Vitamin A Supplementation Induces AMFK Production to Promote Cartilage Proliferation and Antler Growth in Sika Deer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experiment Design
2.2. Sample Collection and Measurement
2.3. DNA and RNA Extraction and Sequencing
2.4. Untargeted Metabolome of Feces and Serum and Analysis
2.5. Bioinformatics Analysis
2.6. Isolation and Culture of Antler Chondrocytes
2.7. Cell Proliferation Assays
2.8. Immunofluorescence Assay
2.9. Cell RNA Extraction and qPCR
2.10. Statistical Analysis
3. Results
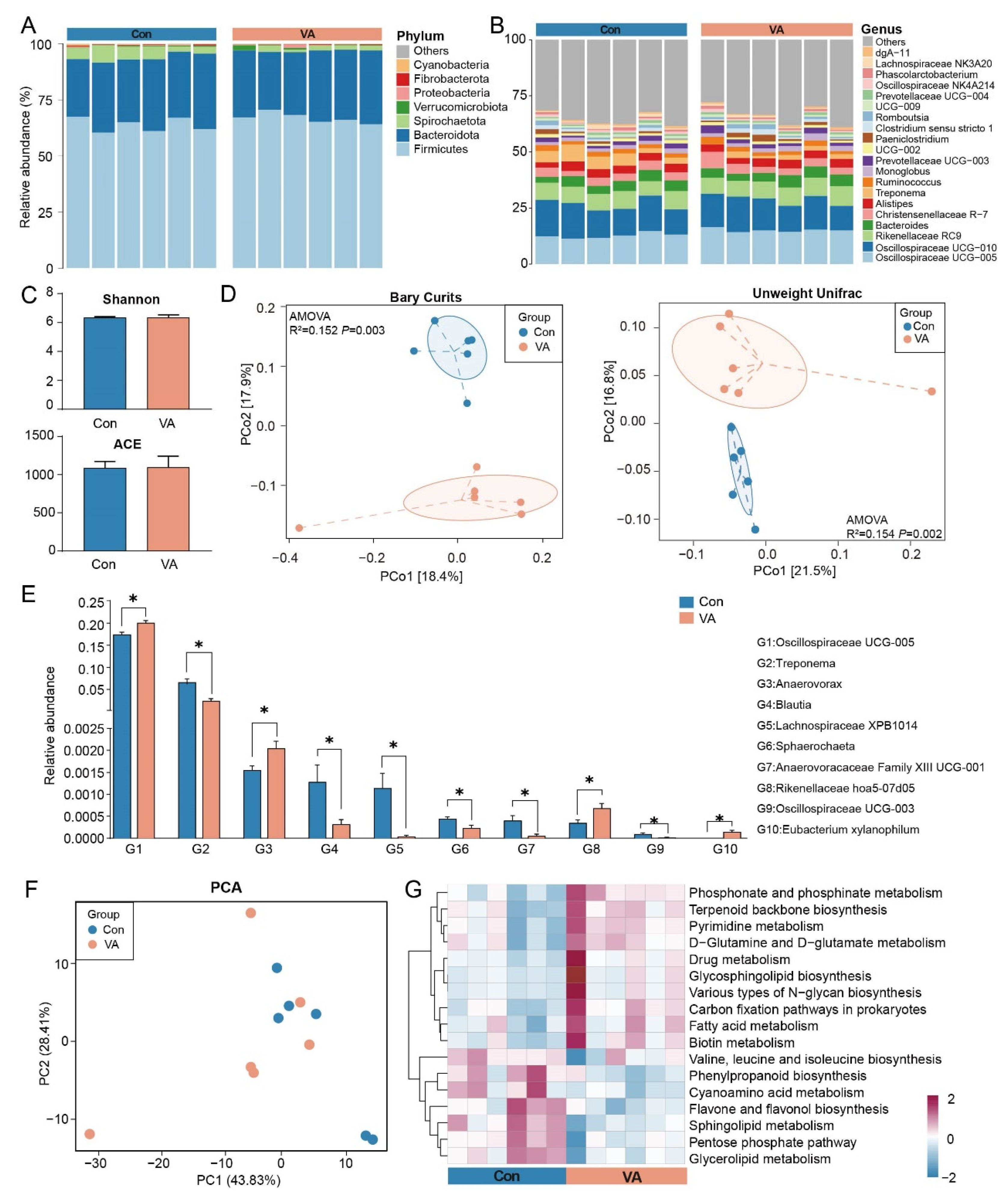
3.1. VA Supplementation Alters Gut Microbiota Composition
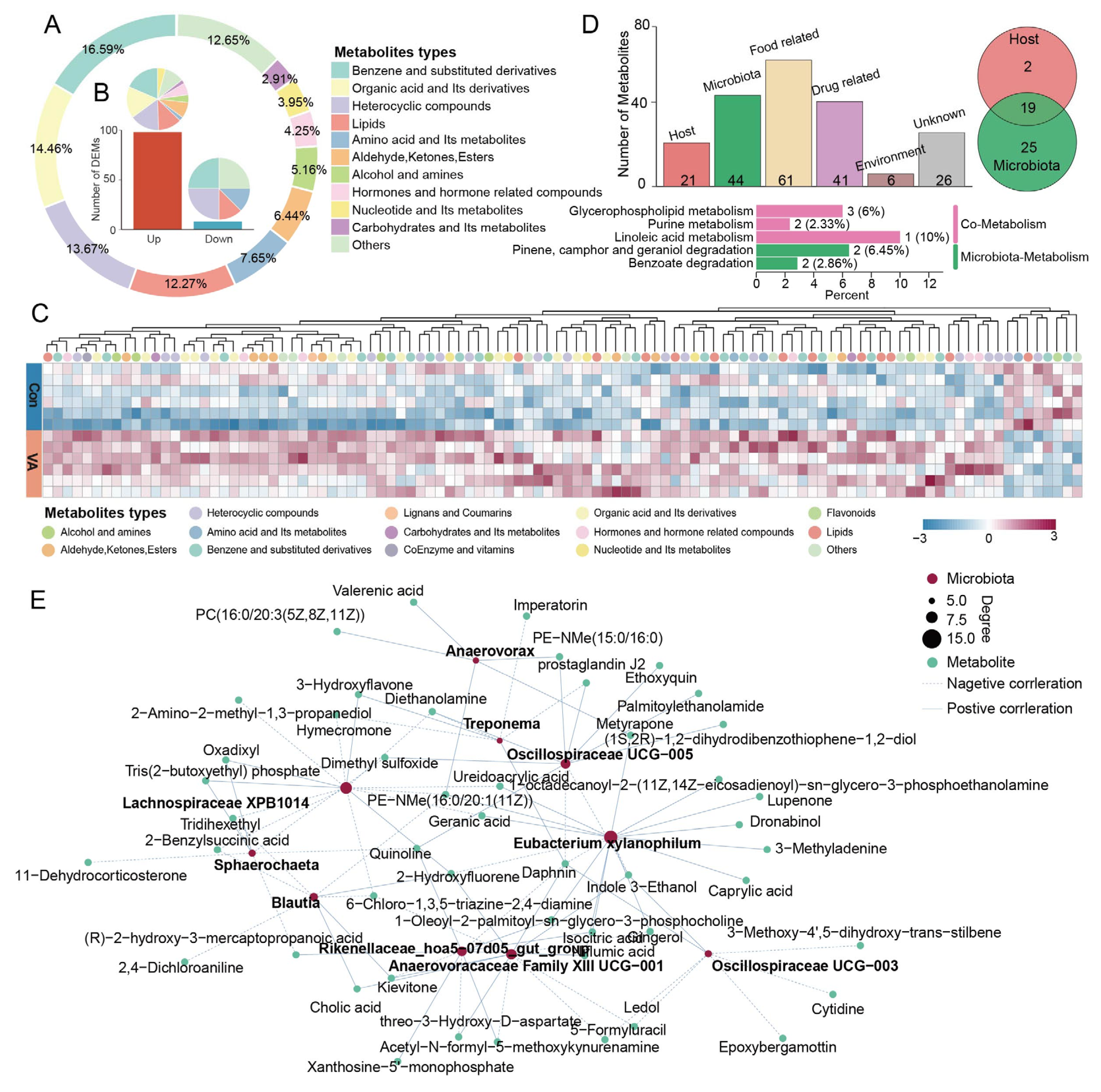
3.2. Effects of VA Supplementation on Fecal Metabolites
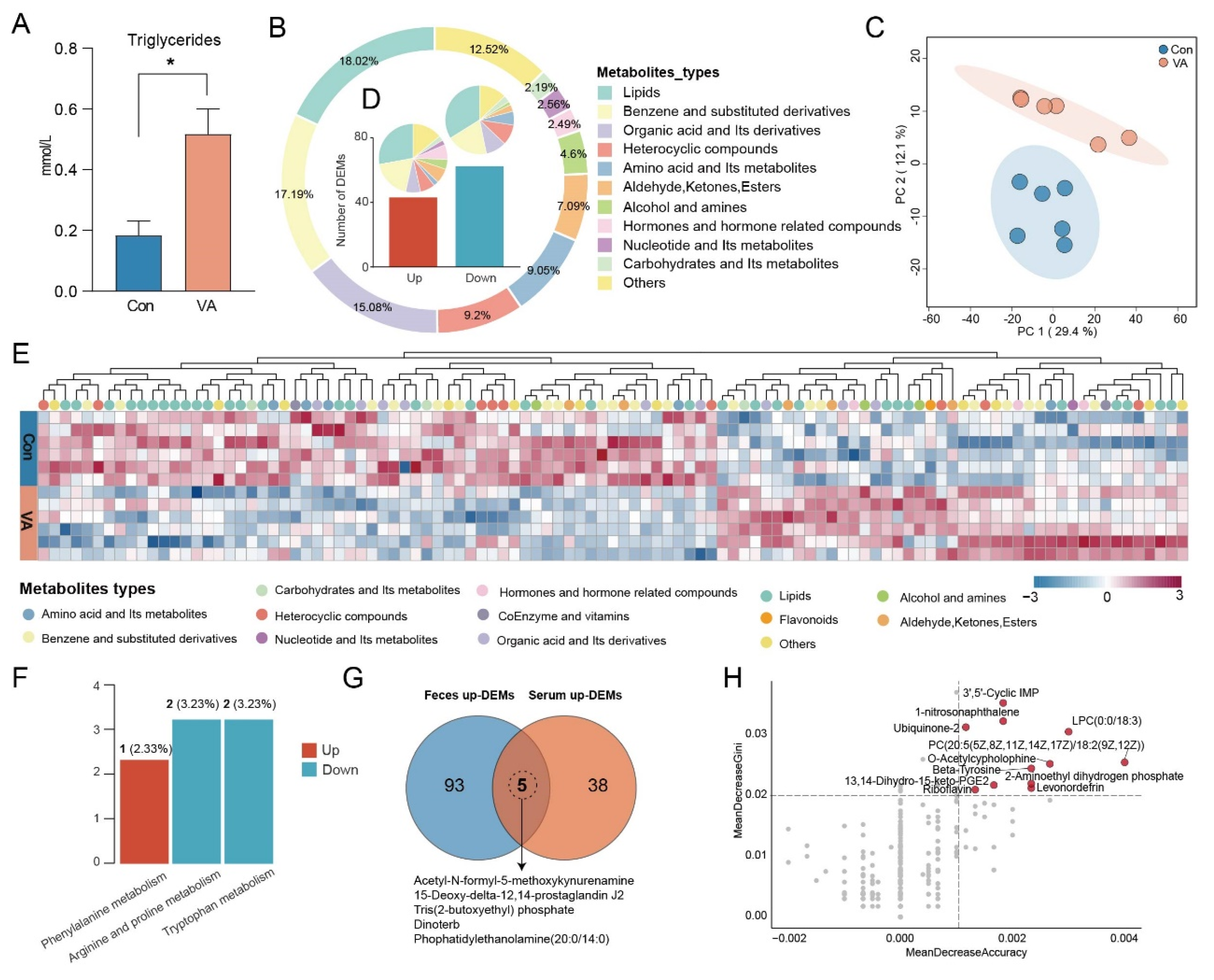
3.3. VA Alters Serum Lipid Metabolic Profiles
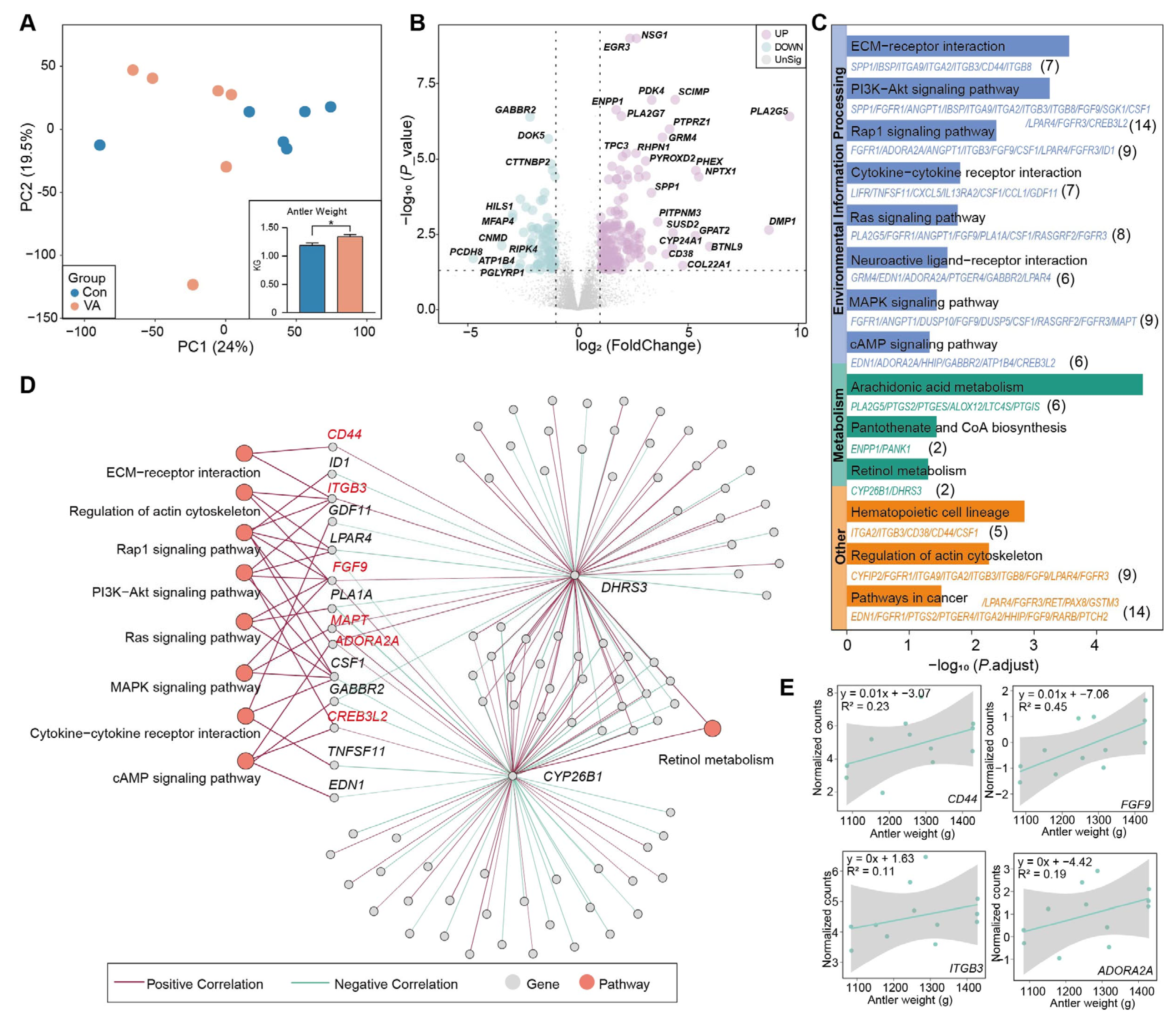
3.4. Distinct Gene Expression of Antler Cartilage
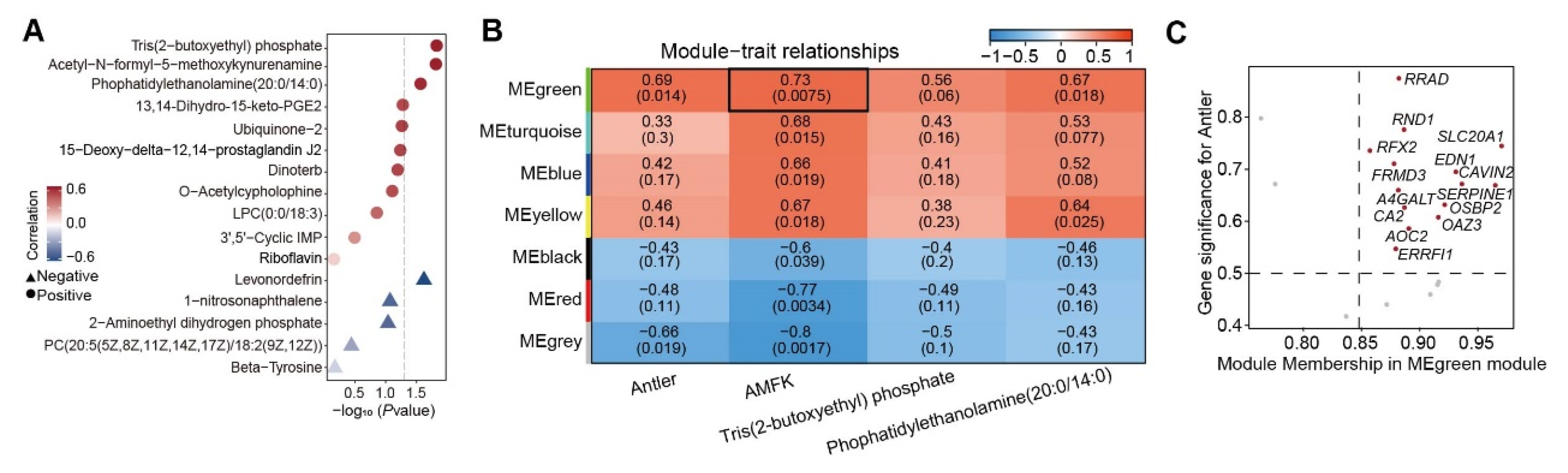
3.5. AMFK-Linked Gene Module Associated with Antler Growth
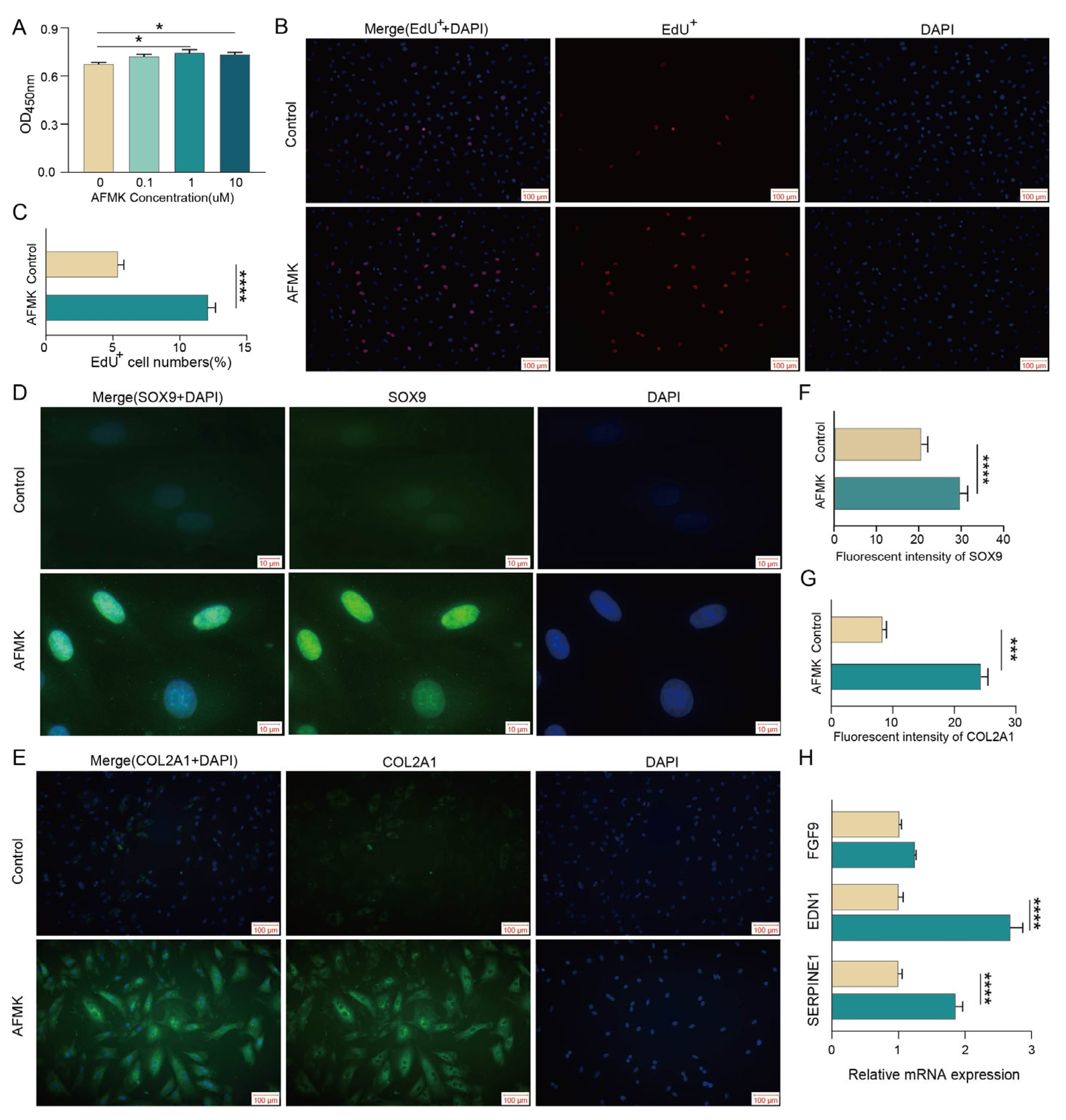
3.6. AMFK Promotes Chondrocyte Proliferation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landete, C.T.; Kierdorf, H.; Gomez, S.; Luna, S.; García, A.J.; Cappelli, J.; Pérez-Serrano, M.; Pérez-Barbería, J.; Gallego, L.; Kierdorf, U. Antlers-evolution, development, structure, composition, and biomechanics of an outstanding type of bone. Bone 2019, 128, 115046. [Google Scholar] [CrossRef]
- Qin, T.; Zhang, G.; Zheng, Y.; Li, S.; Yuan, Y.; Li, Q.; Hu, M.; Si, H.; Wei, G.; Gao, X.; et al. A population of stem cells with strong regenerative potential discovered in deer antlers. Science 2023, 379, 840–847. [Google Scholar] [CrossRef]
- Fennessy, P.F. Deer antlers: Regeneration, function and evolution. J. R. Soc. N. Z. 1984, 14, 290–291. [Google Scholar] [CrossRef]
- Muir, P.D.; Sykes, A.R.; Barrell, G.K. Growth and mineralisation of antlers in red deer (Cervus elaphus). N. Z. J. Agric. Res. 1987, 30, 305–315. [Google Scholar] [CrossRef]
