Simple Summary
Bovine mastitis is a significant disease impacting dairy cows worldwide, primarily due to the substantial economic losses it causes. Among the pathogens involved, Prototheca spp. has gained increasing attention due to the rising number of protothecal mastitis cases and the associated treatment challenges, often resulting in the culling of affected animals. In Chile, only two outdated studies from 2011 have reported protothecal infections in cattle, underscoring the need for updated data to understand the epidemiology of this infection. This study aimed to characterize Prototheca isolates from Chilean dairy cows through molecular identification at the species level and assessment of their genetic diversity, antimicrobial susceptibility, and biofilm formation capacity. A total of 66 Prototheca spp. isolates were analyzed, with 65 identified as P. bovis and one as P. ciferrii. Genetic analysis revealed 18 distinct P. bovis genotypes without clustering by farm origin, indicating high genetic heterogeneity. All isolates exhibited resistance to most antibiotics tested except aminoglycosides. Additionally, the isolates demonstrated weak to moderate biofilm production, which may contribute to the persistence of infections. This work represents the first comprehensive study of Prototheca in Chile and the third worldwide to assess P. bovis genetic diversity, providing valuable insights for the development of alternative therapeutic strategies.
Abstract
Background: Bovine mastitis caused by Prototheca spp. is the most significant animal disease of algal origin, with an increasing number of cases reported worldwide. Currently, there is no effective treatment, so control requires the culling of infected animals. In Chile, information is limited, and a discrepancy remains in the literature regarding the Prototheca species involved in bovine mastitis. Methods: This study aimed to molecularly type and phenotypically characterize Prototheca isolates associated with bovine mastitis in Chile. Sixty-six Prototheca isolates obtained from individual bovine mastitis milk samples and bulk tank milk samples were analyzed through cytochrome b gene (cytb) sequencing, Random Amplified Polymorphic DNA–Polymerase Chain Reaction (RAPD-PCR) analysis, and phenotypic evaluation (morphology, antimicrobial susceptibility, and biofilm formation). Results: Sixty-five isolates were identified as P. bovis and one as P. ciferrii, marking the first report of the latter in bovine mastitis in Chile. RAPD analysis revealed a high genetic diversity in P. bovis. All strains exhibited resistance to the antibiotics tested from the Fluoroquinolone, β-lactam, and sulfonamide groups; however, 100% of the strains showed susceptibility to aminoglycosides, with gentamicin standing out as a potential therapeutic option. Most P. bovis strains formed weak (81.5%, 53/65) or moderate (15.4%, 10/65) biofilms, which could favor the persistence of infection. Conclusions: These findings provide novel insights into the molecular and phenotypic characteristics of Prototheca spp. in Chile, highlighting the predominance of P. bovis, the emergence of P. ciferri, and the implications for antimicrobial management and disease control.
1. Introduction
Bovine mastitis is one of the most prevalent and challenging diseases worldwide, causing substantial economic losses due to reduced milk yield and poor quality [1,2]. Unlike many other animal diseases, it can be caused by a wide range of microorganisms, including bacteria, viruses, fungi, and unicellular algae [3]; among them, the genus Prototheca has gained increasing importance, being ranked among the major etiological agents of bovine mastitis in countries such as Canada, Italy, and Poland [4,5,6,7]. However, globally, protothecal mastitis remains relatively uncommon [8,9].
The genus Prototheca (family Chlorellaceae, order Chlorellales) comprises achlorophyllous microalgae that evolved from the green alga Chlorella through the loss of photosynthetic capacity, leading to their adaptation to heterotrophic conditions, which enables them to cause opportunistic infections collectively referred to as Protothecosis in humans and other animals [4]. Recent taxonomic revisions, based on the analysis of the mitochondrial cytochrome b gene (cytb), have led to a reclassification of the species of the genus Prototheca, providing higher resolution compared to conventional ribosomal DNA (rDNA) markers [10,11]. Consequently, 18 Prototheca species have been recognized, among which P. ciferrii (formerly P. zopfii genotype 1), P. bovis (formerly P. zopfii genotype 2), P. blaschkeae, P. cutis, P. miyajii, and P. wickerhamii have been identified as etiological agents of diseases in humans and animals [4,12]. Protothecal infection in humans generally triggers three main clinical manifestations: cutaneous lesions, olecranon bursitis, or systemic infections, with P. wickerhamii associated with cutaneous cases, while P. bovis is principally involved in systemic protothecal infection [4].
P. bovis is undoubtedly recognized as the principal agent involved in bovine protothecal mastitis [13,14], and the presence of P. blaschkeae is occasionally reported [15]. In contrast, the role of P. wickerhamii [16,17] and P. ciferrii [8] in bovine protothecal mastitis remains unclear [8,18]. Since the first detection of Prototheca infection in cattle by Lerche (1952) [19], these pathogens have been reported as emerging pathogens across all continents, causing acute or chronic bovine mastitis with visibly abnormal milk, swelling of the mammary gland, and reduced milk yield, resulting in economic losses [5,7,20]. They may also represent a serious problem due to the inherent resistance of these microalgae to different classes of antimicrobial drugs [14]. Currently, there are no established treatment guidelines for protothecal infections in humans and animals. Due to the similarity in the cell membrane composition of Prototheca and yeast (the presence of ergosterol), the treatment of human protothecosis typically includes antifungal agents in combination with surgical approaches [4]. Nevertheless, the administration of these agents in dairy cattle is considered impractical, primarily due to their high economic cost and toxicity to mammary gland tissue [13,21,22].
It is noteworthy that there is no effective treatment for mastitis caused by Prototheca spp.; thus, culling infected cows is recommended to prevent the spread of infection to other cows and to avoid unnecessary antibiotic use [23].
Additionally, some authors have noticed the ability of Prototheca to produce biofilm, suggesting that it enhances pathogenicity and contributes to antimicrobial resistance and a reduced effectiveness of the immune response [23]. Although most antimicrobials exhibit limited efficacy, some antibiotics and antifungals have demonstrated variable in vitro activity [14,17,24,25].
Current knowledge about the genetic polymorphism of Prototheca strains is limited to two studies using Random Amplified Polymorphic DNA (RAPD)-PCR, which have shown significant heterogeneity among the Prototheca isolates and highlighted the potential of RAPD-PCR for identifying P. bovis strains at the genetic level [14,24].
Despite the growing global importance of protothecal bovine mastitis [8,9], available information remains limited in many countries. Chile is not an exception, as there are only two dated studies from 2011 that reported the first identification of P. zopfii associated with bovine mastitis in the country [26], and the genotyping and characterization of the antimicrobial and antifungal susceptibility of Prototheca isolates during a bovine mastitis outbreak, revealing that P. zopfii genotype 2 was involved in 46.6% (26/58) of the affected animals [27]. Although both studies demonstrated the presence and potential significance of this microalga in cases of bovine mastitis, no subsequent studies have been conducted in Chile. Updated research is necessary to revise the existing information based on the new taxonomic nomenclature, as well as to incorporate advanced techniques for species-level identification and genotyping. This would enable a more accurate identification of the Prototheca species and variants currently affecting dairy cattle in Chile, recognizing that the precise identification of Prototheca species is crucial, as they differ in their pathogenicity and antimicrobial sensitivity patterns [17,24]. This study aims to perform molecular typing and phenotypic characterization of Prototheca isolates associated with bovine mastitis cases in Chile. The approach involves species identification, analysis of genetic diversity using RAPD-PCR, and phenotypic characterization, which encompasses morphological analysis, antimicrobial sensitivity testing, and assessment of biofilm formation capacity. This comprehensive approach aims to provide relevant information on the epidemiology of protothecal mastitis in Chilean dairy herds and contribute to the design of more effective control strategies.
2. Materials and Methods
2.1. Sample Origin
A convenience sampling study was performed for the analysis of milk samples collected from animals with clinical or subclinical bovine mastitis that arrived at the Bovine Mastitis Laboratory of the Universidad Austral de Chile (UACh), Valdivia, Chile, between January 2023 and October 2024. A total of 6442 individual or composite milk samples from 8 dairy farms in Southern Chile (farms A, B, C, D, E, F, G, H) and 104 bulk tank milk samples from 7 farms in Central and Southern Chile were analyzed (farms H, I, J, K, L, M, N). Additionally, 58 milk samples from cows affected by an outbreak of clinical mastitis refractory to treatment that occurred in January 2010 at a dairy farm in the metropolitan region of Central Chile (farm O) were included in this study.
