Molecular and Phenotypic Characterization of Prototheca Species Isolates Associated with Bovine Mastitis Cases in Chile †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Origin
2.2. Prototheca spp. Isolation
2.3. Molecular Identification of Prototheca Species
2.3.1. DNA Extraction and Amplification of Prototheca spp. Cytb Partial Gen
2.3.2. Phylogenetic Analysis
2.4. RAPD-PCR Genotyping
2.5. Antibiotic Susceptibility
2.6. Biofilm Formation
2.7. Statistical Analysis
3. Results
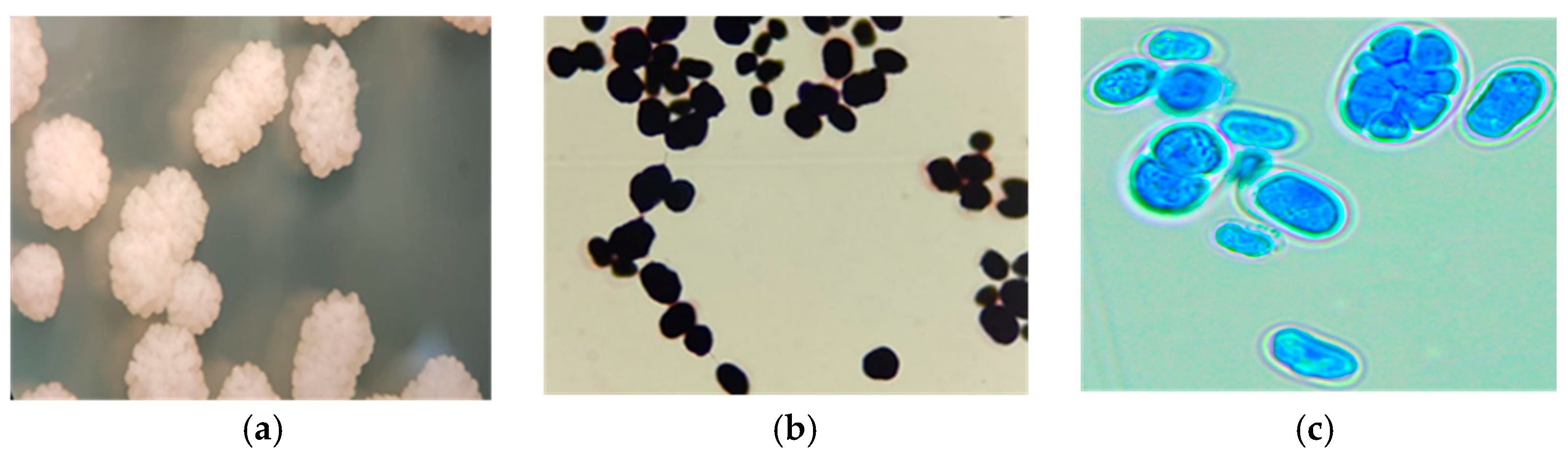
3.1. Prototheca Isolation
3.2. Molecular Identification of Prototheca Species
3.2.1. DNA Extraction and Amplification of Prototheca spp. Cytb Partial Gene
3.2.2. Phylogenetic Analysis
3.3. RAPD-PCR Genotyping
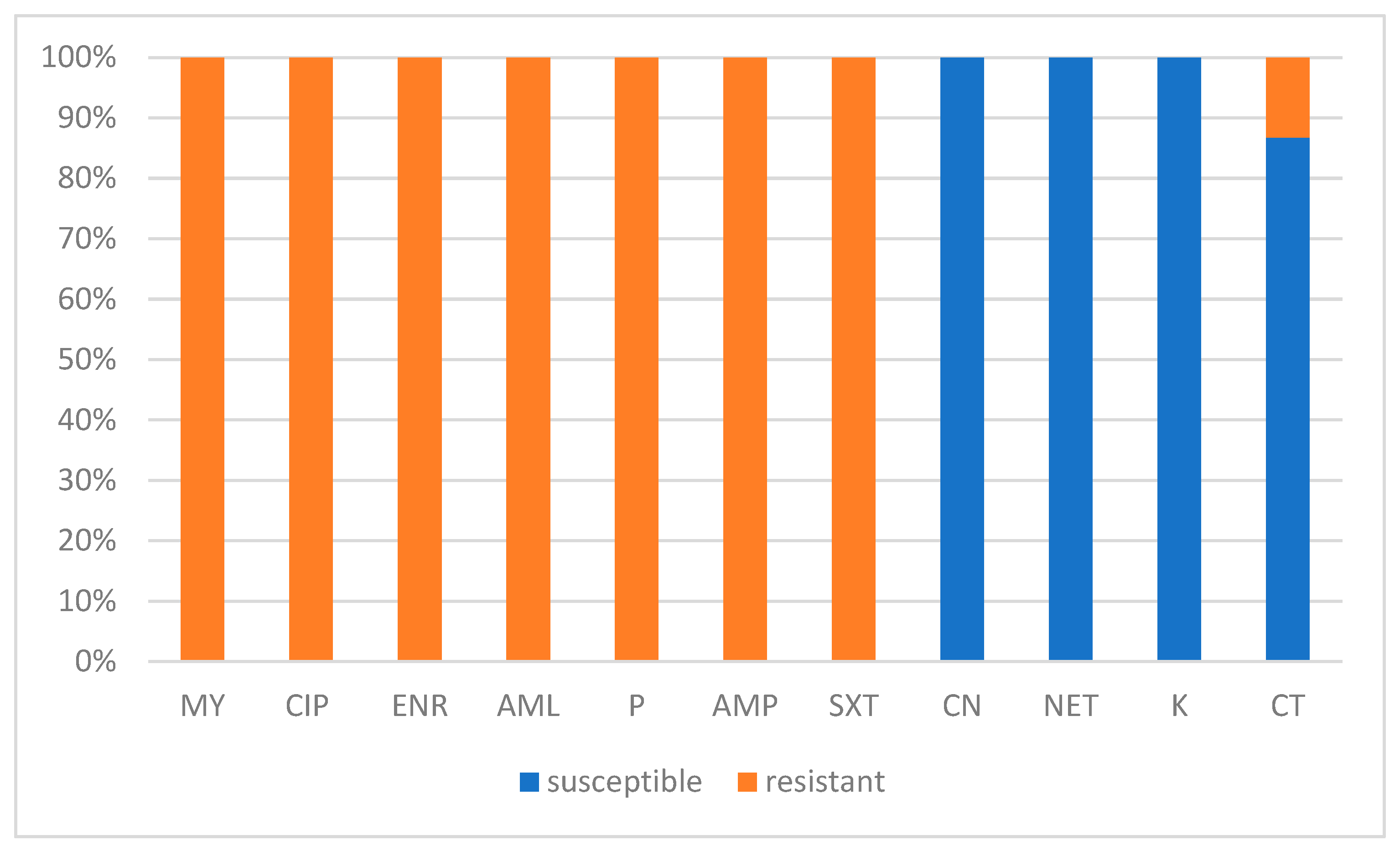
3.4. Antibiotic Susceptibility
3.5. Biofilm Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DNA | Deoxyribonucleic acid |
| DNAr | Ribosomal DNA |
| BLASTn | Basic Local Alignment Nucleotide Search tool |
| CFU | Colony Forming Units |
| cytb | Cytochrome b gene |
| PCR | Polymerase Chain Reaction |
| RAPD-PCR | Random Amplified Polymorphic DNA–Polymerase Chain Reaction |
| SAG | Culture Collection of Algae |
| MY | Lincomycin |
| CIP | Ciprofloxacin |
| ENR | Enrofloxacin |
| AML | Amoxicillin |
| P | Penicillin |
| AMP | Ampicillin |
| SXT | Sulfamethoxazole/Trimethoprim |
| CT | Colistin Sulfate |
| NET | Netilmicin |
| K | Kanamycin |
| CN | Gentamicin |
| ML | Maximum Likelihood Method for phylogenetic inference |
| BY | Bayesian Inference for phylogenetic inference |
| UPGMA | Unweighted Pair Group Method with Arithmetic Mean |
| SDA | Sabouraud Dextrose Agar |
| SDB | Sabouraud Dextrose Broth |
| PIM | Prototheca Isolation Medium |
| NMC | National Mastitis Council |
| CLSI | Clinical and Laboratory Standards Institute |
Appendix A
| Isolate | Species | Farm | MY | CIP | ENR | AML | P | AMP | SXT | CT | NET | K | CN | Biofilm | RAPD-PCR Genotype | Mastitis Type | Sample Type |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 ORO | P. bovis | A | R | R | R | R | R | R | R | S | S | S | S | W | 14 | SCM | Individual milk |
| 2 ORO | P. bovis | A | R | R | R | R | R | R | R | S | S | S | S | W | 1 | SCM | Individual milk |
| 6 ORO | P. bovis | A | R | R | R | R | R | R | R | S | S | S | S | W | 15 | SCM | Individual milk |
| 3 LP | P. ciferrii | B | R | R | R | R | R | R | R | S | S | S | S | W | SCM | Individual milk | |
| 4 QUI | P. bovis | C | R | R | R | R | R | R | R | R | S | S | S | W | 2 | MC | Individual milk |
| 5 QUI | P. bovis | C | R | R | R | R | R | R | R | R | S | S | S | W | 1 | MC | Individual milk |