- Li, C.; Yang, F.; Sheppard, A. Adult stem cells and mammalian epimorphic regeneration-insights from studying annual renewal of deer antlers. Curr. Stem Cell Res. Ther. 2009, 4, 237–251. [Google Scholar] [CrossRef]
- Puttoo, M.; Dryden, G.M.; McCosker, J.E. Performance of weaned rusa (Cervus timorensis) deer given concentrates of varying protein content with sorghum hay. Aust. J. Exp. Agric. 1998, 38, 33–39. [Google Scholar] [CrossRef]
- Allen, S.; Maden, M.; Price, J. A role for retinoic acid in regulating the regeneration of deer antlers. Dev. Biol. 2002, 251, 409–423. [Google Scholar] [CrossRef]
- Landete, C.T.; Currey, J.D.; Ceacero, F.; García, A.J.; Gallego, L.; Gomez, S. Does nutrition affect bone porosity and mineral tissue distribution in deer antlers? The relationship between histology, mechanical properties and mineral composition. Bone 2012, 50, 245–254. [Google Scholar] [CrossRef]
- Dryden, G.M. Nutrition of antler growth in deer. Anim. Prod. Sci. 2016, 56, 962–970. [Google Scholar] [CrossRef]
- Lin, W.; Jia, X.; Shi, X.; He, Q.; Zhang, P.; Zhang, X.; Zhang, L.; Wu, M.; Ren, T.; Liu, Y.; et al. Reactivation of mammalian regeneration by turning on an evolutionarily disabled genetic switch. Science 2025, 388, eadp0176. [Google Scholar] [CrossRef]
- Ősz, J.; McEwen, A.; Bourguet, M.; Przybilla, F.; Peluso, I.C.; Poussin-Courmontagne, P.; Mély, Y.; Cianférani, S.; Jeffries, C.M.; I Svergun, D.; et al. Structural basis for DNA recognition and allosteric control of the retinoic acid receptors RAR–RXR. Nucleic Acids Res. 2020, 48, 9969–9985. [Google Scholar] [CrossRef]
- Chambon, P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996, 10, 940–954. [Google Scholar] [CrossRef]
- Sekiya, I.; Tsuji, K.; Koopman, P.; Watanabe, H.; Yamada, Y.; Shinomiya, K.; Nifuji, A.; Noda, M. SOX9 enhances aggrecan gene promoter/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. J. Biol. Chem. 2000, 275, 10738–10744. [Google Scholar] [CrossRef]