2.2. Prototheca spp. Isolation
For Prototheca spp. isolation, 0.1 mL of milk samples was plated on 5% sheep blood agar, while samples from bulk tanks were plated on Sabouraud dextrose agar supplemented with chloramphenicol (100 mg/mL). The plates were incubated aerobically at 37 °C for 48–72 h, following the National Mastitis Council (NMC) guidelines [28].
The macroscopic morphology (shape, size, color, and opacity) of the colonies was analyzed under a stereomicroscope. The resulting Prototheca suspicious colonies (cream-white, pasty colonies) were examined through Gram-stain and wet mounts with lactophenol blue using optical microscopy. According to the NMC, the presence of spherical-oval sporangia, with or without endospores, is considered indicative of Prototheca [28]. All Prototheca spp. suspected colonies were cryopreserved in liquid Prototheca isolation medium (PIM) with glycerol 20% for subsequent analyses. Additionally, two reference strains were included: P. bovis SAG 2021T and P. ciferrii SAG 2063T, acquired from the Culture Collection of Algae (SAG) at the University of Göttingen, Germany.
2.3. Molecular Identification of Prototheca Species
2.3.1. DNA Extraction and Amplification of Prototheca spp. Cytb Partial Gen
Genomic DNA was extracted with modifications using a Cetyltrimethylammonium Bromide (CTAB)-based protocol [29] in which an additional lysis step was incorporated. This step involved mechanical lysis followed by chemical lysis with SDS/Proteinase K to facilitate disruption of the Prototheca wall cell. Briefly, three to five colonies were resuspended in a microcentrifuge tube containing 400 μL of 1× TE buffer and 0.5 mm zirconia/silica beads (BioSpec Products) and were subjected to mechanical lysis by agitation in a microtube homogenizer (Mini BeadBeaster-16, BioSpec Products) for 1 min. Subsequently, the suspension was digested with SDS/Proteinase K at 65 °C for 10 min. DNA extraction was performed with a chloroform/isoamyl alcohol mixture (24:1), followed by DNA precipitation with isopropanol. The pellet was washed with 70% ethanol, dried, and resuspended in TE buffer. DNA Samples were stored at −20 °C for subsequent analyses.
To identify Prototheca spp. at the level of species, a PCR was performed as previously described by [11]. Briefly, a partial fragment (644 bp) of the cytb gene was amplified using the primers cytb_F1 (5′-GYGTWGAACAYATTATGAGAG-3′) and cytb_R2 (5′-WACCCATAARAARTACCATTCWGG-3′) in a reaction mixture (50 μL) containing 25 μL of Promega GoTaq® DNA polymerase (Promega, Madison, WI, USA), 0.2 μM of each primer, 18 μL of Milli-Q ultrapure water, and 5 μL of template DNA, under the thermal protocol consisting of: initial denaturation at 95 °C for 3 min followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 50 °C for 30 s, and extension at 72 °C for 30 s, with a final extension at 72 °C for 5 m in a SureCycler 8800 thermocycler (Agilent, Santa Clara, CA 95051, USA) [11,13]. PCR products were visualized by electrophoresis on 1% (w/v) agarose gel stained with SYBR Safe (Invitrogen, Carlsbad, CA 92008, USA). The amplicons were sent for purification and sequencing to Macrogen Chile (Santiago, Chile), where they were sequenced by the Sanger method in both directions using the same PCR primers.
2.3.2. Phylogenetic Analysis
Initial sequence analysis was performed using Geneious Prime 2025.2 [30] to assess sequence quality, trim the sequence, and generate the consensus sequence. The resulting cytb gene sequences were analyzed using the BLASTn tool [31] to determine the identity percentage compared with sequences in the GenBank database. Subsequently, the sequences obtained in the present study, together with sequences retrieved from GenBank, were aligned using the MAFFT (Multiple Alignments by fast Fourier Transform) method [32] and analyzed using BMGE (Block Mapping and Gathering with Entropy) software to remove ambiguously aligned regions [33]. The same procedure was performed, involving only the sequence of the present study to estimate the identity distance among them, generating a distance matrix. From those sequences with 100% identity, a single sequence was randomly selected and submitted to GenBank [34].
Two phylogenetic trees were constructed based on two different phylogenetic inference methods: Maximum Likelihood (ML) and Bayesian Inference (BI). Prior to the ML phylogenetic tree inference, the best nucleotide substitution model was selected according to the Bayesian Information Criterion (BIC) [35] for each codon position (partition) [36] using ModelFinder [37]. Thereafter, the tree was inferred by the ML method on IQ-TREE [38,39]. The topology’s robustness was evaluated with 1000 bootstrapping replicates, employing the rapid-hill-climbing and stochastic disturbance methods with IQ-TREE [38,39].
For phylogenetic construction based on the BI method, the appropriate nucleotide substitution models were independently selected for each partition using the Reversible Jump Markov Chain Monte Carlo (MCMC) algorithm [40]. Then, the phylogenetic tree was inferred by running 20,000 generations in Mr. Bayes version 3.2 available on CIPRES [41]. Bayesian posterior probabilities (BPP) with values ≥ 0.70 were considered to provide strong statistical support [40]. Finally, a consensus tree (among the ML and BI resulting phylogenetic construction) was generated using the Consensus Tree Builder in Geneious Prime 2025.2 [30].
2.4. RAPD-PCR Genotyping
The genetic diversity of Prototheca spp. isolates was analyzed using the molecular marker Random Amplified Polymorphic DNA-Polymerase Chain Reaction (RAPD-PCR). The study also included DNA from reference strains P. bovis SAG 2021T and P. ciferrii SAG 2063T.
Based on previous RAPD-PCR studies [14,24], the OPA-4 primer (5′-AATCGGGCTG-3′) was selected for the present analysis due to evidence of its ability to generate distinguishable band patterns for genetic discrimination among the isolates [14]. Amplification was carried out under the following conditions: initial denaturation at 94 °C for 2 min, followed by 55 cycles of 94 °C for 1 min, 40 °C for 2 min, and 72 °C for 2 min, with a final extension at 72 °C for 5 min. The dendrogram was generated using Phoretix 1.0 software and the Unweighted Pair Group Method with Arithmetic Mean (UPGMA) clustering method. According to Morandi et al. (2016) [24], strains with a similarity coefficient equal to or greater than 90% can be considered genotypically identical.
2.5. Antibiotic Susceptibility
The antibiotic susceptibility evaluation of Prototheca spp. isolates were performed using the disk diffusion method on Müller-Hinton agar (Liofilchem, Roseto degli Abruzzi, Italy), in triplicate, following the guidelines of the Clinical and Laboratory Standards Institute (CLSI) [42]. The panel of antibiotics (Oxoid, Basingstoke, UK) evaluated comprised representatives of different families: gentamicin (CN) (10 μg), netilmicin (NET) (30 μg), kanamycin (K) (30 μg), colistin sulfate (CT) (50 μg), lincomycin (MY) (2 μg), ciprofloxacin (CIP) (5 μg), enrofloxacin (ENR) (5 μg), amoxicillin (AML) (25 μg), penicillin (P) (10 IU), ampicillin (AMP) (10 μg), and sulfamethoxazole/trimethoprim (SXT) (25 μg). The plates were incubated at 37 °C for 48 h, after which the diameters of the growth inhibition zones (mm) were measured. Since there are no specific standardized criteria for interpreting susceptibility tests in Prototheca spp., the classification system previously proposed by Morandi et al. (2016) [24] was adopted, categorizing strains according to the inhibition halo diameter as: susceptible (≥9 mm), intermediate (3–8 mm), and resistant (≤2 mm). The reference strain Escherichia coli ATCC 25923 was used as a control.