| 11 QUI | P. bovis | C | R | R | R | R | R | R | R | S | S | S | S | W | 2 | CM | Individual milk |
| 12 QUI | P. bovis | C | R | R | R | R | R | R | R | R | S | S | S | W | 2 | SCM | Individual milk |
| 13 QUI | P. bovis | C | R | R | R | R | R | R | R | S | S | S | S | N | 3 | CM | Individual milk |
| 14 QUI | P. bovis | C | R | R | R | R | R | R | R | S | S | S | S | W | 5 | CM | Individual milk |
| 16 QUI | P. bovis | C | R | R | R | R | R | R | R | R | S | S | S | W | 4 | CM | Individual milk |
| 7 SG | P. bovis | D | R | R | R | R | R | R | R | S | S | S | S | W | 3 | CM | Individual milk |
| 8 CAF | P. bovis | E | R | R | R | R | R | R | R | S | S | S | S | W | 2 | - | Individual milk |
| 9 CAF | P. bovis | E | R | R | R | R | R | R | R | S | S | S | S | W | 1 | - | Individual milk |
| 10 COL | P. bovis | F | R | R | R | R | R | R | R | S | S | S | S | W | 11 | CM | Individual milk |
| 15 LL | P. bovis | G | R | R | R | R | R | R | R | R | S | S | S | W | 9 | CM | Individual milk |
| 6 AN | P. bovis | H | R | R | R | R | R | R | R | S | S | S | S | W | 3 | - | Bulk tank milk |
| 7 AN | P. bovis | H | R | R | R | R | R | R | R | S | S | S | S | W | 3 | - | Bulk tank milk |
| 8AN | P. bovis | H | R | R | R | R | R | R | R | S | S | S | S | W | 1 | - | Bulk tank milk |
| 17 AN | P. bovis | H | R | R | R | R | R | R | R | S | S | S | S | W | 7 | SCM | Individual milk |
| 18 AN | P. bovis | H | R | R | R | R | R | R | R | S | S | S | S | W | 7 | CM | Individual milk |
| 19 AN | P. bovis | H | R | R | R | R | R | R | R | S | S | S | S | W | 2 | CM | Individual milk |
| 20 AN | P. bovis | H | R | R | R | R | R | R | R | S | S | S | S | W | 8 | SCM | Individual milk |
| 21 AN | P. bovis | H | R | R | R | R | R | R | R | S | S | S | S | W | 2 | CM | Individual milk |
| 22 AN | P. bovis | H | R | R | R | R | R | R | R | S | S | S | S | W | 5 | CM | Individual milk |
| 23 AN | P. bovis | H | R | R | R | R | R | R | R | S | S | S | S | W | 3 | CM | Individual milk |
| 1LV | P. bovis | I | R | R | R | R | R | R | R | S | S | S | S | W | 1 | - | Bulk tank milk |
| 2LV | P. bovis | I | R | R | R | R | R | R | R | S | S | S | S | W | 3 | - | Bulk tank milk |
| 3LV | P. bovis | I | R | R | R | R | R | R | R | S | S | S | S | W | 2 | - | Bulk tank milk |
| 4 SS | P. bovis | J | R | R | R | R | R | R | R | S | S | S | S | W | 2 | - | Bulk tank milk |
| 5 SS | P. bovis | J | R | R | R | R | R | R | R | S | S | S | S | N | 6 | - | Bulk tank milk |
| 9 SS | P. bovis | J | R | R | R | R | R | R | R | S | S | S | S | W | 1 | - | Bulk tank milk |