- Tian, Y.; Nichols, R.; Cai, J.; Patterson, A.; Cantorna, M. Vitamin A deficiency in mice alters host and gut microbial metabolism leading to altered energy homeostasis. J. Nutr. Biochem. 2018, 54, 28–34. [Google Scholar] [CrossRef]
- Petersen, C.; Bell, R.; Klag, K.; Lee, S.H.; Soto, R.; Ghazaryan, A.; Buhrke, K.; Atakan Ekiz, H.; Ost, K.S.; Boudina, S.; et al. T cell-mediated regulation of the microbiota protects against obesity. Science 2019, 365, eaat9351. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the genetic basis of fibrolytic specialization by lachnospiraceae and ruminococcaceae in diverse gut communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Ashton, A.; Stoney, P.; McCaffery, P. Investigating the role of vitamin A in melatonin production in the pineal gland. Proc. Nutr. Soc. 2015, 74, E195. [Google Scholar] [CrossRef]
- Sun, X.; Gu, X.; Li, K.; Li, M.; Peng, J.; Zhang, X.; Yang, L.; Xiong, J. Melatonin promotes antler growth by accelerating mt1-mediated mesenchymal cell differentiation and inhibiting VEGF-induced degeneration of chondrocytes. Int. J. Mol. Sci. 2022, 23, 759. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Li, B.; Wang, C.; Yang, Z.; Guo, B.; Yue, Z. Melatonin promotes the proliferation and differentiation of antler chondrocytes via RUNX2 dependent on the interaction between NOTCH1 and SHH signaling pathways. Cell Biol. Int. 2024, 49, 329–342. [Google Scholar] [CrossRef]
- Kelly, P.E.; Ng, H.J.; Farrell, G.; McKirdy, S.; Russell, R.K.; Hansen, R.; Rattray, Z.; Gerasimidis, K.; Rattray, N.J.W. An optimised monophasic faecal extraction method for LC-MS analysis and its application in gastrointestinal disease. Metabolites 2022, 12, 1110. [Google Scholar] [CrossRef]
- Ewald, J.D.; Zhou, G.; Lu, Y.; Kolic, J.; Ellis, C.; Johnson, J.D.; Macdonald, P.E.; Xia, J. Web-based multi-omics integration using the Analyst software suite. Nat. Protoc. 2024, 19, 1467–1497. [Google Scholar] [CrossRef]
