Characterization of Maturation-Associated Genes in Ovary–Hepatopancreas Transcriptome and Vitellogenin Expression in Pacific Blue Swimming Crab Callinectes arcuatus During Gonadal Maturity Stages
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. RNA Isolation, Illumina Sequencing and Bioinformatic Analyses
2.2. mRNA Expression of C. arcuatus Vtg
2.3. Statistical Analysis
3. Results
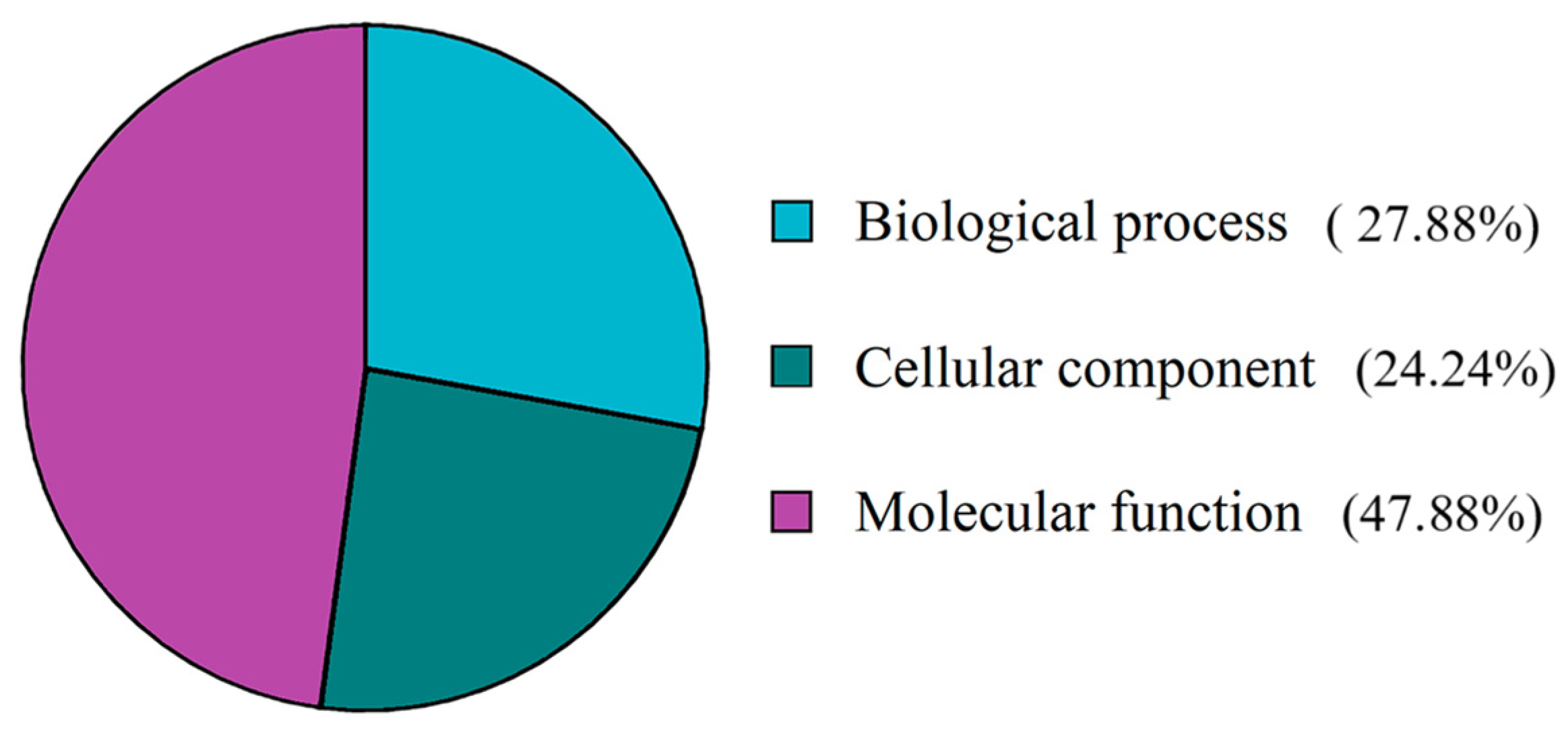
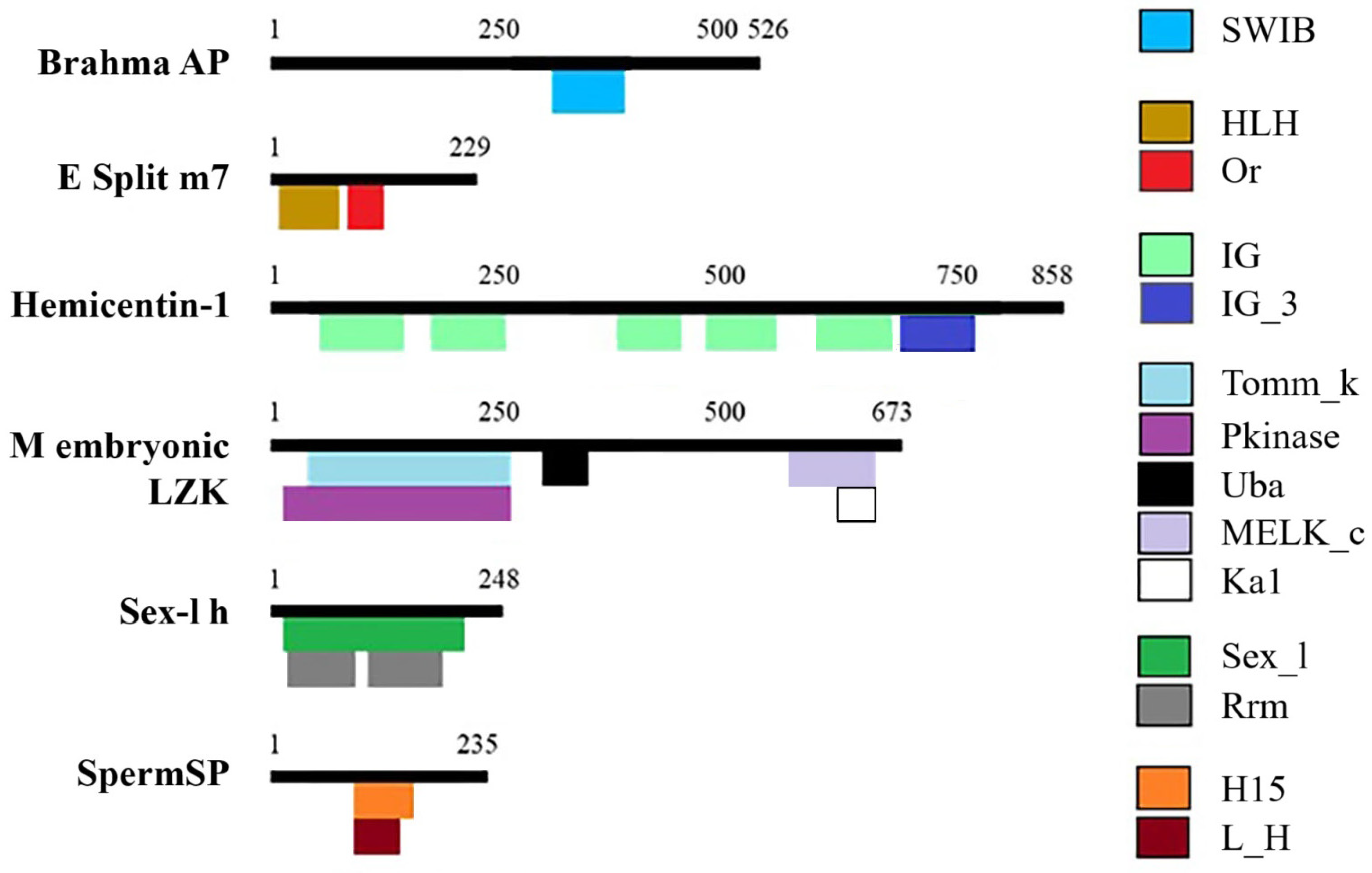
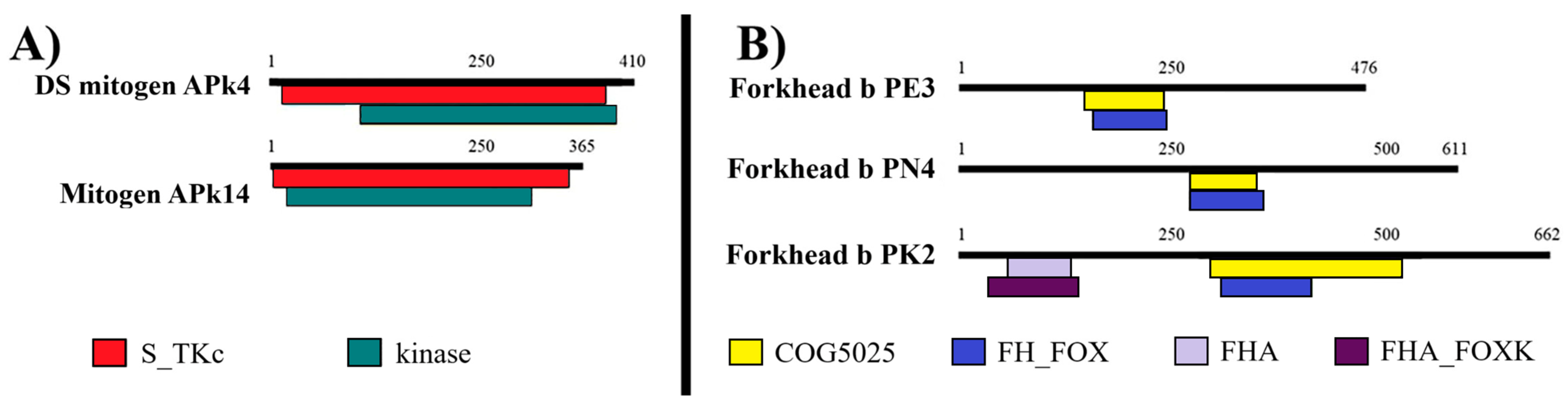
3.1. C. arcuatus Transcriptome
3.2. mRNA Expression of C. arcuatus Vtg
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
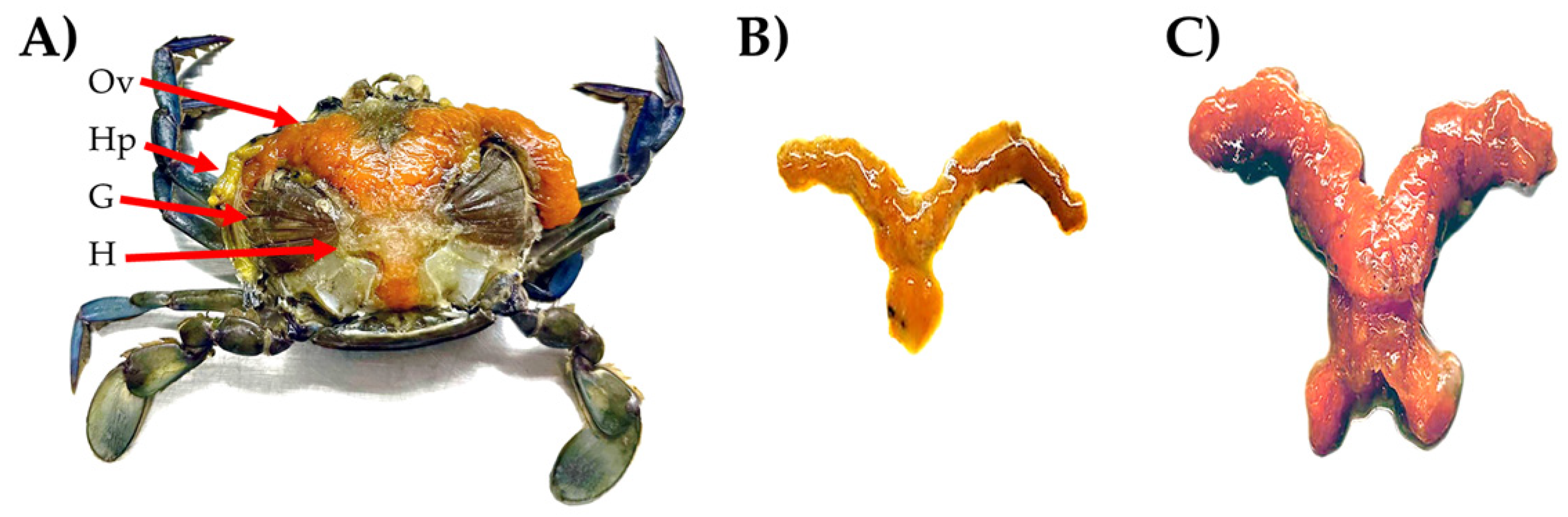
Appendix B
| Gen | Sequence | PCR Product (bp) | Reference |
|---|---|---|---|
| β-actin | Fw > AAAATGTGTGACGACGAGG | 987 | DQ084066.1 |