| 10 SS | P. bovis | J | R | R | R | R | R | R | R | S | S | S | S | W | 2 | - | Bulk tank milk |
| 11 SS | P. bovis | J | R | R | R | R | R | R | R | S | S | S | S | W | 5 | - | Bulk tank milk |
| 12 PA | P. bovis | K | R | R | R | R | R | R | R | R | S | S | S | W | 1 | - | Bulk tank milk |
| 13 PA | P. bovis | K | R | R | R | R | R | R | R | R | S | S | S | W | 1 | - | Bulk tank milk |
| 14 | P. bovis | L | R | R | R | R | R | R | R | S | S | S | S | W | 5 | - | Bulk tank milk |
| 15 CM | P. bovis | M | R | R | R | R | R | R | R | S | S | S | S | W | 2 | - | Bulk tank milk |
| 16 R | P. bovis | N | R | R | R | R | R | R | R | R | S | S | S | W | 1 | - | Bulk tank milk |
| P4 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | M | 3 | CM | Individual milk |
| P12 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 1 | CM | Individual milk |
| P15 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 3 | CM | Individual milk |
| P18 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 1 | CM | Individual milk |
| P19 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 2 | CM | Individual milk |
| P21 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 1 | CM | Individual milk |
| P22 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | M | 2 | CM | Individual milk |
| P23 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 1 | CM | Individual milk |
| P25 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 15 | CM | Individual milk |
| P26 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | M | 2 | CM | Individual milk |
| P30 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 1 | CM | Individual milk |
| P31 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | M | 13 | CM | Individual milk |
| P33 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 1 | CM | Individual milk |
| P35 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 2 | CM | Individual milk |
| P38 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | M | 16 | CM | Individual milk |
| P39 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 2 | CM | Individual milk |
| P40 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 1 | CM | Individual milk |
| P42 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | M | 2 | CM | Individual milk |
| P43 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 1 | CM | Individual milk |
| P45 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 2 | CM | Individual milk |
| P47 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 10 | CM | Individual milk |
| P49 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | M | 18 | CM | Individual milk |
