Cytokine Expression and Haptoglobin Levels in Bovine Fetuses Spontaneously Aborted by Intracellular Infectious Agents and by Probable Infectious Etiology
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design and Selection of Fetuses
2.2. Diagnosis
2.2.1. Neospora caninum
2.2.2. Brucella abortus
2.2.3. Bovine Viral Diarrhea Virus
2.2.4. Other Pathogens
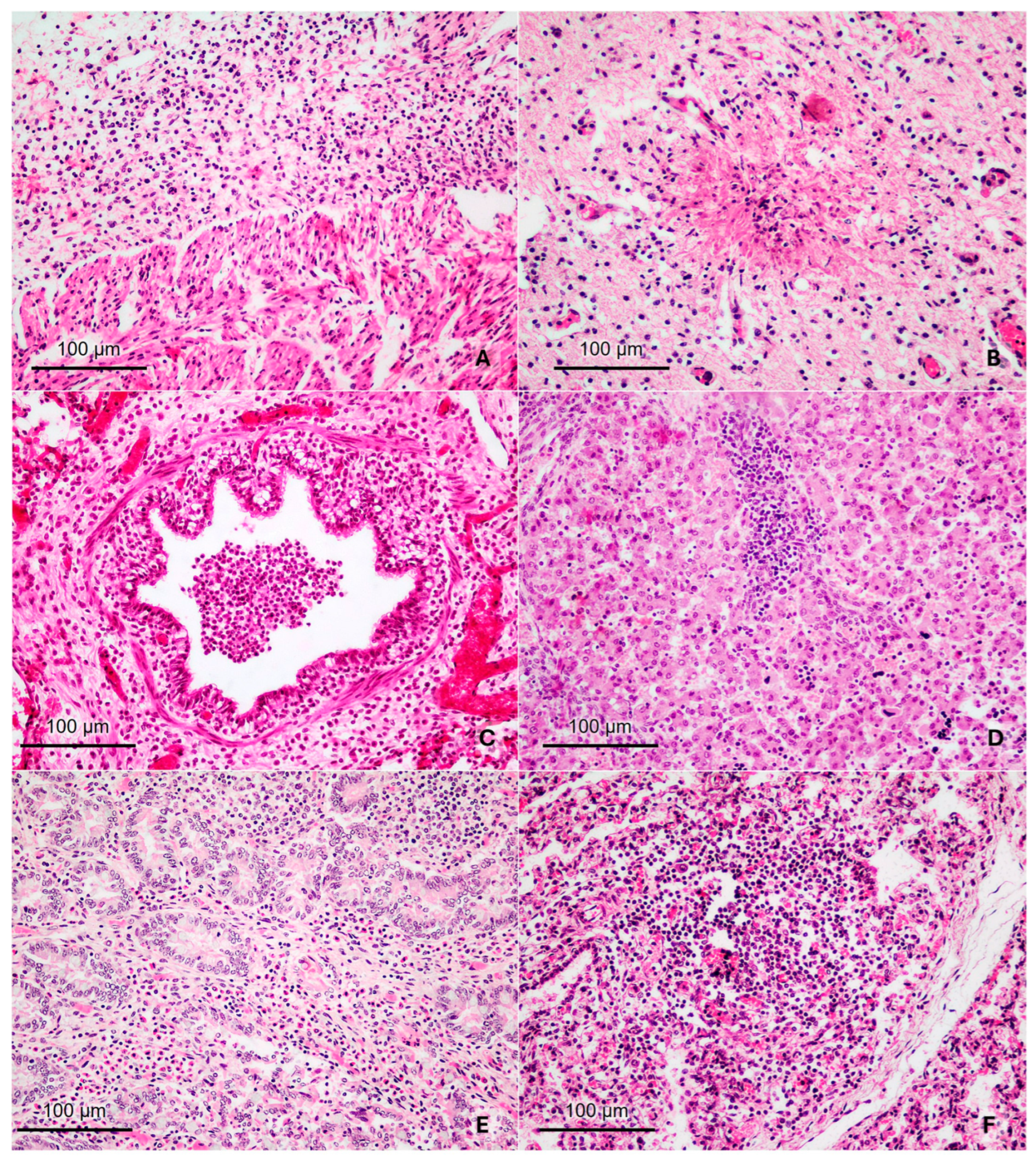
2.3. Histopathology
2.4. Haptoglobin Determination
2.5. Cytokine Gene Expression
2.6. Statistical Analysis
3. Results
3.1. Fetuses Aborted Due to Intracellular Agents and Probable Infectious Etiology
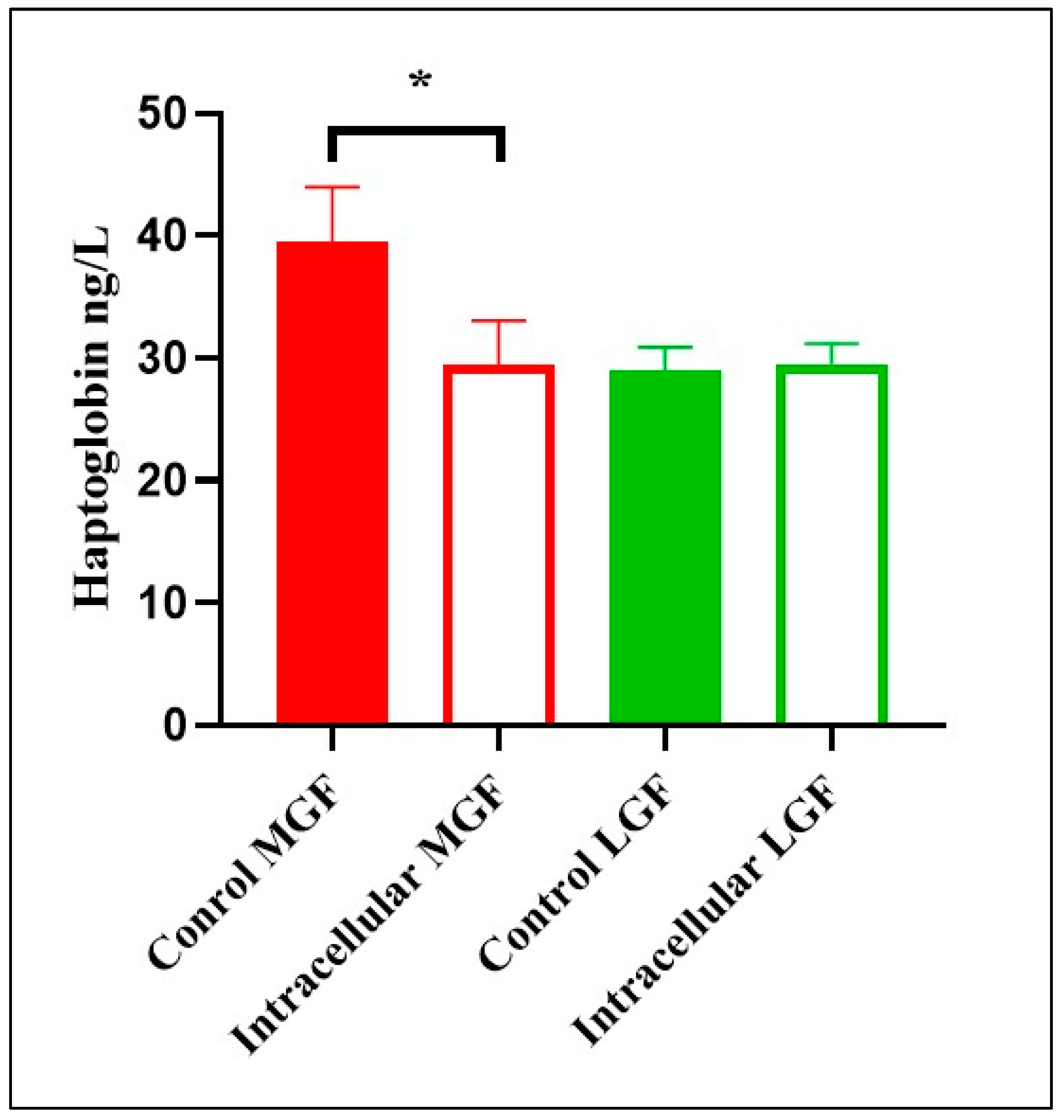
3.2. Haptoglobin
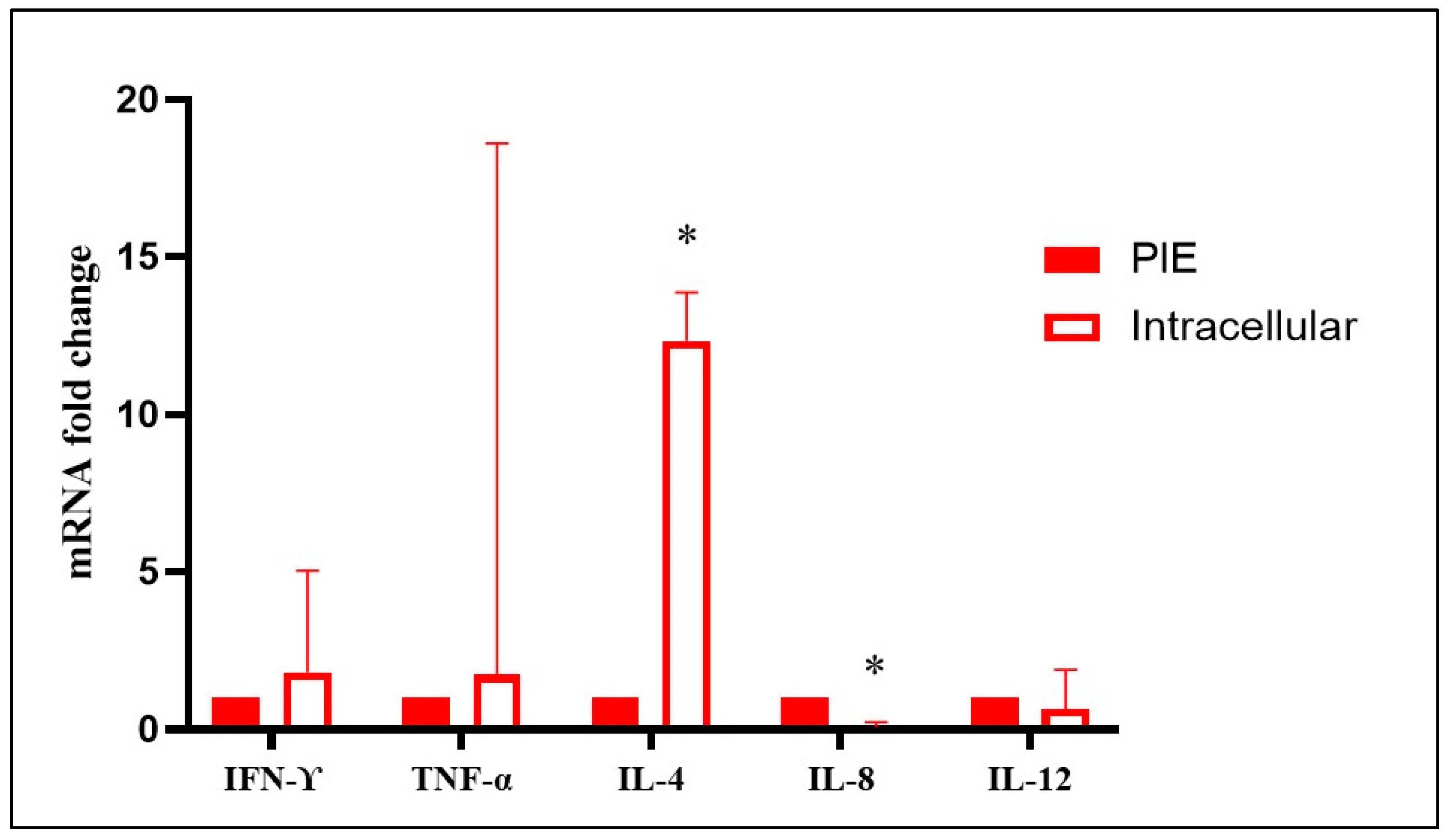
3.3. Cytokine Gene Expression in Fetal Spleen
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cantón, G.J.; Moreno, F.; Fiorentino, M.A.; Hecker, Y.P.; Spetter, M.; Fiorani, F.; Monterubbianesi, M.G.; García, J.A.; Altamiranda, E.G.; Cirone, K.M.; et al. Spatial-temporal trends and economic losses associated with bovine abortifacients in central Argentina. Trop. Anim. Health Prod. 2022, 54, 242. [Google Scholar] [CrossRef]
- Carrillo Parraguez, M.; Ponssa, E.; Caffarena, D.; Artagaveytia, J.; Sotelo, F.; Fariña, S.; Mendoza, A.; Giannitti, F. Estimation of direct economic and productive losses due to abortions caused by Neospora caninum in the primary dairy sector of Uruguay. Front. Vet. Sci. 2025, 12, 1502742. [Google Scholar] [CrossRef]
- Campero, C.M.; Moore, D.P.; Odeón, A.C.; Cipolla, A.L.; Odriozola, E. Aetiology of bovine abortion in Argentina. Vet. Res. Commun. 2003, 27, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.W.; Lee, K.-K.; Lee, K.; Ku, B.-K.; Kim, H.-Y. Laboratory investigation of causes of bovine abortion and stillbirth in the Republic of Korea, 2014-2020. J. Vet. Diagn. Investig. 2024, 36, 428–437. [Google Scholar] [CrossRef]
- Morrell, E.L.; Campero, C.M.; Cantón, G.J.; Odeón, A.C.; Moore, D.P.; Odriozola, E.; Paolicchi, F.; Fiorentino, M.A. Current trends in bovine abortion in Argentina. Pesq. Vet. Bras. 2019, 39, 12–19. [Google Scholar] [CrossRef]
- Van Loo, H.; Pascottini, O.B.; Ribbens, S.; Hooyberghs, J.; Pardon, B.; Opsomer, G. Retrospective study of factors associated with bovine infectious abortion and perinatal mortality. Prev. Vet. Med. 2021, 191, 105366. [Google Scholar] [CrossRef]
- Dubey, J.P.; Buxton, D.; Wouda, W. Pathogenesis of Bovine Neosporosis. J. Comp. Pathol. 2006, 134, 267–289. [Google Scholar] [CrossRef] [PubMed]