- Yu, G.; Xu, C.; Wang, X.; Ju, F.; Fu, J.; Ni, Y. MetOrigin 2.0: Advancing the discovery of microbial metabolites and their origins. iMeta 2024, 3, e246. [Google Scholar] [CrossRef]
- Hall, M.; Beiko, R.G. 16S rRNA gene analysis with QIIME2. In Microbiome Analysis: Methods and Protocols; Springer: New York, NY, USA, 2018; pp. 113–129. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, D1, 590–596. [Google Scholar] [CrossRef]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. Microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef]
- Wemheuer, F.; Taylor, J.A.; Daniel, R.; Johnston, E.; Meinicke, P.; Thomas, T.; Wemheuer, B. Tax4Fun2: Prediction of habitat-specific functional profiles and functional redundancy based on 16S rRNA gene sequences. Environ. Microbiome 2020, 15, 11. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Guo, B.; Wang, S.; Duan, C.C.; Li, D.; Tian, X.; Wang, Q.Y.; Yue, Z.-P. Effects of PTHrP on chondrocytes of sika deer antler. Cell Tissue Res. 2013, 354, 451–460. [Google Scholar] [CrossRef]
- Li, S.; Mu, R.; Zhang, Y.; Wang, S.; Wright, A.D.; Si, H.; Li, Z. Dynamics of intestinal mucosa microbiota in juvenile sika deer during early growth. Int. J. Mol. Sci. 2025, 26, 892. [Google Scholar] [CrossRef]
- Yang, J.; Jacobs, J.; Hwang, M.; Sabui, S.; Liang, F.; Said, H.; Skupsky, J. Biotin deficiency induces intestinal dysbiosis associated with an inflammatory bowel disease-like phenotype. Nutrients 2023, 15, 264. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Yang, J.; Zhao, S.; Jin, S.; Ao, N.; Yang, J.; Liu, H.-X.; Du, J. Vitamin D alleviates non-alcoholic fatty liver disease via restoring gut microbiota and metabolism. Front. Microbiol. 2023, 14, 1117644. [Google Scholar] [CrossRef]
- Nguyen, T.; Watanabe, A.; Burleigh, S.; Ghaffarzadegan, T.; Kanklai, J.; Prykhodko, O.; Hållenius, F.F.; Nyman, M. Monobutyrin and monovalerin improve gut-blood-brain biomarkers and alter gut microbiota composition in high-fat fed apolipoprotein-E-knockout rats. Sci. Rep. 2022, 12, 15454. [Google Scholar] [CrossRef]