| P53 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 17 | CM | Individual milk |
| P54 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 16 | CM | Individual milk |
| P55 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | M | 13 | CM | Individual milk |
| P58 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | M | 12 | CM | Individual milk |
| P59 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | M | 13 | CM | Individual milk |
| Accession Number | Isolated | Distance Matrix 100% |
|---|---|---|
| PV768537 | 1LV | 1ORO; 2LV; 3LV; 4SS; 5SS; 6AN; 6ORO; 7AN; 8AN; 8CAF; 9CAF; 9SS; 10COL; 10SS; 11QUI; 11SS; 13PA; 14BTM; 15CM; 15LL; 16R; 18AN; 20AN; 23AN; P4; P18; P19; P21; P22; P23; P25; P26; P30; P31; P35; P38; P39; P40; P42; P43; P45; P47; P49; P53; P54; P55; P58; P59; 7SG; 12PA; 13QUI; 17AN; 19AN; 22AN |
| PV768539 | 5QUI | 4QUI; 12QUI; 14QUI; 16ORO |
| PV768538 | P33 | |
| PV768540 | 21AN | 2ORO |
| PV768541 | P12 | |
| PV768542 | P15 | |
| PV768543 | 3LP |
References
- Cheng, W.N.; Han, S.G. Bovine Mastitis: Risk Factors, Therapeutic Strategies, and Alternative Treatments—A Review. Asian-Australas. J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef]
- Gomes, F.; Henriques, M. Control of Bovine Mastitis: Old and Recent Therapeutic Approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef]
- Sharma, N.; Rho, G.J.; Hong, Y.H.; Kang, T.Y.; Lee, H.K.; Hur, T.-Y.; Jeong, D.K. Bovine Mastitis: An Asian Perspective. Asian J. Anim. Vet. Adv. 2012, 7, 454–476. [Google Scholar] [CrossRef]
- Libisch, B.; Picot, C.; Ceballos-garzon, A.; Moravkova, M.; Klimesová, M.; Telkes, G.; Chuang, S.T.; Pape, P.L. Prototheca Infections and Ecology from a One Health Perspective. Microorganisms 2022, 10, 938. [Google Scholar] [CrossRef]
- Pieper, L.; Godkin, A.; Roesler, U.; Polleichtner, A.; Slavic, D.; Leslie, K.E.; Kelton, D.F. Herd Characteristics and Cow-Level Factors Associated with Prototheca Mastitis on Dairy Farms in Ontario, Canada. J. Dairy Sci. 2012, 95, 5635–5644. [Google Scholar] [CrossRef]
- Buzzini, P.; Turchetti, B.; Facelli, R.; Baudino, R.; Cavarero, F.; Mattalia, L.; Mosso, P.; Martini, A. First Large-Scale Isolation of Prototheca zopfii from Milk Produced by Dairy Herds in Italy. Mycopathologia 2004, 158, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Jagielski, T.; Roeske, K.; Bakuła, Z.; Piech, T.; Wlazło, Ł.; Bochniarz, M.; Woch, P.; Krukowski, H. A Survey on the Incidence of Protothecal Mastitis in Dairy Herds in Lublin Province, Poland. J. Dairy Sci. 2019, 102, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Bozzo, G.; Bonerba, E.; Pinto, A.D.; Bolzoni, G.; Ceci, E.; Mottola, A.; Tantillo, G.; Terio, V. Occurrence of Prototheca spp. in Cow Milk Samples. New Microbiol. 2014, 37, 459–464. [Google Scholar] [PubMed]
