determined by in vitro assays in municipal waste waters can be considered as safe? Environ. Intern. 2014, 64, 98–109. [Google Scholar] [CrossRef] [PubMed]
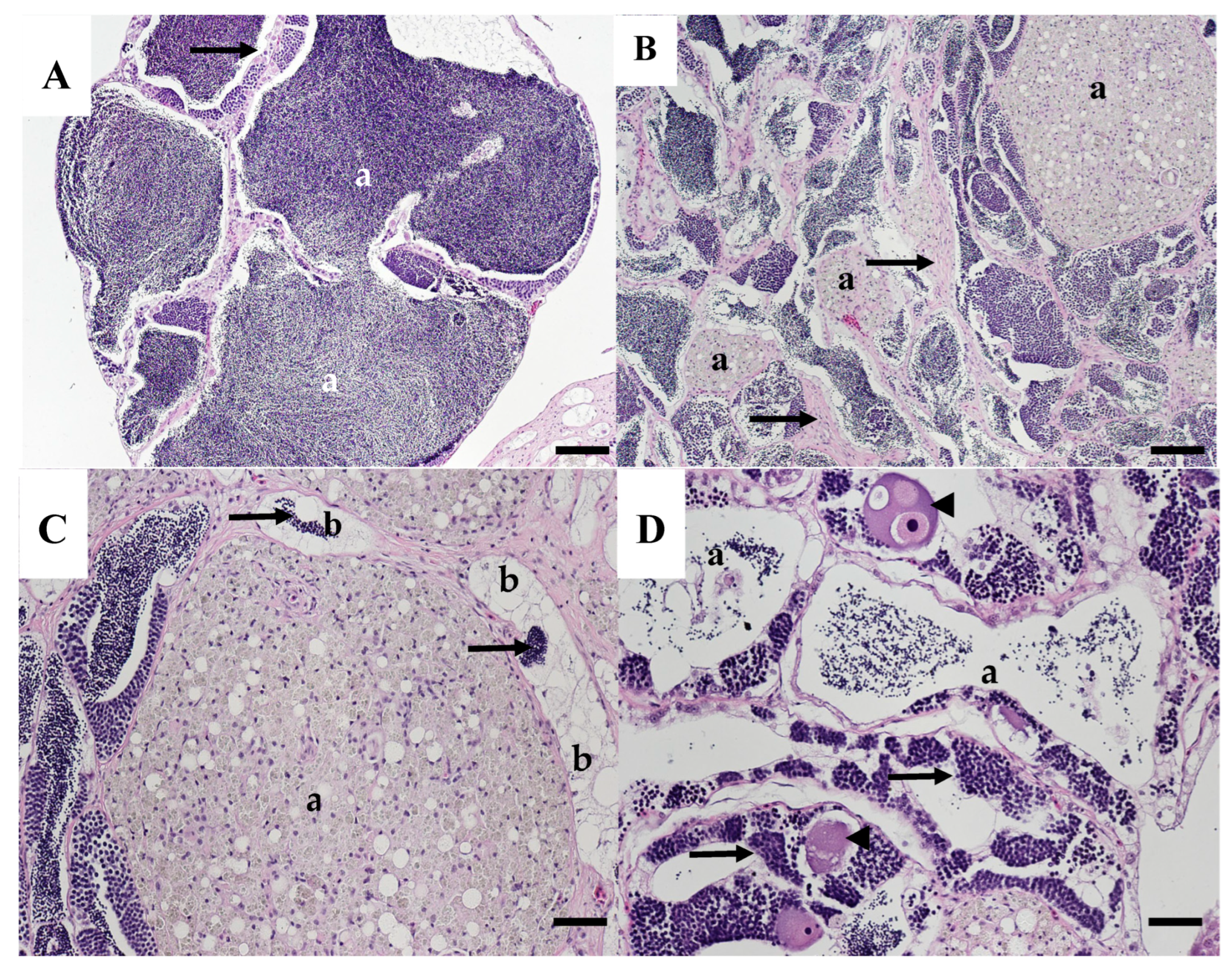

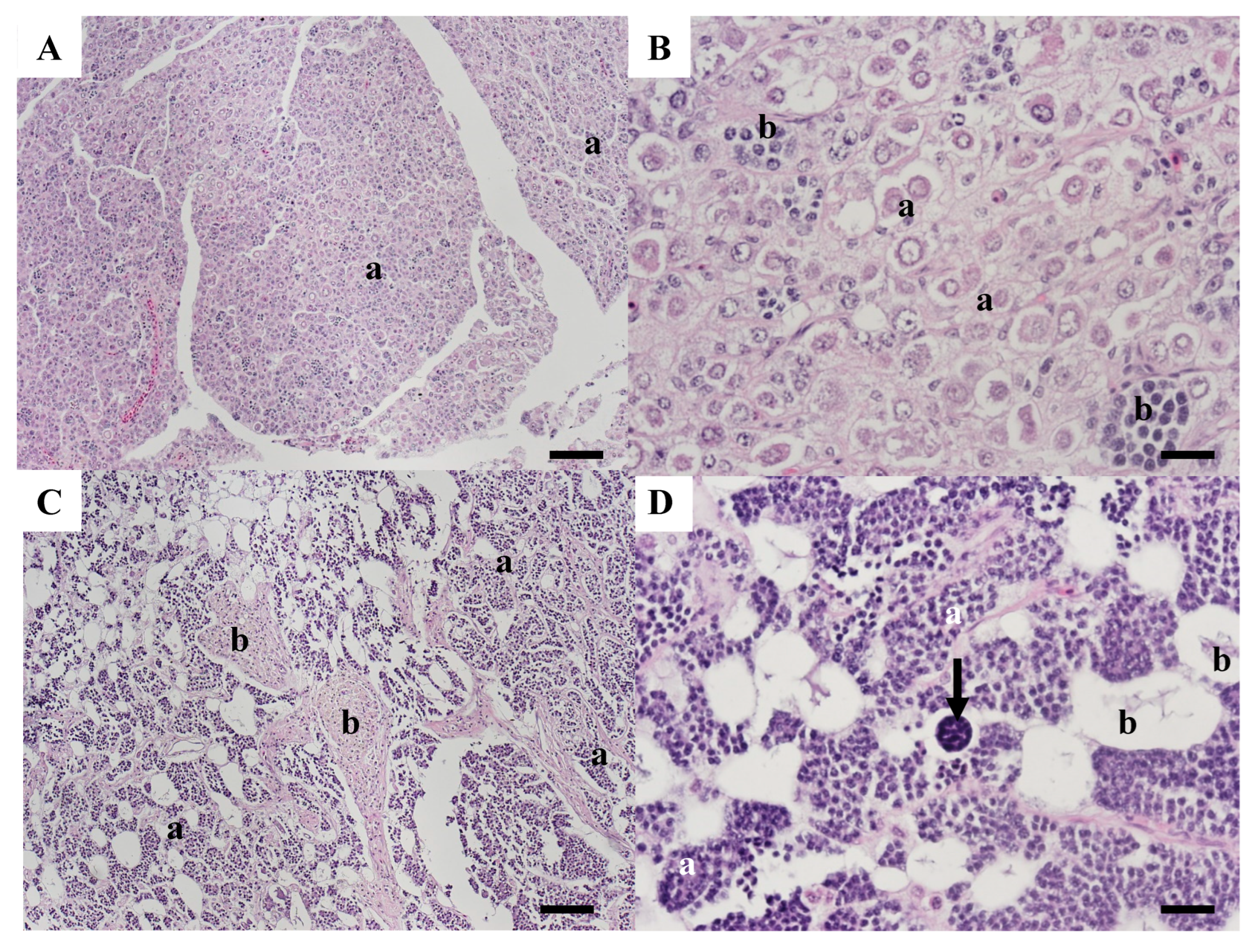
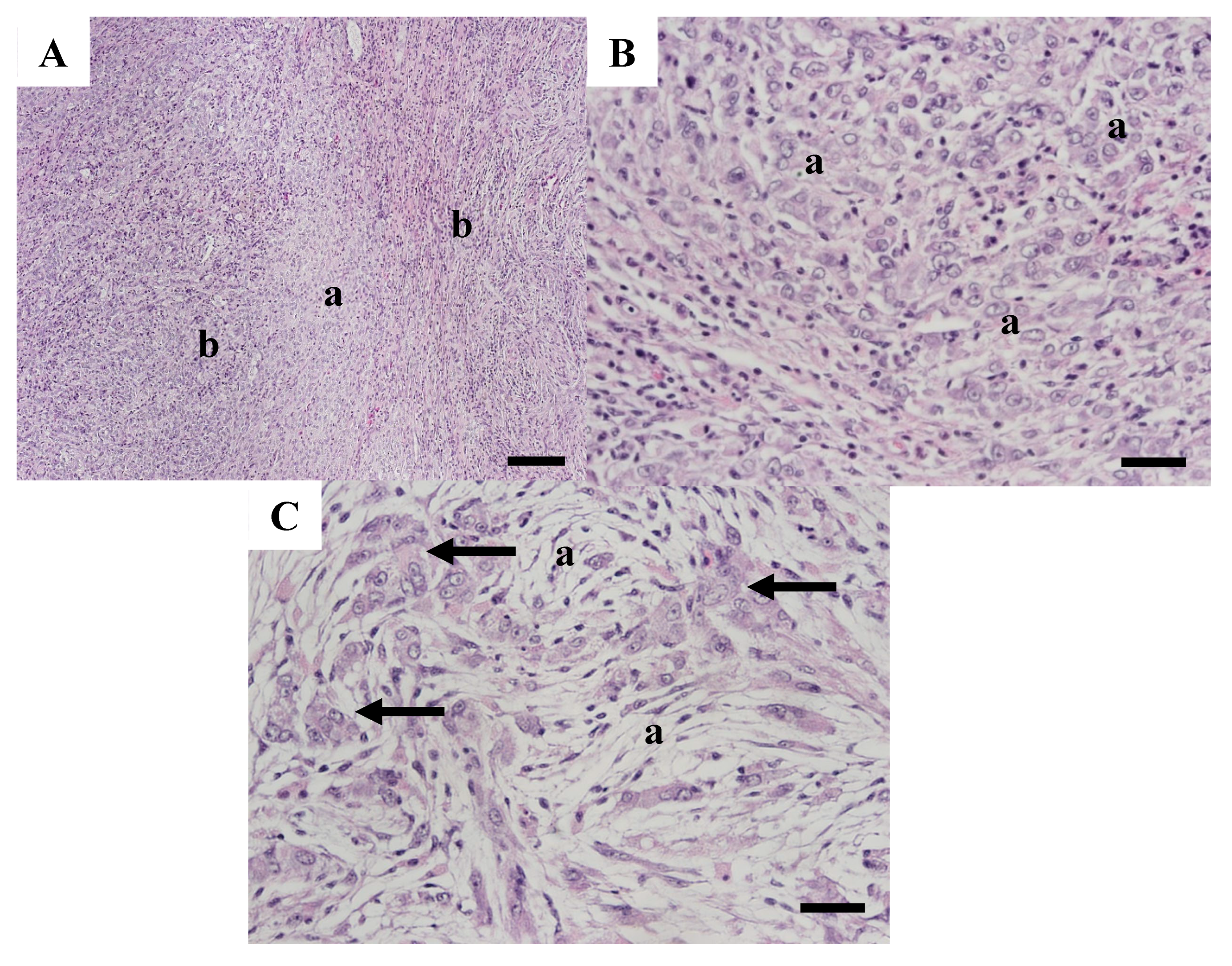
| Year Collected | Length Millimeters | Age Years | Sample Number | Gonad Stage | Observations |
|---|---|---|---|---|---|
| 2003 | 467 | 35 | 320-2 | 4 | MA 1 |
| 2003 | 500 | 42 | 320-3 | 3 | Normal |
| 2003 | 510 | 53 | 320-6 | 3 | MA, Sertoli cell proliferation |
| 2003 | 575 | 47 | 320-7 | 3 | Normal |
| 2003 | 525 | 50 | 320-9 | 3 | MA |
| 2003 | 550 | 39 | 320-10 | 3 | MA |
| 2003 | 465 | 38 | 320-11 | 3 | MA |
| 2003 | 590 | 37 | 320-12 | 4 | Sertoli cell proliferation |