- Ruhn, K.; Li, Y.; Hu, Z.; Gattu, S.; Raj, P.; Herz, J.; Hooper, L.V. Serum amyloid A delivers retinol to intestinal myeloid cells to promote adaptive immunity. Science 2021, 373, eabf9232. [Google Scholar] [CrossRef]
- Ziouzenkova, O.; Plutzky, J. Retinoid metabolism and nuclear receptor responses: New insights into coordinated regulation of the PPAR-RXR complex. FEBS Lett. 2008, 582, 32–38. [Google Scholar] [CrossRef]
- Li, X.; Sun, Q.; Xiao, M.; Cheng, B.; Wang, S.; Wei, X. Vitamin A deficiency from maternal gestation may contribute to autistic-like behaviors and gastrointestinal dysfunction in rats through the disrupted purine and tryptophan metabolism. Behav. Brain Res. 2023, 452, 114520. [Google Scholar] [CrossRef]
- Song, X.; Geng, Y.; Wang, Y. Effect of IC50 dose of retinol on metabolomics of RAW264.7 cells. J. Food Biochem. 2020, 44, e13327. [Google Scholar]
- Das, P.; Chakravortty, D.; Lahiri, A.; Lahiri, A. Modulation of the arginase pathway in the context of microbial pathogenesis: A Metabolic enzyme moonlighting as an immune modulator. PLoS Pathog. 2010, 6, 1000899. [Google Scholar] [CrossRef]
- Wuelling, M.; Vortkamp, A. Chondrocyte proliferation and differentiation. Endocr. Dev. 2011, 21, 1–11. [Google Scholar]
- Li, M.; Chang, Q.; Luo, Y.; Pan, J.; Hu, Y.; Liu, B.; Ma, M.; Wang, Q.; Guo, Y.; Wang, Q. The gut microbial composition in polycystic ovary syndrome with hyperandrogenemia and its association with steroid hormones. Front. Cell Dev. Biol. 2024, 12, 1384233. [Google Scholar] [CrossRef]
- Ahmadi, S.; Taghizadieh, M.; Mehdizadehfar, E.; Hasani, A.; Fard, J.K.; Feizi, H.; Hamishehkar, H.; Ansarin, M.; Yekani, M.; Memar, M.Y. Gut microbiota in neurological diseases: Melatonin plays an important regulatory role. Biomed Pharmacother 2024, 174, 116487. [Google Scholar] [CrossRef]
- Garashchenko, N.; Semenova, N.; Kolesnikova, L. Melatonin and gut microbiome. Acta Biomed. Sci. 2024, 9, 12–23. [Google Scholar] [CrossRef]