- Melville, P.A.; Watanabe, E.T.; Benites, N.R.; Ribeiro, A.R.; Garino, F., Jr.; Costa, E.O. Evaluation of the Susceptibility of Prototheca zopfii to Milk Pasteurization. Mycopathologia 1999, 146, 79–82. [Google Scholar] [CrossRef]
- Jagielski, T. The Genus Prototheca (Trebouxiophyceae, Chlorophyta) Revisited_ Implications from Molecular Taxonomic Studies. Algal Res. 2019, 43, 101639. [Google Scholar] [CrossRef]
- Jagielski, T.; Gawor, J.; Bakuła, Z.; Decewicz, P.; Maciszewski, K.; Karnkowska, A. Cytb as a New Genetic Marker for Differentiation of Prototheca Species. J. Clin. Microbiol. 2018, 56, e00584-18. [Google Scholar] [CrossRef]
- Masuda, M.; Jagielski, T.; Danesi, P.; Falcaro, C.; Bertola, M.; Krockenberger, M.; Malik, R.; Kano, R. Protothecosis in Dogs and Cats—New Research Directions. Mycopathologia 2021, 186, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Huilca-Ibarra, M.P.; Vasco-Julio, D.; Ledesma, Y.; Guerrero-Freire, S.; Zurita, J.; Castillejo, P.; Blasco, F.B.; Yanez, L.; Changoluisa, D.; Echeverría, G.; et al. High Prevalence of Prototheca bovis Infection in Dairy Cattle with Chronic Mastitis in Ecuador. Vet. Sci. 2022, 9, 659. [Google Scholar] [CrossRef] [PubMed]
- Tashakkori, N.; Rahmani, H.K.; Khoramian, B. Genotypic and Phenotypic Diversity of Prototheca spp. Recovered from Bovine Mastitis in Terms of Antimicrobial Resistance and Biofilm Formation Ability. BMC Vet. Res. 2022, 18, 452. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.; Silva, E.; Kraft, C.; Carvalheira, J.; Videira, A.; Huss, V.A.R.; Thompson, G. Bovine Mastitis Associated with Prototheca blaschkeae. J. Clin. Microbiol. 2008, 46, 1941–1945. [Google Scholar] [CrossRef]
- Capra, E.; Cremonesi, P.; Cortimiglia, C.; Bignoli, G.; Ricchi, M.; Moroni, P.; Pesce, A.; Luini, M.; Castiglioni, B. Simultaneous Identification by Multiplex PCR of Major Prototheca spp. Isolated from Bovine and Buffalo Intramammary Infection and Bulk Tank. Lett. Appl. Microbiol. 2014, 59, 642–647. [Google Scholar] [CrossRef]
- Marques, S.; Silva, E.; Carvalheira, J.; Thompson, G. Short Communication: In Vitro Antimicrobial Susceptibility of Prototheca Wickerhamii and Prototheca Zopfii Isolated from Bovine Mastitis. J. Dairy Sci. 2006, 89, 4202–4204. [Google Scholar] [CrossRef]
- Ito, T.; Kano, R.; Sobukawa, H.; Ogawa, J.; Honda, Y.; Hosoi, Y.; Shibuya, H.; Sato, T.; Hasegawa, A.; Kamata, H. Experimental Infection of Bovine Mammary Gland with Prototheca zopfii Genotype 1. J. Vet. Med. Sci. 2011, 73, 117–119. [Google Scholar] [CrossRef]