2.6. Biofilm Formation
Biofilm formation was evaluated following the methodology described by Morandi et al. (2016) and Tashakkori et al. (2022) [14,24]. Briefly, Prototheca spp. cell suspensions were prepared in Sabouraud dextrose broth (SDB) (BD DifcoTM, Le Pont de Claix, France) and incubated for 48 h at 37 °C. In 96-well flat-bottom cell culture plates, 200 μL of the culture was inoculated and diluted in a 1:9 ratio in SDB. The assays were performed in triplicate, including negative controls with SDB. After incubating the plates without agitation at 37 °C for 24 h, the medium was removed by aspiration, and the wells were washed with PBS. Following the established protocol, the plates were dried at 45 °C for 3 h, stained with 200 μL of 2% crystal violet for 20 min., rinsed with sterile water, and dried at room temperature. The dye adhered to the biofilm was solubilized with 200 μL of 33% acetic acid. Absorbance readings were quantified at 490 nm (OD490) in a SynergyTM 2 microplate reader (Bio Tek, USA). The ability of strains to produce biofilm was classified as weak (OD NC < OD ≤ 2 × OD NC), moderate (2 × OD NC < OD ≤ 4 × OD NC), or strong (OD > 4 × OD NC), where OD NC is the optical density of the negative control.
2.7. Statistical Analysis
Data were analyzed according to the type of variable assessed. Antimicrobial susceptibility and biofilm formation results were summarized using descriptive statistics, reporting categorical outcomes, frequencies, and percentages. Genotyping analysis by RAPD-PCR was interpreted through similarity coefficients and UPGMA clustering, applying a ≥90% threshold to define identical profiles. For phylogenetic inference, Maximum Likelihood and Bayesian approaches were used, and node support was evaluated with 1000 bootstrap replicates and Bayesian posterior probability values, considering thresholds ≥ 0.70 as strong statistical support. No additional inferential analyses were applied, as the study was intended to provide a descriptive characterization of the isolates.
3. Results
3.1. Prototheca Isolation
The identification of positive Prototheca culture was initially achieved based on the macroscopic characteristics of the colonies observed under a stereomicroscope, as well as microscopic observations of the cells using Gram-staining and wet mount preparation with lactophenol [28]. The macroscopic examination revealed colonies with irregular morphology, whitish coloration, shiny appearance, pronounced elevation, wavy edge, and granular surface (mulberry or cauliflower-like) (Figure 1a). The microscopic evaluation showed oval, spherical, or reniform structures, individual cells or small groups stained purple with Gram-staining (internal structures are not evidenced) (Figure 1b) and blue in wet preparation with lactophenol, showing sporangial-type cells (characteristic of Prototheca) containing 2 to 8 or more sporangiospores and an evident cell wall (Figure 1c).
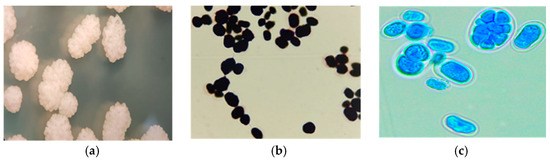
Figure 1.
Morphological characteristics of Prototheca spp. (a) Colonies on SDA observed under a stereomicroscope, appreciating the whitish coloration, pronounced elevation, and a cauliflower-like shape. (b) Gram-stain (100×) of Prototheca cells that are completely purple staining (internal structures are not distinguishable). (c) Preparation with lactophenol blue (100×). Sporangial-type cells (characteristic of Prototheca) containing 2 to 8 or more sporangiospores and a well-defined cell wall are observed.
Was observed a culture positivity occurrence of the culture of 0.36% (23/6442) in individual or composite milk samples from dairy farms in southern Chile; 15.38% (16/104) in bulk tank milk samples from 7 farms in Central and Southern Chile; and 46.55% (27/58) in an outbreak of clinical mastitis in a farm of Metropolitan Region of Chile (farm O). Thus, a total of 66 Prototheca spp. isolates were analyzed from individual milk samples (50 isolates) and bulk tank milk samples (16 isolates) (Table A1).
3.2. Molecular Identification of Prototheca Species
3.2.1. DNA Extraction and Amplification of Prototheca spp. Cytb Partial Gene
All Prototheca spp. suspected isolates (n = 66) showed a positive amplification of the cytb partial gene, reflected in the presence of the expected size band (644 bp), resulting in 66 PCR products successfully sequenced. The BLASTn analysis of the obtained sequences showed that 65 sequences exhibited high identity percentages (98.52 to 100%) with P. bovis cytb gene detected in bovines from Germany (MF163469), Japan (AP038925), Spain (MZ604423, MZ404428), China (OP748363), and P. bovis from a dog in Australia (MT240530). Based on the estimation of distance among the 65 cytb-Prototheca spp. sequences, six genetic variants were observed and deposited in GenBank under the accession numbers PV768537-42 (Table A2). One of the sequences showed 100% identity with P. ciferrii identified in a human from Puerto Rico (MK452796) and was deposited in GenBank under the accession number PV768543 (Table A2).
3.2.2. Phylogenetic Analysis
The resulting phylogenetic trees from the BI and ML methods supported the BLASTn analysis outcomes. The consensus tree showed that the sequences of the present study (PV768537-43) were allocated into two distinct clades, one that grouped P. bovis sequences and the other comprising P. ciferrii. Within the P. bovis group, most of the P. bovis sequences clustered closely together with P. bovis originating from Germany, Australia, Japan, and Spain, except for sequence PV768539, which was placed in a different clade together with P. bovis from China. In contrast, the sequence PV768543 was placed in the P. ciferrii clade, closely related to P. ciferrii from a dog in Italy, a bovine in Germany, and humans from Puerto Rico and Belgium (Figure 2).
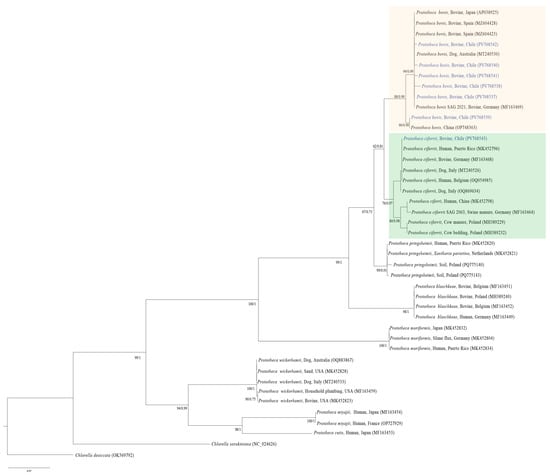
Figure 2.
Consensus Maximum likelihood (ML) and Bayesian Inference (BI) phylogenetic tree based on alignment of 594 bp of the partial cytb gene of a subset of Prototheca spp. sequences. The best substitution models estimated for each one of the codon positions (partition) were HKY+F+G4 for partitions 1 and 2, and TIM+F+G4 for partition 3, based on Bayesian Information Criterion (BIC) for the ML inference method. The selected substitution models using the Reversible Jump Markov Chain Monte Carlo (MCMC) algorithm were: M15, M90 for partition 1; M20, M107 for partition 2; and M88, M150, M185, M197, M187 for partition 3, for the phylogenetic construction inferred by BI. The green box indicates the clade comprising the P. ciferrii sequence isolated in this study, together with P. ciferrii sequences obtained from the database. Similarly, the beige box frames the clade corresponding to P. bovis.
3.3. RAPD-PCR Genotyping
The RAPD-PCR analysis with the OPA-4 primer generated band profiles that enabled the evaluation of genetic diversity among the 66 Prototheca spp. isolates, together with the two reference strains. The resulting dendrogram, constructed using the UPGMA method, revealed that the 65 P. bovis isolates were sorted into 18 different genotypes with a distribution independent of the origin farm (Figure 3).