- Li, J.; Guo, C.; Wu, J. 15-Deoxy-∆-12,14-Prostaglandin J2, an Endogenous Ligand of PPAR-γ: Function and Mechanism. PPAR Res. 2019, 174, 116487. [Google Scholar] [CrossRef]
- Hoffer, B.; Siggins, G.; Bloom, F. Prostaglandins E1 and E2 antagonize norepinephrine effects on cerebellar purkinje cells: Microelectrophoretic study. Science 1969, 166, 1418–1420. [Google Scholar] [CrossRef]
- Zoref, S.E.; Sperling, O.; Shainberg, A. Characterization of purine nucleotide metabolism in primary rat muscle cultures. Biochim. Biophys. Acta 1982, 716, 324–330. [Google Scholar] [CrossRef]
- Chen, H.; Jin, C.; Jing, L.; Qi, B.; Zhao, D.; Su, H.; Yang, C. Comparative metabolomics study revealed difference in central carbon metabolism between sika deer and red deer antler. Int. J. Genom. 2020, 25, 7192896. [Google Scholar]
- Hardeland, R. Atioxidative protection by melatonin. Endocrine 2007, 27, 119–130. [Google Scholar] [CrossRef]
- Obrochta, K.; Kane, M.; Napoli, J. Effects of diet and strain on mouse serum and tissue retinoid concentrations. PLoS ONE 2014, 9, e99435. [Google Scholar] [CrossRef]
- Liu, L.; Wang, D.; Du, Y.; Yang, X.; Li, N.; Ying, C.J.; Liu, L. Suppression of retinoic acid receptors may contribute to embryonic skeleton hypoplasia in maternal rats with chronic vitamin A deficiency. J. Nutr. Biochem. 2010, 21, 710–716. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Z.; Li, G.; Sun, W.; Zhang, T.; Li, R.; Li, G. Effects of vitamin A on antioxidant functions, immune functions and production performance in male sika deer (Cervus nippon) during the first antler growth period. Ital. J. Anim. Sci. 2019, 18, 98–104. [Google Scholar] [CrossRef]
- Toyosawa, S.; Kobata, M.; Yuki, M.; Shintani, S.; Kishino, M.; Ijuhin, N.; Komori, T. Expression of dentin matrix protein 1 (DMP1) during fracture healing. Bone 2004, 35, 553–561. [Google Scholar] [CrossRef]