- Hecker, Y.P.; González-Ortega, S.; Cano, S.; Ortega-Mora, L.M.; Horcajo, P. Bovine infectious abortion: A systematic review and meta-analysis. Front. Vet. Sci. 2023, 10, 1249410. [Google Scholar] [CrossRef]
- Anderson, M.L. Infectious causes of bovine abortion during mid- to late-gestation. Theriogenology 2007, 68, 474–486. [Google Scholar] [CrossRef]
- Baumgartner, W. Fetal Disease and Abortion: Diagnosis and Causes. In Bovine Reproduction; Hopper, R.M., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2021; p. 671. [Google Scholar] [CrossRef]
- Khurana, S.K.; Sehrawat, A.; Tiwari, R.; Prasad, M.; Gulati, B.; Shabbir, M.Z.; Chhabra, R.; Karthik, K.; Patel, S.K.; Pathak, M.; et al. Bovine brucellosis–a comprehensive review. Vet. Q. 2021, 41, 61–88. [Google Scholar] [CrossRef]
- Lanyon, S.R.; Hill, F.I.; Reichel, M.P.; Brownlie, J. Bovine brucellosis–A comprehensive review. Vet. J. 2014, 199, 201–209. [Google Scholar] [CrossRef]
- Spetter, M.J.; Louge Uriarte, E.L.; Armendano, J.I.; Morrell, E.L.; Cantón, G.J.; Verna, A.E.; Dorsch, M.A.; Pereyra, S.B.; Odeón, A.C.; Saliki, J.T.; et al. Detection methods and characterization of bovine viral diarrhea virus in aborted fetuses and neonatal calves over a 22-year period. Braz. J. Microbiol. 2020, 51, 2077–2086. [Google Scholar] [CrossRef]
- Mee, J.F. Investigation of bovine abortion and stillbirth/perinatal mortality—Similar diagnostic challenges, different approaches. Ir. Vet. J. 2020, 73, 20. [Google Scholar] [CrossRef] [PubMed]
- Barrington, G.M.; Parish, S.M. Bovine neonatal immunology. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 463–476. [Google Scholar] [CrossRef]
- Schultz, R.D. Developmental aspects of the fetal bovine immune response: A review. Cornell Vet. 1973, 63, 507–535. [Google Scholar]
- Mee, J.F.; Hayes, C.; Stefaniak, T.; Jawor, P. Review: Bovine foetal mortality—risk factors, causes, immune responses and immuno-prophylaxis. Animal 2023, 17, 100774. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Saif, L.J. Bovine Immunology: Implications for Dairy Cattle. Front. Immunol. 2021, 12, 643206. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.C.L.; Rice-Ficht, A.C.; Estes, D.M. Bovine type 1 and type 2 responses. Vet. Immunol. Immunopathol. 1998, 63, 45–55. [Google Scholar] [CrossRef]
- Ceciliani, F.; Ceron, J.J.; Eckersall, P.D.; Sauerwein, H. Acute phase proteins in ruminants. J. Proteom. 2012, 75, 4207–4231. [Google Scholar] [CrossRef]
- Fouz, R.; Rodríguez-Bermúdez, R.; Rodríguez-Godina, I.J.; Rodríguez-Domínguez, M.; Rico, M.; Diéguez, F.J. Evaluation of haptoglobin concentration in clinically healthy dairy cows: Correlation between serum and milk levels. J. Appl. Anim. Res. 2024, 52, 2300624. [Google Scholar] [CrossRef]