- Lerche, M. Eine Durch Algen (Prototheca) Hervorgerufene Mastitis Der Kuh. Berl. Münchener Tierärztliche Wochenschr. 1952, 65, 64–69. [Google Scholar]
- Costa, E.O.; Carciofi, A.C.; Melville, P.A.; Prada, M.S.; Schalch, U. Prototheca sp. Outbreak of Bovine Mastitis. J. Vet. Med. B 1996, 43, 321–324. [Google Scholar] [CrossRef]
- Tomanić, D.; Božić, D.D.; Kladar, N.; Samardžija, M.; Apić, J.; Baljak, J.; Kovačević, Z. Clinical Evidence on Expansion of Essential Oil-Based Formulation’s Pharmacological Activity in Bovine Mastitis Treatment: Antifungal Potential as Added Value. Antibiotics 2024, 13, 575. [Google Scholar] [CrossRef]
- Kuczyńska, M.; Kot, M.; Stocki, M.; Zapora, E.; Jagielski, T.; Perlińska-Teresiak, M.; Kalińska, A. In Vitro Determination of Cytotoxic Effects of Ten Essential Oils on Prototheca bovis, Which Causes Mastitis in Dairy Cows. Int. J. Mol. Sci. 2025, 26, 5451. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J. Biofilm Formation by Pathogenic Prototheca Algae. Lett. Appl. Microbiol. 2015, 61, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Morandi, S.; Cremonesi, P.; Capra, E.; Silvetti, T.; Decimo, M.; Bianchini, V.; Alves, A.C.; Vargas, A.C.; Costa, G.M.; Ribeiro, M.G.; et al. Molecular Typing and Differences in Biofilm Formation and Antibiotic Susceptibilities among Prototheca Strains Isolated in Italy and Brazil. J. Dairy Sci. 2016, 99, 6436–6445. [Google Scholar] [CrossRef]
- Shahid, M.; Ali, T.; Zhang, L.; Hou, R.; Zhang, S.; Ding, L.; Han, D.; Deng, Z.; Rahman, A.; Han, B. Characterization of Prototheca zopfii Genotypes Isolated from Cases of Bovine Mastitis and Cow Barns in China. Mycopathologia 2016, 181, 185–195. [Google Scholar] [CrossRef]
- Zaror, L.; Valenzuela, K.; Kruze, J. Bovine Mastitis Caused by Prototheca zopfii: First Isolation in Chile. Arch. Med. Vet. 2011, 176, 173–176. [Google Scholar] [CrossRef]
- Vásquez, M. Identificación y Caracterización del Alga Prototheca spp. Aisladas en Un Brote de Mastitis Bovina; Universidad Austral de Chile: Valdivia, Chile, 2011. [Google Scholar]
- National Mastitis Council. Laboratory Handbook on Bovine Mastitis, 3rd ed.; National Mastitis Council: New Prague, MN, USA, 2017. [Google Scholar]
- Jagielski, T.; Gawor, J.; Bakuła, Z.; Zuchniewicz, K.; Żak, I.; Gromadka, R. An Optimized Method for High Quality DNA Extraction from Microalga Prototheca wickerhamii for Genome Sequencing. Plant Methods 2017, 13, 77. [Google Scholar] [CrossRef]
- Available online: http://www.geneious.com (accessed on 6 June 2025).