- Upadhyay, U.; Kolla, S.; Chelluri, L. Extracellular matrix composition analysis of human articular cartilage for the development of organ-on-a-chip. Biochem. Biophys. Res. Commun. 2023, 667, 81–88. [Google Scholar] [CrossRef]
- Ishida, O.; Tanaka, Y.; Morimoto, I.; Takigawa, M.; Eto, S. Chondrocytes are regulated by cellular adhesion through CD44 and hyaluronic acid pathway. J. Bone Miner. Res. 1997, 12, 1657–1663. [Google Scholar] [CrossRef]
- Zhang, X.; Weng, M.; Chen, Z. Fibroblast growth factor 9 (FGF9) negatively regulates the early stage of chondrogenic differentiation. PLoS ONE 2021, 16, e0241281. [Google Scholar] [CrossRef]
- Clyman, R.; Mauray, F.; Kramer, R. Beta 1 and beta 3 integrins have different roles in the adhesion and migration of vascular smooth muscle cells on extracellular matrix. Exp. Cell Res. 1992, 200, 272–284. [Google Scholar] [CrossRef]
- Endres, K. Retinoic acid and the gut microbiota in alzheimer’s disease: Fighting back-to-back? Curr. Alzheimer Res. 2019, 16, 405–417. [Google Scholar] [CrossRef]
- Barbieri, O.; Astigiano, S.; Morini, M.; Tavella, S.; Schito, A.; Corsi, A.; Di Martino, D.; Bianco, P.; Cancedda, R.; Garofalo, S. Depletion of cartilage collagen fibrils in mice carrying a dominant negative COL2A1 transgene affects chondrocyte differentiation. Am. J. Physiol. Physiol. 2003, 285, C1504–C1512. [Google Scholar] [CrossRef]
- Song, H.; Park, K.H. Regulation and function of SOX9 during cartilage development and regeneration. Semin. Cancer Biol. 2020, 67, 12–23. [Google Scholar] [CrossRef]
- Pietropaolo, C.; Adornetto, A.; Masullo, M.; Sarnataro, D.; Pagliara, V.; Arcone, R.; Mammì, M. Protease Nexin-1 affects the migration and invasion of C6 glioma cells through the regulation of urokinase plasminogen activator and matrix metalloproteinase-9/2. Biochim. Biophys. Acta 2014, 1843, 2631–2644. [Google Scholar]
- Sottile, J. Regulation of angiogenesis by extracellular matrix. Biochim. Biophys. Acta 2004, 1654, 13–22. [Google Scholar] [CrossRef]