- Jain, S.; Gautam, V.; Naseem, S. Acute-phase proteins: As diagnostic tool. J. Pharm. Bioallied Sci. 2011, 3, 118–127. [Google Scholar] [CrossRef]
- Naryzny, S.N.; Legina, O.K. Haptoglobin as a Biomarker. Biochem. Mosc. Suppl. B Biomed. Chem. 2021, 15, 184–198. [Google Scholar] [CrossRef]
- Moore, D.P.; Campero, C.M.; Odeón, A.C.; Bardón, J.C.; Silva-Paulo, P.; Paolicchi, F.A.; Cipolla, A.L. Humoral immune response to infectious agents in aborted bovine fetuses in Argentina. Rev. Argent. Microbiol. 2003, 35, 143–148. [Google Scholar]
- Kirkbride, C.A. Examination of bovine and ovine fetuses. Vet. Clin. N. Am. Food Anim. Pract. 1986, 2, 61–83. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, R.; Ferre, I.; Re, M.; Vázquez, P.; Ferrer, L.M.; Blanco-Murcia, J.; Regidor-Cerrillo, J.; Pizarro Díaz, M.; González-Huecas, M.; Tabanera, E.; et al. Safety and efficacy of the bumped kinase inhibitor BKI-1553 in pregnant sheep experimentally infected with Neospora caninum tachyzoites. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Hecker, Y.P.; Burucúa, M.M.; Fiorani, F.; Maldonado Rivera, J.E.; Cirone, K.M.; Dorsch, M.A.; Cheuquepán, F.A.; Campero, L.M.; Cantón, G.J.; Marían, M.S.; et al. Reactivation and foetal infection in pregnant heifers infected with Neospora caninum live tachyzoites at prepubertal age. Vaccines 2022, 10, 1175. [Google Scholar] [CrossRef] [PubMed]
- Buxton, D.; Maley, S.W.; Wright, S.; Thomson, K.M.; Rae, A.G.; Innes, E.A. The pathogenesis of experimental neosporosis in pregnant sheep. J. Comp. Pathol. 1998, 118, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Alton, G.G.; Jones, L.M.; Angus, R.D.; Verger, J.M. Techniques for the brucellosis laboratory. In Techniques for the Brucellosis Laboratory; Institut National de la Recherche Agronomique (INRA), Ed.; CABI: Paris, France, 1988; p. 190. ISBN 978-2-7380-0042-2. [Google Scholar]
- Terzolo, H.R.; Paolicchi, F.A.; Moreira, A.R.; Homse, A. Skirrow agar for simultaneous isolation of Brucella and Campylobacter species. Vet. Rec. 1991, 129, 531–532. [Google Scholar]
- Fiorentino, M.A.; Campos, E.; Cravero, S.; Arese, A.; Paolicchi, F.; Campero, C.; Rossetti, O. Protection levels in vaccinated heifers with experimental vaccines Brucella abortus M1-luc and INTA 2. Vet. Microbiol. 2008, 132, 302–311. [Google Scholar] [CrossRef]
- Saunders, V.F.; Reddacliff, L.A.; Berg, T.; Hornitzky, M. Multiplex PCR for the detection of Brucella ovis, Actinobacillus seminis and Histophilus somni in ram semen. Aust. Vet. J. 2007, 85, 72–77. [Google Scholar] [CrossRef]