| Rv > GATCTTCATGGTGGAGGG | |||
| Vtg | Fw > TGTCCTTCACCACAGGC | 800 | MN105880.2 |
| Rv > CAGAGCTGGAGCAAACC |
Appendix C
References
- CONAPESCA. Anuario Estadístico de Acuacultura y Pesca 2023; Comisión Nacional de Acuacultura y Pesca: Mazatlán, Mexico, 2023; p. 278.
- SonPlayas. Inician Capturas de Jaiba en Sinaloa y Sonora; Levantan Veda Anticipada. Available online: https://sonplayas.com/pesca/inician-capturas-de-jaiba-en-sinaloa-y-sonora-levantan-veda-de-forma-anticipada/ (accessed on 23 June 2025).
- CONAPESCA. Captura de Jaiba, Opción Nutritiva Ante la Veda del Camarón. Available online: https://www.gob.mx/conapesca/articulos/captura-de-jaiba-opcion-nutritiva-ante-la-veda-del-camaron-267557 (accessed on 23 June 2025).
- INAPESCA. Acuerdo por el que se da a Conocer el Plan de Manejo Pesquero de Jaiba (Callinectes spp.) de Sinaloa y Sonora; Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación INAPESCA: Ciudad de México, Mexico, 2014; p. 42.
- Rivera-Velázquez, P.J.; Aragón-Noriega, E.A.; Rodríguez-Domínguez, G.; Pérez-González, R.; Castillo-Vargasmachuca, S.G. Growth, maturity and mortality of the blue crab Callinectes arcuatus Ordway, 1863 (Decapoda, Portunidae) in a Mexican coastal lagoon. Crustaceana 2018, 91, 659–675. [Google Scholar] [CrossRef]
- Diarte-Plata, G. Aspectos poblacionales de las jaibas del género Callinectes (Decápoda: Portunidae) en la laguna El Colorado, Ahome, Sinaloa, México. College Thesis, Universidad Autónoma de Baja California Sur, La Paz, Mexico, 2016. [Google Scholar]