| 2003 | 468 | 44 | 320-15 | 3 | Normal |
| 2007 | 438 | ND 2 | WBCC-46 | 3 | MA, Sertoli cell proliferation, neoplasia |
| 2007 | 521 | ND | WBCC-50 | Unknown | MA, neoplasia |
| 2007 | 442 | ND | WBCC-52 | 3 | MA, intersex |
| 2007 | 471 | ND | WBCC-57 | Unknown | Neoplasia |
| 2007 | 442 | ND | WBCC-66 | 3 | MA, Sertoli cell proliferation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blazer, V.S.; Goodbred, S.L.; Walsh, H.L.; Wichman, D.; Johnson, D.; Patiño, R. Testicular Neoplasms and Other Abnormalities in Common Carp Cyprinus carpio from the Lower Colorado River, United States. Animals 2025, 15, 2887. https://doi.org/10.3390/ani15192887
Blazer VS, Goodbred SL, Walsh HL, Wichman D, Johnson D, Patiño R. Testicular Neoplasms and Other Abnormalities in Common Carp Cyprinus carpio from the Lower Colorado River, United States. Animals. 2025; 15(19):2887. https://doi.org/10.3390/ani15192887
Chicago/Turabian StyleBlazer, Vicki S., Steven L. Goodbred, Heather L. Walsh, Dylan Wichman, Darren Johnson, and Reynaldo Patiño. 2025. "Testicular Neoplasms and Other Abnormalities in Common Carp Cyprinus carpio from the Lower Colorado River, United States" Animals 15, no. 19: 2887. https://doi.org/10.3390/ani15192887
APA StyleBlazer, V. S., Goodbred, S. L., Walsh, H. L., Wichman, D., Johnson, D., & Patiño, R. (2025). Testicular Neoplasms and Other Abnormalities in Common Carp Cyprinus carpio from the Lower Colorado River, United States. Animals, 15(19), 2887. https://doi.org/10.3390/ani15192887