- Spetter, M.J.; Louge Uriarte, E.L.; Armendano, J.I.; Álvarez, I.; Norero, N.S.; Storani, L.; Pereyra, S.B.; Verna, A.E.; Odeón, A.C.; González Altamiranda, E.A. Frequency of bovine viral diarrhea virus (BVDV) in Argentinean bovine herds and comparison of diagnostic tests for BVDV detection in bovine serum samples: A preliminary study. Braz. J. Microbiol. 2021, 52, 467–475. [Google Scholar] [CrossRef]
- Odeón, A.C.; Leunda, M.R.; Faverin, C.; Boynak, N.; Vena, M.M.; Zabal, O. In vitro amplification of BVDV field strains isolated in Argentina: Effect of cell line and culture conditions. Rev. Argent. Microbiol. 2009, 41, 79–85. [Google Scholar]
- Altamiranda, E.G.; Kaiser, G.G.; Mucci, N.C.; Verna, A.E.; Campero, C.M.; Odeón, A.C. Effect of bovine viral diarrhea virus on the ovarian functionality and in vitro reproductive performance of persistently infected heifers. Vet. Microbiol. 2013, 165, 326–332. [Google Scholar] [CrossRef]
- Merien, F.; Amouriaux, P.; Perolat, P.; Baranton, G.; Girons, I.S. Polymerase chain reaction for detection of Leptospira spp. in clinical samples. J. Clin. Microbiol. 1992, 30, 2219–2224. [Google Scholar] [CrossRef] [PubMed]
- Campero, C.; Catena, M.; Medina, D. Caldo infusión hígado para cultivo de Tritrichomonas foetus. Vet. Arg. 1986, 3, 80–81. [Google Scholar]
- Jiménez-Pelayo, L.; García-Sánchez, M.; Regidor-Cerrillo, J.; Horcajo, P.; Collantes-Fernández, E.; Gómez-Bautista, M.; Hambruch, N.; Pfarrer, C.; Ortega-Mora, L.M. Immune response profile of caruncular and trophoblast cell lines infected by high- (Nc-Spain7) and low-virulence (Nc-Spain1H) isolates of Neospora caninum. Parasites Vectors 2019, 12, 218. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- McGuire, K.; Manuja, A.; Russell, G.C.; Springbett, A.; Craigmile, S.C.; Nichani, A.K.; Malhotra, D.V.; Glass, E.J. Quantitative analysis of pro-inflammatory cytokine mRNA expression in Theileria annulata-infected cell lines derived from resistant and susceptible cattle. Vet. Immunol. Immunopathol. 2004, 99, 87–98. [Google Scholar] [CrossRef]
- Waldvogel, A.S.; Hediger-Weithaler, B.M.; Eicher, R.; Zakher, A.; Zarlenga, D.S.; Gasbarre, L.C.; Heussler, V.T. Interferon-γ and Interleukin-4 mRNA expression by peripheral blood mononuclear cells from pregnant and non-pregnant cattle seropositive for bovine viral diarrhea virus. Vet. Immunol. Immunopathol. 2000, 77, 201–212. [Google Scholar] [CrossRef]