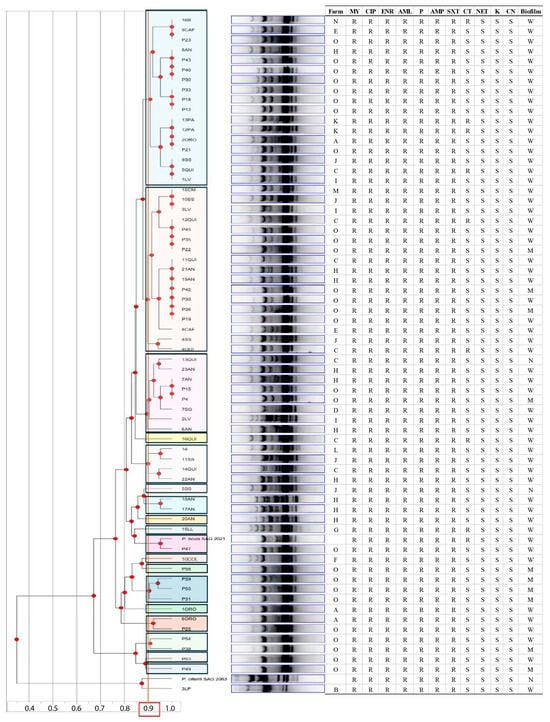
Figure 3.
Dendrogram obtained from the amplification profiles generated with the OPA-4 primer, using the Unweighted Pair Group Method with Arithmetic Mean (UPGMA). Strains with a similar coefficient equal to or greater than 90% can be considered genotypically identical (clustered within the same color) [24]. The analysis includes the Prototheca isolates studied, along with the reference strains Prototheca bovis SAG 2021T and Prototheca ciferrii SAG 2063T. The information for each isolate is indicated: farm, susceptibility (R: resistant and S: susceptible) to tested antibiotic (MY: lincomycin, CIP: ciprofloxacin, ENR: enrofloxacin, AML: amoxicillin, P: penicillin, AMP: ampicillin, SXT: sulfamethoxazole/trimethoprim, CT: colistin sulfate, NET: netilmicin, K: kanamycin, CN: gentamicin) and biofilm production (W: weak; M: moderate; N: nonproduction).
The analysis successfully grouped the isolates according to their species. The 3LP isolate clustered with the type of strain P. ciferrii SAG 2063T, confirming its identity as P. ciferrii. The remaining isolates clustered with the type of strain P. bovis SAG 2021T, indicating their assignment to this species.
3.4. Antibiotic Susceptibility
All isolates showed resistance to lincomycin (MY), ciprofloxacin (CIP), enrofloxacin (ENR), amoxicillin (AML), penicillin (P), ampicillin (AMP), and sulfamethoxazole/trimethoprim (SXT), with no inhibition zones observed (inhibition diameter = 0 mm). Conversely, gentamicin (CN), netilmicin (NET), and kanamycin (K) exhibited the highest antimicrobial activity, with all strains classified as susceptible. Colistin sulfate (CT) showed variable activity, with 13.24% (9/68) of the isolate exhibiting resistance. No intermediate susceptibility was observed for any of the antibiotics tested (Figure 4). The antibiotic susceptibility results of the isolates appeared independent of their RAPD-genotype or farm origin. The individualized antibiotic susceptibility outcomes by isolate are detailed in Figure 3.
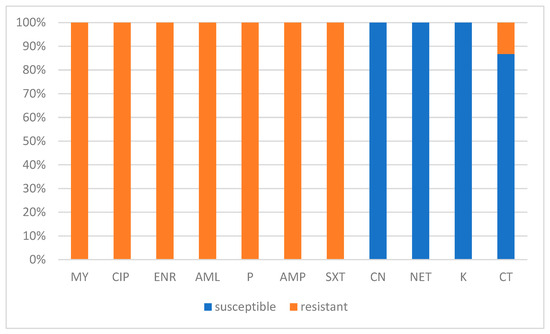
Figure 4.
Percentage of Prototheca isolates classified as susceptible or resistant to antibiotics tested in this study. CIP: ciprofloxacin, ENR: enrofloxacin, AML: amoxicillin, P: penicillin, AMP: ampicillin, SXT: sulfamethoxazole/trimethoprim, CN: gentamicin, NET: netilmicin, K: kanamycin, CT: Colistin sulfate.
3.5. Biofilm Formation
It was observed that the majority of P. bovis isolates in the present study were classified as weak biofilm producers (81.5%, 53/65). A moderate biofilm production was observed in 15.4% (10/65) of the isolates, while only two isolates (3.1%) showed no evidence of biofilm formation. The reference strains, P. bovis SAG 2021T, showed weak biofilm production. The only P. ciferrii isolate obtained in the present study exhibited weak biofilm production, whereas the reference strain P. ciferrii SAG 2063T was classified as a non-producer (Table 1).

Table 1.
Biofilm formation capacity in field isolates and reference strains.
4. Discussion
The present study revealed the identification of P. bovis as the principal species involved in bovine protothecal mastitis cases in different farms from Central and Southern Chile, identifying 65 of the 66 isolates as P. bovis through molecular techniques, which is consistent with previous studies that have identified this species as the main etiological agent in several regions of the world. The isolation of a P. ciferrii strain from a quarter of a cow with subclinical mastitis, without detecting any other mammary pathogens, suggests that P. ciferrii could be a potential causal agent of mastitis. However, this idea should be approached with caution since most studies have demonstrated that the isolation of P. ciferrii from mastitis cow milk samples is unusual [5,7,25,43,44], and it has been isolated more frequently from cow barn surroundings. Consequently, it is proposed that P. ciferrii is not involved in bovine mastitis [25,43]. Furthermore, the presence of P. ciferrii in stool and rectal swabs from healthy dairy cows suggests its role as part of the non-pathogenic microbiota of the bovine gut [7]. Nonetheless, a few studies have challenged the suggestion of non-pathogenicity, reporting the isolation of P. ciferrii from individual milk samples from cows with mastitis from different family dairy herds in Italy [8]. Additionally, mammary gland tissue from a cow experimentally infected with P. ciferrii, as analyzed histopathologically, exhibited the development of typical features of mastitis similar to those induced by P. bovis [18]. Recent studies have also documented its presence in human [45] and dog [46,47] infections. In this context, this study represents the second global record of P. ciferrii presence in milk samples from bovine mastitis cases and the first reported in Chile. However, the potential pathogenicity of P. ciferrii remains controversial, warranting further research to elucidate its role in bovine mastitis.
The analysis of genetic diversity by RAPD-PCR in 66 Prototheca spp. strains enabled the evaluation of genotypic variability within the Chilean population of this genus. The dendrogram generated with the OPA-4 primer, clustered using the UPGMA method, clearly separated the two species (P. bovis and P. ciferrii), confirming the utility of this technique for inter-specific differentiation within Prototheca. These findings are consistent with those reported by Morandi et al. (2016) [24], who observed a grouping of strains according to their species [24].
Regarding intra-specific diversity, the classification of the P. bovis isolates into 18 different genotypes was observed, evidencing the genetic heterogeneity of this species. Similar results were reported in two previous studies [14,24]. One of these studies identified 28 distinct genotypes of P. bovis [24], while the other found that only four pairs of isolates were genotypically identical among 48 P. bovis isolates [14]. Additionally, the dendrogram created from the RAPD-PCR banding patterns showed that the genotype distribution did not correlate with the farm of origin, antibiotic susceptibility, or biofilm production. Indeed, the isolates obtained from the protothecal mastitis outbreak were clustered indiscriminately. To the best of the authors’ knowledge, only two studies to date have explored intraspecific genetic variation in Prototheca spp. using RAPD-PCR, which limits the possibility of drawing conclusive comparisons. One of these studies [14] reported similar findings, revealing high genetic heterogeneity: only four pairs of P. bovis isolates were genotypically identical out of 48 tested using the OPA-4 primer. Moreover, the technique was unable to cluster the resulting genotypes based on farm origin, antibiotic susceptibility, or biofilm production. The study by Morandi et al. (2016) [24] also aligns with the present findings regarding the genetic variability of P. bovis, identifying 28 distinct genotypes. However, unlike the present study, which used only the OPA-4 primer, Morandi et al. (2016) differentiated strains based on geographical origin by analyzing the combined banding profiles generated by three primers (M-13, OPA-4, and OPA-18) and comparing isolates from two different countries (Brazil and Italy). It has been reported that combining multiple primers increases the discriminatory power of RAPD-PCR. For example, Idil & Bilkay (2014) [48] found that some strains from different hospitals could not be distinguished using a single primer. Therefore, the use of multiple primers is recommended to enhance the discriminatory resolution of RAPD-PCR and potentially enable the differentiation of isolates according to their origin. Additionally, future studies should consider incorporating high-resolution methodologies, such as sequencing-based molecular markers, to allow a more comprehensive characterization of the P. bovis population structure in Chile.