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Katoh, K. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Criscuolo, A.; Gribaldo, S. BMGE (Block Mapping and Gathering with Entropy): A New Software for Selection of Phylogenetic Informative Regions from Multiple Sequence Alignments. BMC Ecol. Evol. 2010, 10, 210. [Google Scholar] [CrossRef]
- Benson, D.A. GenBank: Update. Nucleic Acids Res. 2004, 32, 23D–26D. [Google Scholar] [CrossRef]
- Schwarz, G. Estimatibg the Dimension of a Model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Chernomor, O.; Von Haeseler, A.; Minh, B.Q. Terrace Aware Data Structure for Phylogenomic Inference from Supermatrices. Syst. Biol. 2016, 65, 997–1008. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Haeseler, A.V.; Jermiin, L.S. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Haeseler, A.V.; Lanfear, R.; Teeling, E. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; Haeseler, A.V.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P. Bayesian Phylogenetic Model Selection Using Reversible Jump Markov Chain Monte Carlo. Mol. Biol. Evol. 2004, 21, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. CLSI Supplement M100, 33rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023. [Google Scholar]
- Osumi, T.; Kishimoto, Y.; Kano, R.; Maruyama, H.; Onozaki, M.; Makimura, K.; Ito, T.; Matsubara, K.; Hasegawa, A. Prototheca zopfii Genotypes Isolated from Cow Barns and Bovine Mastitis in Japan. Vet. Microbiol. 2008, 131, 419–423. [Google Scholar] [CrossRef]
- Möller, A.; Truyen, U.; Roesler, U. Prototheca zopfii Genotype 2-The Causative Agent of Bovine Protothecal Mastitis? Vet. Microbiol. 2007, 120, 370–374. [Google Scholar] [CrossRef]
- Hirose, N.; Hua, Z.; Kato, Y.; Zhang, Q.; Li, R.; Nishimura, K.; Masuda, M. Molecular Characterization of Prototheca Strains Isolated in China Revealed the First Cases of Protothecosis Associated with Prototheca zopfii Genotype 1. Med. Mycol. 2018, 56, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Falcaro, C.; Furlanello, T.; Binanti, D.; Fondati, A.; Bonfanti, U.; Krockenberger, M.; Malik, R.; Danesi, P. Molecular Characterization of Prototheca in 11 Symptomatic Dogs. J. Vet. Diagn. Investig. 2021, 33, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Jagielski, T.; Proskurnicka, A.; Iskra, M.; Wronka, S.; Bakuła, Z.; Danesi, P.; De Farias, M.R.; Ramos Portilho, F.V.; Garcia Ribeiro, M.; Rösler, U.; et al. Protothecosis in Dogs: A Narrative Review. Vet. Intern. Med. 2025, 39, e70025. [Google Scholar] [CrossRef]
- Idil, N.; Bilkay, I.S. Application of RAPD-PCR for Determining the Clonality of Methicillin Resistant Staphylococcus aureus Isolated from Different Hospitals. Braz. Arch. Biol. Technol. 2014, 57, 548–553. [Google Scholar] [CrossRef]
- Lopes, M.M.; Ribeiro, R.; Carvalho, D.; Freitas, G. In Vitro Antimicrobial Susceptibility of Prototheca spp. Isolated from Bovine Mastitis in a Portugal Dairy Herd. J. Mycol. Médicale 2008, 18, 205–209. [Google Scholar] [CrossRef]
- Wawron, W.; Bochniarz, M.; Piech, T.; Wysocki, J.; Kocik, M. Antimicrobial Susceptibility of Prototheca zopfii Isolated from Bovine Mastitis. Bull. Vet. Inst. Pulawy 2013, 57, 485–488. [Google Scholar] [CrossRef][Green Version]