- Regidor-Cerrillo, J.; Arranz-Solís, D.; Benavides, J.; Gómez-Bautista, M.; Castro-Hermida, J.A.; Mezo, M.; Pérez, V.; Ortega-Mora, L.M.; González-Warleta, M. Neospora caninum infection during early pregnancy in cattle: How the isolate influences infection dynamics, clinical outcome and peripheral and local immune responses. Vet. Res. 2014, 45, 10. [Google Scholar] [CrossRef] [PubMed]
- Konnai, S.; Usui, T.; Ikeda, M.; Kohara, J.; Hirata, T.; Okada, K.; Ohashi, K.; Onuma, M. Tumor necrosis factor-alpha up-regulation in spontaneously proliferating cells derived from bovine leukemia virus-infected cattle. Arch. Virol. 2006, 151, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Refaai, W.; Ducatelle, R.; Geldhof, P.; Mihi, B.; El-shair, M.; Opsomer, G. Digital dermatitis in cattle is associated with an excessive innate immune response triggered by the keratinocytes. BMC Vet. Res. 2013, 9, 193. [Google Scholar] [CrossRef]
- Fleige, S.; Pfaffl, M.W. RNA integrity and the effect on the real-time qRT-PCR performance. Mol. Aspects Med. 2006, 27, 126–139. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, 36. [Google Scholar] [CrossRef]
- Zhou, J.; Tong, J.; Ru, X.; Teng, Y.; Geng, M.; Yan, S.; Tao, F.; Huang, K. Placental inflammatory cytokines mRNA expression and preschool children’s cognitive performance: A birth cohort study in China. BMC Med. 2023, 21, 449. [Google Scholar] [CrossRef]
- Dorsch, M.A.; Moore, D.P.; Regidor-Cerrillo, J.; Scioli, M.V.; Morrell, E.L.; Cantón, G.J.; Ortega-Mora, L.M.; Hecker, Y.P. Morphometric study of encephalic lesions in aborted bovine fetuses naturally infected by two subpopulations of Neospora caninum. Parasitol. Res. 2021, 120, 2995–3000. [Google Scholar] [CrossRef]
- Priyanka; Shringi, B.N.; Choudhary, O.P.; Kashyap, S.K. Cytokines in brucellosis: Biological rhythm at the interface of innate and adaptive immunity. Biol. Rhythm. Res. 2021, 52, 1031–1043. [Google Scholar] [CrossRef]
- Almeria, S.; Serrano-Pérez, B.; Darwich, L.; Mur-Novales, R.; Garcia-Ispierto, I.; Cabezón, O.; López-Gatius, F. Cytokine gene expression in aborting and non-aborting dams and in their foetuses after experimental infection with Neospora caninum at 110 days of gestation. Vet. Parasitol. 2016, 227, 138–142. [Google Scholar] [CrossRef]