The antimicrobial susceptibility profile of Prototheca spp. strains examined in this study showed broad resistance to most antibiotics tested, except for aminoglycosides and, to a lesser degree, polymyxins. These results align with previous research indicating significant resistance of Prototheca spp. to conventional antibiotics, especially β-lactams, fluoroquinolones, and Sulfonamides/Trimethoprim [49,50]. β-lactams work by blocking bacterial cell wall synthesis through inhibiting transpeptidases, preventing the transformation of immature peptidoglycan into its mature, functional form [51]. Fluoroquinolones target bacterial DNA gyrase and topoisomerase IV, enzymes vital for DNA replication [51]. Sulfonamides and trimethoprim inhibit the synthesis of tetrahydrofolic acid, the active form of folic acid necessary for the synthesis of thymidine, purines, and ultimately DNA [52].
These mechanisms are crucial for the survival of most prokaryotic organisms. However, they are absent or significantly different in eukaryotic cells, supporting the hypothesis that Prototheca spp., as eukaryotic algae, lack specific cellular targets for these antibiotics, therefore possess an intrinsic resistance mechanism to these antimicrobial classes. This is a noteworthy consideration, as the indiscriminate use of antibiotics in dairy cattle can contribute to the development of antimicrobial resistance. This highlights the importance of raising awareness about Prototheca spp. as a causative agent of bovine mastitis. In many cases, bovine mastitis (including Prototheca spp.) is treated with antibiotics without an etiological diagnosis, which are ineffective against Prototheca spp. Consequently, such misdiagnosis often leads to refractory cases and unnecessary antibiotic use [4].
On the other hand, aminoglycosides proved to be the most effective antibiotics, with all strains found to be susceptible to gentamicin, kanamycin, and netilmicin. This finding is consistent with previous studies that have reported high susceptibility of Prototheca spp. to aminoglycosides [14,24,25,49,53]. Furthermore, in a murine model of protothecal mastitis, administration of gentamicin at doses of 20–30 mg/kg significantly reduced Prototheca load in mammary glands, supporting its potential therapeutic use for Prototheca infections [54]. This finding is particularly relevant given the availability of commercial formulations of gentamicin, both parenteral and intramammary, approved for use in cattle in veterinary practice. The convergence of in vitro and in vivo results suggests that gentamicin could be considered as a promising therapeutic alternative for treating bovine protothecal mastitis. However, controlled clinical trials in cattle with protothecal mastitis are necessary to validate its efficacy and establish optimal treatment protocols.
Colistin sulfate showed variable activity, with 13.2% of strains found to be resistant. This result is comparable to the findings of Tashakkori et al. (2022) [14], who reported 89.58% susceptibility to colistin, suggesting that this antibiotic may play a role in controlling Prototheca spp. infections depending on the strain and clinical context. This heterogeneity in the response to polymyxins has also been reported by Wawron et al. (2013) [50], who found that 29.6% of Prototheca strains exhibited intermediate susceptibility to colistin.
Regarding the biofilm production observed in P. bovis isolates of the present study, most of the isolates (81.5%) exhibited weak biofilm-forming capacity, while 15.4% showed moderate production, and only 3.1% did not display this ability. Unfortunately, to the best of our knowledge, studies that assess Prototheca spp. biofilm production is limited and modified from procedures created to assess biofilm production in bacteria; thus, it is necessary to generate a standardized assay to evaluate biofilm production in this microalga [14,24,55]. The outcomes obtained in the present study are similar to those reported by Gonçalves et al. (2015) [55], with most isolates classified as weak biofilm producers, followed by moderate biofilm producers. Otherwise, discrepancies in the degree of production among studies have been noted. Morandi et al. (2016) [24] reported that a remarkable percentage of P. bovis isolates exhibit strong production at 37 °C, whereas Tashakkori et al. (2022) [14] found a higher proportion of non-producing strains (41.66%), followed by moderate and weak producers (25% each). These inconsistencies among studies could be partially explained by differences in Prototheca spp. strain, Morandi et al. (2016) [24] suggested that the ability to produce biofilm is strain-dependent.
Regarding P. ciferrii, our field isolate exhibited weak biofilm production, while P. ciferrii SAG 2063T did not form biofilms. This observation partially agrees with Morandi et al. (2016) [24], who classified the P. ciferrii reference strain as a weak producer, supporting previous reports on this species’ lower pathogenicity [43,56].
It is noteworthy that all moderate biofilm producers isolates originated from a mastitis outbreak, suggesting a possible role of biofilm in Prototheca infections, as mentioned by Kwiecinski (2015) [23], who concluded that biofilm production property could contribute to promoting Prototheca infection, partly by preventing IL-6 signaling and the initiation of the immune response.
The biofilm-forming ability in 96.9% of our strains, although predominantly weak, suggests that this trait may be relevant to the pathogenesis of P. bovis-associated mastitis in Chilean herds. According to Kwiecinski (2015) [23], this property may contribute to the chronicity of the infection and to resistance against antimicrobial treatments, as well as to the host immune response factors frequently associated with protothecal mastitis. In a nutshell, studies on Prototheca spp. biofilm formation are scarce; nonetheless, the available reports noticed the ability of Prototheca strain to produce biofilm in variable degrees, highlighting their role in the persistence of this alga in sustaining infection [14,24,55]. Nevertheless, further research is needed to elucidate the association between biofilm production and the complexity of clinical manifestation, as well as its impact on disinfection procedures and milk pasteurization. These aspects are particularly relevant for public health, as they involve understanding the zoonotic potential of this alga and the possibility of transmission through the consumption of contaminated milk or dairy products [8,9].
5. Conclusions
The present study confirmed the relevance of P. bovis as the principal species involved in l mastitis cases in Chile and reports the first identification of P. ciferrii in mastitis cow’s milk samples. Additionally, the RAPD-PCR analysis revealed a high level of genetic heterogeneity among P. bovis, with 18 distinct genotypes distributed randomly across the different farms. Furthermore, Prototheca spp. were found to be resistant to most of the tested antimicrobials, except for aminoglycosides (gentamycin, netilmicin, kanamycin), suggesting their potential use as a therapeutic agent for bovine protothecosis. Finally, varying degrees of biofilm production were observed, with most isolates classified as weak producers, followed by moderate producers. Notably, all isolates from the protothecal mastitis outbreak exhibited moderate biofilm production, supporting the notion that biofilm involvement is a key factor in the pathogenicity and infection process of Prototheca. Further studies are required to assess the accuracy of aminoglycosides as a therapy against bovine protothecal mastitis and to determine the correlation among biofilm production, Prototheca pathogenicity, and antimicrobial resistance.
Author Contributions
Conceptualization, J.R. and A.M.; methodology, J.R., C.C. and M.G.; software, J.R. and P.S.-G.; validation, A.M., N.C. and M.G.; formal analysis, J.R.; investigation, J.R.; resources, A.M.; data curation, Á.G.M. and P.S.-G.; writing—original draft preparation, J.R.; writing—review and editing, P.S.-G., Á.G.M. and L.C.; visualization, A.M.; supervision, A.M. and Á.G.M.; project administration, A.M.; funding acquisition, A.M. and C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the FONDEF project ID21I10176 and by cost center number 1121101426 of the Bovine Mastitis Laboratory at the Universidad Austral de Chile.