- Hooper, D.C. Mechanisms of Action of Antimicrobials: Focus on Fluoroquinolones. Clin. Infect. Dis. 2001, 32, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Masters, P.A.; O’Bryan, T.A.; Zurlo, J.; Miller, D.Q.; Joshi, N. Trimethoprim-Sulfamethoxazole Revisited. Arch. Intern. Med. 2003, 163, 402. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, H.; He, J.; He, Y.; Li, S.; Hou, R.; Wu, Q.; Gao, Y.; Han, B. Characterization of Prototheca zopfii Associated with Outbreak of Bovine Clinical Mastitis in Herd of Beijing, China. Mycopathologia 2012, 173, 275–281. [Google Scholar] [CrossRef]
- Chang, R.; Yang, Q.; Liu, G.; Liu, Y.; Zheng, B.; Su, J.; Han, B. Treatment with Gentamicin on a Murine Model of Protothecal Mastitis. Mycopathologia 2013, 175, 241–248. [Google Scholar] [CrossRef]
- Gonçalves, L.J.; Lee, S.H.I.; De Paula Arruda, E.; Galles, D.P.; Caetano, V.C.; De Oliveira, C.A.F.; Fernandes, A.M.; Dos Santos, M.V. Biofilm-Producing Ability and Efficiency of Sanitizing Agents against Prototheca zopfii Isolates from Bovine Subclinical Mastitis. J. Dairy Sci. 2015, 98, 3613–3621. [Google Scholar] [CrossRef] [PubMed]
- Jagielski, T.; Lassa, H.; Ahrholdt, J.; Malinowski, E.; Roesler, U. Genotyping of Bovine Prototheca Mastitis Isolates from Poland. Vet. Microbiol. 2011, 149, 283–287. [Google Scholar] [CrossRef] [PubMed]


| Species | Isolate Type | Biofilm Production | Number of Isolates (%) | OD 490 Range * |
|---|---|---|---|---|
| P. bovis | Field (n = 65) | Moderate | 10 (15.4%) | 0.176–0.293 |
| Weak | 53 (81.5%) | 0.070–0.149 | ||
| Non-producer | 2 (3.1%) | <0.078 | ||
| Reference ** | Weak | 1 (100%) | 0.096 | |
| P. ciferrii | Field (n = 1) | Weak | 1 (100%) | 0.081 |
| Reference *** | Non-producer | 1 (100%) | 0.075 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, J.; Sepúlveda-García, P.; Canales, N.; Goddard, M.; Cornuy, C.; Morales, Á.G.; Collado, L.; Mella, A. Molecular and Phenotypic Characterization of Prototheca Species Isolates Associated with Bovine Mastitis Cases in Chile. Animals 2025, 15, 2869. https://doi.org/10.3390/ani15192869
Rodriguez J, Sepúlveda-García P, Canales N, Goddard M, Cornuy C, Morales ÁG, Collado L, Mella A. Molecular and Phenotypic Characterization of Prototheca Species Isolates Associated with Bovine Mastitis Cases in Chile. Animals. 2025; 15(19):2869. https://doi.org/10.3390/ani15192869
Chicago/Turabian StyleRodriguez, Jaime, Paulina Sepúlveda-García, Nivia Canales, Matías Goddard, Carlo Cornuy, Álvaro G. Morales, Luis Collado, and Armin Mella. 2025. "Molecular and Phenotypic Characterization of Prototheca Species Isolates Associated with Bovine Mastitis Cases in Chile" Animals 15, no. 19: 2869. https://doi.org/10.3390/ani15192869
APA StyleRodriguez, J., Sepúlveda-García, P., Canales, N., Goddard, M., Cornuy, C., Morales, Á. G., Collado, L., & Mella, A. (2025). Molecular and Phenotypic Characterization of Prototheca Species Isolates Associated with Bovine Mastitis Cases in Chile. Animals, 15(19), 2869. https://doi.org/10.3390/ani15192869