- Andrianarivo, A.; Barr, B.; Anderson, M.; Rowe, J.; Packham, A.; Sverlow, K.; Conrad, P. Immune responses in pregnant cattle and bovine fetuses following experimental infection with Neospora caninum. Parasitol. Res. 2001, 87, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Almería, S.; Nogareda, C.; Santolaria, P.; Garcia-Ispierto, I.; Yániz, J.L.; López-Gatius, F. Specific anti-Neospora caninum IgG1 and IgG2 antibody responses during gestation in naturally infected cattle and their relationship with gamma interferon production. Vet. Immunol. Immunopathol. 2009, 130, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Dion, S.; Germon, S.; Guiton, R.; Ducournau, C.; Dimier-Poisson, I. Functional activation of T cells by dendritic cells and macrophages exposed to the intracellular parasite Neospora caninum. Int. J. Parasitol. 2011, 41, 685–695. [Google Scholar] [CrossRef]
- Al-Rahman Riyadh, H.; Abdullah, F.A.; Al-Saad, K.M. Expression of IL1β Gene in the Placentas from Humans and Cows during Brucella Infection. Arch. Razi Inst. 2022, 77, 1575. [Google Scholar] [CrossRef] [PubMed]
- Priyanka Shringi, B.N.; Choudhary, O.P.; Kashyap, S.K. Expression profiling of cytokine-related genes in Brucella abortus infected cattle. Biol. Rhythm. Res. 2021, 52, 654–665. [Google Scholar] [CrossRef]
- Dornand, J.; Gross, A.; Lafont, V.; Liautard, J.; Oliaro, J.; Liautard, J.P. The innate immune response against Brucella in humans. Vet. Microbiol. 2002, 90, 383–394. [Google Scholar] [CrossRef]
- Tsai, A.Y.; Byndloss, M.X.; Seyffert, N.; Winter, M.G.; Young, B.M.; Tsolis, R.M. Tumor Necrosis Factor Alpha Contributes to Inflammatory Pathology in the Placenta during Brucella abortus Infection. Infect. Immun. 2022, 90, e00013-22. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhong, L.; Wang, M.; Wang, M.; Han, Y.; Wang, Y.; Zhou, J.; Song, J.; Li, Y.; Xu, Y. Up-regulated lncRNA CYLD as a ceRNA of miR-2383 facilitates bovine viral diarrhea virus replication by promoting CYLD expression to counteract RIG-I-mediated type-I IFN production. Int. J. Biol. Macromol. 2023, 253, 127351. [Google Scholar] [CrossRef]
- Hansen, T.R.; Smirnova, N.P.; Van Campen, H.; Shoemaker, M.L.; Ptitsyn, A.A.; Bielefeldt-Ohmann, H. Maternal and fetal response to fetal persistent infection with bovine viral diarrhea virus. Am. J. Reprod. Immunol. 2010, 64, 295–306. [Google Scholar] [CrossRef]
- Smirnova, N.P.; Bielefeldt-Ohmann, H.; Van Campen, H.; Austin, K.J.; Han, H.; Montgomery, D.L.; Shoemaker, M.L.; van Olphen, A.L.; Hansen, T.R. Acute non-cytopathic bovine viral diarrhea virus infection induces pronounced type I interferon response in pregnant cows and fetuses. Virus Res. 2008, 132, 49–58. [Google Scholar] [CrossRef]
- Smirnova, N.P.; Webb, B.T.; McGill, J.L.; Schaut, R.G.; Bielefeldt-Ohmann, H.; Van Campen, H.; Sacco, R.E.; Hansen, T.R. Induction of interferon-gamma and downstream pathways during establishment of fetal persistent infection with bovine viral diarrhea virus. Virus Res. 2014, 183, 95–106. [Google Scholar] [CrossRef]