Institutional Review Board Statement
Ethical review and approval were waived for this study because no experiments involved harming animals, as they were not the units of study but rather microbiological isolates (in this case, Prototheca). Although milk samples from the animals were used, these samples were obtained from routine sampling for the detection of mammary pathogens in cows, conducted by Lecherías del Sur spa.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
This research was conducted as part of a Master’s thesis in the Microbiology specialization of the Master’s Program in Science at the Faculty of Sciences, Universidad Austral de Chile. We also thank the “Lecherías del Sur spa” for providing milk samples from cows with mastitis, as well as for facilitating the associated data. During the preparation of this manuscript, the authors used the Grammarly tool to correct grammatical and punctuation errors, with the intention of improving the English writing. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DNA | Deoxyribonucleic acid |
| DNAr | Ribosomal DNA |
| BLASTn | Basic Local Alignment Nucleotide Search tool |
| CFU | Colony Forming Units |
| cytb | Cytochrome b gene |
| PCR | Polymerase Chain Reaction |
| RAPD-PCR | Random Amplified Polymorphic DNA–Polymerase Chain Reaction |
| SAG | Culture Collection of Algae |
| MY | Lincomycin |
| CIP | Ciprofloxacin |
| ENR | Enrofloxacin |
| AML | Amoxicillin |
| P | Penicillin |
| AMP | Ampicillin |
| SXT | Sulfamethoxazole/Trimethoprim |
| CT | Colistin Sulfate |
| NET | Netilmicin |
| K | Kanamycin |
| CN | Gentamicin |
| ML | Maximum Likelihood Method for phylogenetic inference |
| BY | Bayesian Inference for phylogenetic inference |
| UPGMA | Unweighted Pair Group Method with Arithmetic Mean |
| SDA | Sabouraud Dextrose Agar |
| SDB | Sabouraud Dextrose Broth |
| PIM | Prototheca Isolation Medium |
| NMC | National Mastitis Council |
| CLSI | Clinical and Laboratory Standards Institute |
Appendix A

Table A1.
Information on each isolate: farm of origin, Prototheca species, RAPD-PCR genotype, antimicrobial susceptibility (R: resistant; S: susceptible), and biofilm production (W: weak; M: moderate; N: non-producer), mastitis type (SCM: subclinical mastitis; MC: clinical mastitis), and sample type.
Table A1.
Information on each isolate: farm of origin, Prototheca species, RAPD-PCR genotype, antimicrobial susceptibility (R: resistant; S: susceptible), and biofilm production (W: weak; M: moderate; N: non-producer), mastitis type (SCM: subclinical mastitis; MC: clinical mastitis), and sample type.
| Isolate | Species | Farm | MY | CIP | ENR | AML | P | AMP | SXT | CT | NET | K | CN | Biofilm | RAPD-PCR Genotype | Mastitis Type | Sample Type |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 ORO | P. bovis | A | R | R | R | R | R | R | R | S | S | S | S | W | 14 | SCM | Individual milk |
| 2 ORO | P. bovis | A | R | R | R | R | R | R | R | S | S | S | S | W | 1 | SCM | Individual milk |
| 6 ORO | P. bovis | A | R | R | R | R | R | R | R | S | S | S | S | W | 15 | SCM | Individual milk |
| 3 LP | P. ciferrii | B | R | R | R | R | R | R | R | S | S | S | S | W | SCM | Individual milk | |
| 4 QUI | P. bovis | C | R | R | R | R | R | R | R | R | S | S | S | W | 2 | MC | Individual milk |
| 5 QUI | P. bovis | C | R | R | R | R | R | R | R | R | S | S | S | W | 1 | MC | Individual milk |
| 11 QUI | P. bovis | C | R | R | R | R | R | R | R | S | S | S | S | W | 2 | CM | Individual milk |
| 12 QUI | P. bovis | C | R | R | R | R | R | R | R | R | S | S | S | W | 2 | SCM | Individual milk |
| 13 QUI | P. bovis | C | R | R | R | R | R | R | R | S | S | S | S | N | 3 | CM | Individual milk |
| 14 QUI | P. bovis | C | R | R | R | R | R | R | R | S | S | S | S | W | 5 | CM | Individual milk |
| 16 QUI | P. bovis | C | R | R | R | R | R | R | R | R | S | S | S | W | 4 | CM | Individual milk |
| 7 SG | P. bovis | D | R | R | R | R | R | R | R | S | S | S | S | W | 3 | CM | Individual milk |
| 8 CAF | P. bovis | E | R | R | R | R | R | R | R | S | S | S | S | W | 2 | - | Individual milk |
| 9 CAF | P. bovis | E | R | R | R | R | R | R | R | S | S | S | S | W | 1 | - | Individual milk |
| 10 COL | P. bovis | F | R | R | R | R | R | R | R | S | S | S | S | W | 11 | CM | Individual milk |
| 15 LL | P. bovis | G | R | R | R | R | R | R | R | R | S | S | S | W | 9 | CM | Individual milk |
| 6 AN | P. bovis | H | R | R | R | R | R | R | R | S | S | S | S | W | 3 | - | Bulk tank milk |
| 7 AN | P. bovis | H | R | R | R | R | R | R | R | S | S | S | S | W | 3 | - | Bulk tank milk |
| 8AN | P. bovis | H | R | R | R | R | R | R | R | S | S | S | S | W | 1 | - | Bulk tank milk |
| 17 AN | P. bovis | H | R | R | R | R | R | R | R | S | S | S | S | W | 7 | SCM | Individual milk |
| 18 AN | P. bovis | H | R | R | R | R | R | R | R | S | S | S | S | W | 7 | CM | Individual milk |
| 19 AN | P. bovis | H | R | R | R | R | R | R | R | S | S | S | S | W | 2 | CM | Individual milk |
| 20 AN | P. bovis | H | R | R | R | R | R | R | R | S | S | S | S | W | 8 | SCM | Individual milk |
| 21 AN | P. bovis | H | R | R | R | R | R | R | R | S | S | S | S | W | 2 | CM | Individual milk |
| 22 AN | P. bovis | H | R | R | R | R | R | R | R | S | S | S | S | W | 5 | CM | Individual milk |
| 23 AN | P. bovis | H | R | R | R | R | R | R | R | S | S | S | S | W | 3 | CM | Individual milk |
| 1LV | P. bovis | I | R | R | R | R | R | R | R | S | S | S | S | W | 1 | - | Bulk tank milk |
| 2LV | P. bovis | I | R | R | R | R | R | R | R | S | S | S | S | W | 3 | - | Bulk tank milk |
| 3LV | P. bovis | I | R | R | R | R | R | R | R | S | S | S | S | W | 2 | - | Bulk tank milk |
| 4 SS | P. bovis | J | R | R | R | R | R | R | R | S | S | S | S | W | 2 | - | Bulk tank milk |
| 5 SS | P. bovis | J | R | R | R | R | R | R | R | S | S | S | S | N | 6 | - | Bulk tank milk |
| 9 SS | P. bovis | J | R | R | R | R | R | R | R | S | S | S | S | W | 1 | - | Bulk tank milk |
| 10 SS | P. bovis | J | R | R | R | R | R | R | R | S | S | S | S | W | 2 | - | Bulk tank milk |
| 11 SS | P. bovis | J | R | R | R | R | R | R | R | S | S | S | S | W | 5 | - | Bulk tank milk |
| 12 PA | P. bovis | K | R | R | R | R | R | R | R | R | S | S | S | W | 1 | - | Bulk tank milk |
| 13 PA | P. bovis | K | R | R | R | R | R | R | R | R | S | S | S | W | 1 | - | Bulk tank milk |
| 14 | P. bovis | L | R | R | R | R | R | R | R | S | S | S | S | W | 5 | - | Bulk tank milk |
| 15 CM | P. bovis | M | R | R | R | R | R | R | R | S | S | S | S | W | 2 | - | Bulk tank milk |
| 16 R | P. bovis | N | R | R | R | R | R | R | R | R | S | S | S | W | 1 | - | Bulk tank milk |
| P4 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | M | 3 | CM | Individual milk |
| P12 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 1 | CM | Individual milk |
| P15 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 3 | CM | Individual milk |
| P18 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 1 | CM | Individual milk |
| P19 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 2 | CM | Individual milk |
| P21 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 1 | CM | Individual milk |
| P22 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | M | 2 | CM | Individual milk |
| P23 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 1 | CM | Individual milk |
| P25 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 15 | CM | Individual milk |
| P26 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | M | 2 | CM | Individual milk |
| P30 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 1 | CM | Individual milk |
| P31 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | M | 13 | CM | Individual milk |
| P33 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 1 | CM | Individual milk |
| P35 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 2 | CM | Individual milk |
| P38 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | M | 16 | CM | Individual milk |
| P39 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 2 | CM | Individual milk |
| P40 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 1 | CM | Individual milk |
| P42 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | M | 2 | CM | Individual milk |
| P43 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 1 | CM | Individual milk |
| P45 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 2 | CM | Individual milk |
| P47 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 10 | CM | Individual milk |
| P49 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | M | 18 | CM | Individual milk |
| P53 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 17 | CM | Individual milk |
| P54 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | W | 16 | CM | Individual milk |
| P55 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | M | 13 | CM | Individual milk |
| P58 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | M | 12 | CM | Individual milk |
| P59 | P. bovis | O | R | R | R | R | R | R | R | S | S | S | S | M | 13 | CM | Individual milk |

Table A2.