- Harthé, C.; Claudy, D.; Déchaud, H.; Vivien-Roels, B.; Pévet, P.; Claustrat, B. Radioimmunoassay of N-acetyl-N-formyl-5-methoxykynuramine (AFMK): A melatonin oxidative metabolite. Life Sci. 2003, 73, 1587–1597. [Google Scholar] [CrossRef]
- Rozov, S.V.; Filatova, E.V.; Orlov, A.A.; Volkova, A.V.; Zhloba, A.R.; Blashko, E.L.; Pozdeyev, N.V. N1-acetyl-N2-formyl-5-methoxykynuramine is a product of melatonin oxidation in rats. J. Pineal Res. 2003, 35, 245–250. [Google Scholar] [CrossRef]
- Silva, S.O.; Ximenes, V.F.; Livramento, J.A.; Catalani, L.H.; Campa, A. High concentrations of the melatonin metabolite, N1-acetyl-N2-formyl-5-methoxykynuramine, in cerebrospinal fluid of patients with meningitis: A possible immunomodulatory mechanism. J. Pineal Res. 2005, 39, 302–306. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, H.; Li, S.; Liu, H.; Duan, X.; Deng, R.; Zhu, Y.; Zhang, Y.; Chen, S.; Wang, S.; Ma, C.; et al. Vitamin A Supplementation Induces AMFK Production to Promote Cartilage Proliferation and Antler Growth in Sika Deer. Animals 2025, 15, 2879. https://doi.org/10.3390/ani15192879
Si H, Li S, Liu H, Duan X, Deng R, Zhu Y, Zhang Y, Chen S, Wang S, Ma C, et al. Vitamin A Supplementation Induces AMFK Production to Promote Cartilage Proliferation and Antler Growth in Sika Deer. Animals. 2025; 15(19):2879. https://doi.org/10.3390/ani15192879
Chicago/Turabian StyleSi, Huazhe, Songze Li, Huanhuan Liu, Xing Duan, Ruijia Deng, Yuhang Zhu, Yunxi Zhang, Sibo Chen, Shaoying Wang, Cuiliu Ma, and et al. 2025. "Vitamin A Supplementation Induces AMFK Production to Promote Cartilage Proliferation and Antler Growth in Sika Deer" Animals 15, no. 19: 2879. https://doi.org/10.3390/ani15192879
APA StyleSi, H., Li, S., Liu, H., Duan, X., Deng, R., Zhu, Y., Zhang, Y., Chen, S., Wang, S., Ma, C., Li, Y., Sang, J., Gao, X., Liu, H., Nan, W., & Li, Z. (2025). Vitamin A Supplementation Induces AMFK Production to Promote Cartilage Proliferation and Antler Growth in Sika Deer. Animals, 15(19), 2879. https://doi.org/10.3390/ani15192879