- Alsemgeest, S.P.M.; Kalsbeek, H.C.; Wensing, T.; Koeman, J.P.; Van Ederen, A.M.; Gruys, E. Concentrations of serum Amyloid-a (SAA) and haptoglobin (HP) as parameters of inflammatory diseases in cattle. Vet. Q. 1994, 16, 21–23. [Google Scholar] [CrossRef]
- Ercan, N.; Meral, Ö.; Yokuş, B. Serum Haptoglobin Levels in Complicated Pregnancies in Cows. Inst. Health Sci. J. 2022, 7, 230–233. [Google Scholar] [CrossRef]
- Çenesiz, S.; Şahin, B.; Akpinar, R.K.; Kiliçoğlu, Y. Investigation of some acute phase protein levels in cattle infected with Brucella abortus. Comp. Clin. Path. 2023, 32, 963–970. [Google Scholar] [CrossRef]
- Jawor, P.; Mee, J.F.; Stefaniak, T. Perinatal immuno/inflammatory responses in the presence or absence of bovine fetal infection. BMC Vet. Res. 2018, 14, 322. [Google Scholar] [CrossRef] [PubMed]
- Cañón-Beltrán, K.; Cajas, Y.; Gutierrez-Adán, A.; García-Vázquez, F.; Izquierdo-Rico, M.; Rizos, D. Haptoglobin addition during in vitro culture influences gene expression related to oxidative stress and lipid metabolism in bovine embryos. Reprod. Fertil. Dev. 2024, 37, RDv37n1Ab91. [Google Scholar] [CrossRef]
- Aras, Z.; Yavuz, O. Evaluation of fetal heart serum amyloid a concentrations in infectious cattle abortion cases. Heliyon 2022, 8, e11330. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B. Biomarkers as drug development tools: Discovery, validation, qualification and use. Nat. Rev. Rheumatol. 2018, 14, 354–362. [Google Scholar] [CrossRef]


| mRNA | Primer Sense | Size (pb) | 5′-3′ Sequence | Reference | NM Reference |
|---|---|---|---|---|---|
| GAPDH | F | 112 | TTCTGGCAAAGTGGACATCGT | [40] | NM_001034034.2 |
| R | CTTGACTGTGCCGTTGAACTTG | ||||
| IL-4 | F | 83 | CATGCATGGAGCTGCCTGTA | [41] | NM_173921.2 |
| R | AATTCCAACCCTGCAGAAGGT | ||||
| IFN-γ | F | 110 | GATTCAAATTCCGGTGGATG | [42] | NM_174086.1 |
| R | TTCTCTTCCGCTTTCTGAGG | ||||
| TNFα | F | 176 | AGCCTCAAGTAACAAGCC | [43] | NM_173966.3 |
| R | TGAAGAGGACCTGTGAGT | ||||
| IL-8 | F | 60 | GTTGCTCTCTTGGCAGCTTT | [44] | NM_173925.2 |
| R | GGTGGAAAGGTGTGGAATGT | ||||
| IL-12 | F | 157 | AGTACACAGTGGAGTGTCAG | [45] | NM_174356.1 |
| R | TTCTTGGGTGGGTCTGGTTT |
| Group. | Gestation Stage | Infectious Agent | Number | Hp (ng/L) |
|---|---|---|---|---|
| Intracellular agent | Mid-gestation | N. caninum | 1 | 30.37 |
| N. caninum | 2 | 27.36 | ||
| N. caninum | 3 | 3.83 | ||
| N. caninum | 4 | 36.13 | ||