GenBank accession numbers of cytb partial gene sequences from representative Prototheca isolates obtained in the present study. For those sequences with 100% identity among them, only one representative sequence was submitted.
Table A2.
GenBank accession numbers of cytb partial gene sequences from representative Prototheca isolates obtained in the present study. For those sequences with 100% identity among them, only one representative sequence was submitted.
| Accession Number | Isolated | Distance Matrix 100% |
|---|---|---|
| PV768537 | 1LV | 1ORO; 2LV; 3LV; 4SS; 5SS; 6AN; 6ORO; 7AN; 8AN; 8CAF; 9CAF; 9SS; 10COL; 10SS; 11QUI; 11SS; 13PA; 14BTM; 15CM; 15LL; 16R; 18AN; 20AN; 23AN; P4; P18; P19; P21; P22; P23; P25; P26; P30; P31; P35; P38; P39; P40; P42; P43; P45; P47; P49; P53; P54; P55; P58; P59; 7SG; 12PA; 13QUI; 17AN; 19AN; 22AN |
| PV768539 | 5QUI | 4QUI; 12QUI; 14QUI; 16ORO |
| PV768538 | P33 | |
| PV768540 | 21AN | 2ORO |
| PV768541 | P12 | |
| PV768542 | P15 | |
| PV768543 | 3LP |
References
- Cheng, W.N.; Han, S.G. Bovine Mastitis: Risk Factors, Therapeutic Strategies, and Alternative Treatments—A Review. Asian-Australas. J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef]
- Gomes, F.; Henriques, M. Control of Bovine Mastitis: Old and Recent Therapeutic Approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef]
- Sharma, N.; Rho, G.J.; Hong, Y.H.; Kang, T.Y.; Lee, H.K.; Hur, T.-Y.; Jeong, D.K. Bovine Mastitis: An Asian Perspective. Asian J. Anim. Vet. Adv. 2012, 7, 454–476. [Google Scholar] [CrossRef]
- Libisch, B.; Picot, C.; Ceballos-garzon, A.; Moravkova, M.; Klimesová, M.; Telkes, G.; Chuang, S.T.; Pape, P.L. Prototheca Infections and Ecology from a One Health Perspective. Microorganisms 2022, 10, 938. [Google Scholar] [CrossRef]
- Pieper, L.; Godkin, A.; Roesler, U.; Polleichtner, A.; Slavic, D.; Leslie, K.E.; Kelton, D.F. Herd Characteristics and Cow-Level Factors Associated with Prototheca Mastitis on Dairy Farms in Ontario, Canada. J. Dairy Sci. 2012, 95, 5635–5644. [Google Scholar] [CrossRef]
- Buzzini, P.; Turchetti, B.; Facelli, R.; Baudino, R.; Cavarero, F.; Mattalia, L.; Mosso, P.; Martini, A. First Large-Scale Isolation of Prototheca zopfii from Milk Produced by Dairy Herds in Italy. Mycopathologia 2004, 158, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Jagielski, T.; Roeske, K.; Bakuła, Z.; Piech, T.; Wlazło, Ł.; Bochniarz, M.; Woch, P.; Krukowski, H. A Survey on the Incidence of Protothecal Mastitis in Dairy Herds in Lublin Province, Poland. J. Dairy Sci. 2019, 102, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Bozzo, G.; Bonerba, E.; Pinto, A.D.; Bolzoni, G.; Ceci, E.; Mottola, A.; Tantillo, G.; Terio, V. Occurrence of Prototheca spp. in Cow Milk Samples. New Microbiol. 2014, 37, 459–464. [Google Scholar] [PubMed]
- Melville, P.A.; Watanabe, E.T.; Benites, N.R.; Ribeiro, A.R.; Garino, F., Jr.; Costa, E.O. Evaluation of the Susceptibility of Prototheca zopfii to Milk Pasteurization. Mycopathologia 1999, 146, 79–82. [Google Scholar] [CrossRef]
- Jagielski, T. The Genus Prototheca (Trebouxiophyceae, Chlorophyta) Revisited_ Implications from Molecular Taxonomic Studies. Algal Res. 2019, 43, 101639. [Google Scholar] [CrossRef]
- Jagielski, T.; Gawor, J.; Bakuła, Z.; Decewicz, P.; Maciszewski, K.; Karnkowska, A. Cytb as a New Genetic Marker for Differentiation of Prototheca Species. J. Clin. Microbiol. 2018, 56, e00584-18. [Google Scholar] [CrossRef]
- Masuda, M.; Jagielski, T.; Danesi, P.; Falcaro, C.; Bertola, M.; Krockenberger, M.; Malik, R.; Kano, R. Protothecosis in Dogs and Cats—New Research Directions. Mycopathologia 2021, 186, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Huilca-Ibarra, M.P.; Vasco-Julio, D.; Ledesma, Y.; Guerrero-Freire, S.; Zurita, J.; Castillejo, P.; Blasco, F.B.; Yanez, L.; Changoluisa, D.; Echeverría, G.; et al. High Prevalence of Prototheca bovis Infection in Dairy Cattle with Chronic Mastitis in Ecuador. Vet. Sci. 2022, 9, 659. [Google Scholar] [CrossRef] [PubMed]
- Tashakkori, N.; Rahmani, H.K.; Khoramian, B. Genotypic and Phenotypic Diversity of Prototheca spp. Recovered from Bovine Mastitis in Terms of Antimicrobial Resistance and Biofilm Formation Ability. BMC Vet. Res. 2022, 18, 452. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.; Silva, E.; Kraft, C.; Carvalheira, J.; Videira, A.; Huss, V.A.R.; Thompson, G. Bovine Mastitis Associated with Prototheca blaschkeae. J. Clin. Microbiol. 2008, 46, 1941–1945. [Google Scholar] [CrossRef]
- Capra, E.; Cremonesi, P.; Cortimiglia, C.; Bignoli, G.; Ricchi, M.; Moroni, P.; Pesce, A.; Luini, M.; Castiglioni, B. Simultaneous Identification by Multiplex PCR of Major Prototheca spp. Isolated from Bovine and Buffalo Intramammary Infection and Bulk Tank. Lett. Appl. Microbiol. 2014, 59, 642–647. [Google Scholar] [CrossRef]
- Marques, S.; Silva, E.; Carvalheira, J.; Thompson, G. Short Communication: In Vitro Antimicrobial Susceptibility of Prototheca Wickerhamii and Prototheca Zopfii Isolated from Bovine Mastitis. J. Dairy Sci. 2006, 89, 4202–4204. [Google Scholar] [CrossRef]
- Ito, T.; Kano, R.; Sobukawa, H.; Ogawa, J.; Honda, Y.; Hosoi, Y.; Shibuya, H.; Sato, T.; Hasegawa, A.; Kamata, H. Experimental Infection of Bovine Mammary Gland with Prototheca zopfii Genotype 1. J. Vet. Med. Sci. 2011, 73, 117–119. [Google Scholar] [CrossRef]
- Lerche, M. Eine Durch Algen (Prototheca) Hervorgerufene Mastitis Der Kuh. Berl. Münchener Tierärztliche Wochenschr. 1952, 65, 64–69. [Google Scholar]
- Costa, E.O.; Carciofi, A.C.; Melville, P.A.; Prada, M.S.; Schalch, U. Prototheca sp. Outbreak of Bovine Mastitis. J. Vet. Med. B 1996, 43, 321–324. [Google Scholar] [CrossRef]
- Tomanić, D.; Božić, D.D.; Kladar, N.; Samardžija, M.; Apić, J.; Baljak, J.; Kovačević, Z. Clinical Evidence on Expansion of Essential Oil-Based Formulation’s Pharmacological Activity in Bovine Mastitis Treatment: Antifungal Potential as Added Value. Antibiotics 2024, 13, 575. [Google Scholar] [CrossRef]