| B. abortus | 5 | 28.53 | ||
| BVDV | 6 | 24.36 | ||
| Late-gestation | N. caninum | 7 | 29.14 | |
| N. caninum | 8 | 28.53 | ||
| N. caninum | 9 | 30.84 | ||
| N. caninum | 10 | 31.64 | ||
| N. caninum | 11 | 26.69 | ||
| B. abortus | 12 | 30.73 | ||
| BVDV | 13 | 28.98 | ||
| Probable infectious abortion | Late-gestation | - | 14 | 27.74 |
| - | 15 | 31.11 | ||
| - | 16 | 29.19 | ||
| - | 17 | 27.27 | ||
| - | 18 | 27.35 | ||
| Negative control fetuses | Mid-gestation | - | 19 | 44.95 |
| - | 20 | 38.63 | ||
| - | 21 | 34.09 | ||
| Late-gestation | - | 22 | 30.68 | |
| - | 23 | 29.03 | ||
| - | 24 | 29.93 | ||
| - | 25 | 29.31 | ||
| - | 26 | 29.96 | ||
| - | 27 | 29.01 | ||
| - | 28 | 24.73 |
| Haptoglobin Concentration (LGF) | SEM | p-Value | |||
|---|---|---|---|---|---|
| Control | Intracellular | PIE | Group | ||
| Group | 28.9 | 29.5 | 28.5 | 0.714 | 0.64 |
| CI 95% | (27.5–30.4) | (28.1–30.9) | (26.8–30.2) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosa, E.; Pla, N.; Moore, D.P.; García, J.A.; Campero, L.M.; Fiorentino, M.A.; Miqueo, E.; González Altamiranda, E.; Lázaro, F.; Morán, K.; et al. Cytokine Expression and Haptoglobin Levels in Bovine Fetuses Spontaneously Aborted by Intracellular Infectious Agents and by Probable Infectious Etiology. Animals 2025, 15, 2878. https://doi.org/10.3390/ani15192878
Sosa E, Pla N, Moore DP, García JA, Campero LM, Fiorentino MA, Miqueo E, González Altamiranda E, Lázaro F, Morán K, et al. Cytokine Expression and Haptoglobin Levels in Bovine Fetuses Spontaneously Aborted by Intracellular Infectious Agents and by Probable Infectious Etiology. Animals. 2025; 15(19):2878. https://doi.org/10.3390/ani15192878
Chicago/Turabian StyleSosa, Emiliano, Natalia Pla, Dadin Prando Moore, Juan Agustín García, Lucía María Campero, María Andrea Fiorentino, Evangelina Miqueo, Erika González Altamiranda, Fermín Lázaro, Karen Morán, and et al. 2025. "Cytokine Expression and Haptoglobin Levels in Bovine Fetuses Spontaneously Aborted by Intracellular Infectious Agents and by Probable Infectious Etiology" Animals 15, no. 19: 2878. https://doi.org/10.3390/ani15192878
APA StyleSosa, E., Pla, N., Moore, D. P., García, J. A., Campero, L. M., Fiorentino, M. A., Miqueo, E., González Altamiranda, E., Lázaro, F., Morán, K., Bilbao, M. G., Quintana, S., Marín, M. S., & Cantón, G. J. (2025). Cytokine Expression and Haptoglobin Levels in Bovine Fetuses Spontaneously Aborted by Intracellular Infectious Agents and by Probable Infectious Etiology. Animals, 15(19), 2878. https://doi.org/10.3390/ani15192878