- Kuczyńska, M.; Kot, M.; Stocki, M.; Zapora, E.; Jagielski, T.; Perlińska-Teresiak, M.; Kalińska, A. In Vitro Determination of Cytotoxic Effects of Ten Essential Oils on Prototheca bovis, Which Causes Mastitis in Dairy Cows. Int. J. Mol. Sci. 2025, 26, 5451. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J. Biofilm Formation by Pathogenic Prototheca Algae. Lett. Appl. Microbiol. 2015, 61, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Morandi, S.; Cremonesi, P.; Capra, E.; Silvetti, T.; Decimo, M.; Bianchini, V.; Alves, A.C.; Vargas, A.C.; Costa, G.M.; Ribeiro, M.G.; et al. Molecular Typing and Differences in Biofilm Formation and Antibiotic Susceptibilities among Prototheca Strains Isolated in Italy and Brazil. J. Dairy Sci. 2016, 99, 6436–6445. [Google Scholar] [CrossRef]
- Shahid, M.; Ali, T.; Zhang, L.; Hou, R.; Zhang, S.; Ding, L.; Han, D.; Deng, Z.; Rahman, A.; Han, B. Characterization of Prototheca zopfii Genotypes Isolated from Cases of Bovine Mastitis and Cow Barns in China. Mycopathologia 2016, 181, 185–195. [Google Scholar] [CrossRef]
- Zaror, L.; Valenzuela, K.; Kruze, J. Bovine Mastitis Caused by Prototheca zopfii: First Isolation in Chile. Arch. Med. Vet. 2011, 176, 173–176. [Google Scholar] [CrossRef]
- Vásquez, M. Identificación y Caracterización del Alga Prototheca spp. Aisladas en Un Brote de Mastitis Bovina; Universidad Austral de Chile: Valdivia, Chile, 2011. [Google Scholar]
- National Mastitis Council. Laboratory Handbook on Bovine Mastitis, 3rd ed.; National Mastitis Council: New Prague, MN, USA, 2017. [Google Scholar]
- Jagielski, T.; Gawor, J.; Bakuła, Z.; Zuchniewicz, K.; Żak, I.; Gromadka, R. An Optimized Method for High Quality DNA Extraction from Microalga Prototheca wickerhamii for Genome Sequencing. Plant Methods 2017, 13, 77. [Google Scholar] [CrossRef]
- Available online: http://www.geneious.com (accessed on 6 June 2025).
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Katoh, K. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Criscuolo, A.; Gribaldo, S. BMGE (Block Mapping and Gathering with Entropy): A New Software for Selection of Phylogenetic Informative Regions from Multiple Sequence Alignments. BMC Ecol. Evol. 2010, 10, 210. [Google Scholar] [CrossRef]
- Benson, D.A. GenBank: Update. Nucleic Acids Res. 2004, 32, 23D–26D. [Google Scholar] [CrossRef]
- Schwarz, G. Estimatibg the Dimension of a Model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Chernomor, O.; Von Haeseler, A.; Minh, B.Q. Terrace Aware Data Structure for Phylogenomic Inference from Supermatrices. Syst. Biol. 2016, 65, 997–1008. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Haeseler, A.V.; Jermiin, L.S. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Haeseler, A.V.; Lanfear, R.; Teeling, E. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; Haeseler, A.V.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P. Bayesian Phylogenetic Model Selection Using Reversible Jump Markov Chain Monte Carlo. Mol. Biol. Evol. 2004, 21, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. CLSI Supplement M100, 33rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023. [Google Scholar]
- Osumi, T.; Kishimoto, Y.; Kano, R.; Maruyama, H.; Onozaki, M.; Makimura, K.; Ito, T.; Matsubara, K.; Hasegawa, A. Prototheca zopfii Genotypes Isolated from Cow Barns and Bovine Mastitis in Japan. Vet. Microbiol. 2008, 131, 419–423. [Google Scholar] [CrossRef]
- Möller, A.; Truyen, U.; Roesler, U. Prototheca zopfii Genotype 2-The Causative Agent of Bovine Protothecal Mastitis? Vet. Microbiol. 2007, 120, 370–374. [Google Scholar] [CrossRef]
- Hirose, N.; Hua, Z.; Kato, Y.; Zhang, Q.; Li, R.; Nishimura, K.; Masuda, M. Molecular Characterization of Prototheca Strains Isolated in China Revealed the First Cases of Protothecosis Associated with Prototheca zopfii Genotype 1. Med. Mycol. 2018, 56, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Falcaro, C.; Furlanello, T.; Binanti, D.; Fondati, A.; Bonfanti, U.; Krockenberger, M.; Malik, R.; Danesi, P. Molecular Characterization of Prototheca in 11 Symptomatic Dogs. J. Vet. Diagn. Investig. 2021, 33, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Jagielski, T.; Proskurnicka, A.; Iskra, M.; Wronka, S.; Bakuła, Z.; Danesi, P.; De Farias, M.R.; Ramos Portilho, F.V.; Garcia Ribeiro, M.; Rösler, U.; et al. Protothecosis in Dogs: A Narrative Review. Vet. Intern. Med. 2025, 39, e70025. [Google Scholar] [CrossRef]
- Idil, N.; Bilkay, I.S. Application of RAPD-PCR for Determining the Clonality of Methicillin Resistant Staphylococcus aureus Isolated from Different Hospitals. Braz. Arch. Biol. Technol. 2014, 57, 548–553. [Google Scholar] [CrossRef]
- Lopes, M.M.; Ribeiro, R.; Carvalho, D.; Freitas, G. In Vitro Antimicrobial Susceptibility of Prototheca spp. Isolated from Bovine Mastitis in a Portugal Dairy Herd. J. Mycol. Médicale 2008, 18, 205–209. [Google Scholar] [CrossRef]
- Wawron, W.; Bochniarz, M.; Piech, T.; Wysocki, J.; Kocik, M. Antimicrobial Susceptibility of Prototheca zopfii Isolated from Bovine Mastitis. Bull. Vet. Inst. Pulawy 2013, 57, 485–488. [Google Scholar] [CrossRef][Green Version]
- Hooper, D.C. Mechanisms of Action of Antimicrobials: Focus on Fluoroquinolones. Clin. Infect. Dis. 2001, 32, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Masters, P.A.; O’Bryan, T.A.; Zurlo, J.; Miller, D.Q.; Joshi, N. Trimethoprim-Sulfamethoxazole Revisited. Arch. Intern. Med. 2003, 163, 402. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, H.; He, J.; He, Y.; Li, S.; Hou, R.; Wu, Q.; Gao, Y.; Han, B. Characterization of Prototheca zopfii Associated with Outbreak of Bovine Clinical Mastitis in Herd of Beijing, China. Mycopathologia 2012, 173, 275–281. [Google Scholar] [CrossRef]
- Chang, R.; Yang, Q.; Liu, G.; Liu, Y.; Zheng, B.; Su, J.; Han, B. Treatment with Gentamicin on a Murine Model of Protothecal Mastitis. Mycopathologia 2013, 175, 241–248. [Google Scholar] [CrossRef]
- Gonçalves, L.J.; Lee, S.H.I.; De Paula Arruda, E.; Galles, D.P.; Caetano, V.C.; De Oliveira, C.A.F.; Fernandes, A.M.; Dos Santos, M.V. Biofilm-Producing Ability and Efficiency of Sanitizing Agents against Prototheca zopfii Isolates from Bovine Subclinical Mastitis. J. Dairy Sci. 2015, 98, 3613–3621. [Google Scholar] [CrossRef] [PubMed]
- Jagielski, T.; Lassa, H.; Ahrholdt, J.; Malinowski, E.; Roesler, U. Genotyping of Bovine Prototheca Mastitis Isolates from Poland. Vet. Microbiol. 2011, 149, 283–287. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).