- Jimenez-Gutierrez, S.; Cadena-Caballero, C.E.; Barrios-Hernandez, C.; Perez-Gonzalez, R.; Martinez-Perez, F.; Jimenez-Gutierrez, L.R. Crustacean vitellogenin: A systematic and experimental analysis of their genes, genomes, mRNA and proteins; and perspective to next generation sequencing. Crustaceana 2019, 92, 1169–1205. [Google Scholar] [CrossRef]
- Feng, Q.M.; Liu, M.M.; Cheng, Y.X.; Wu, X.G. Comparative proteomics elucidates the dynamics of ovarian development in the Chinese mitten crab Eriocheir sinensis. Comp. Biochem. Physiol. D Genom. Proteom. 2021, 40, 100878. [Google Scholar] [CrossRef] [PubMed]
- Montes-Dominguez, A.L.; Avena-Soto, J.A.; Lizarraga-Rodriguez, J.L.; Perez-Gala, R.J.; Jimenez-Gutierrez, S.; Sotelo-Falomir, J.A.; Pinzon-Miranda, F.M.; Martinez-Perez, F.; Muñoz-Rubi, H.A.; Chavez-Herrera, D.; et al. Comparison between cultured and wild Pacific white shrimp (Penaeus vannamei) vitellogenesis: Next-generation sequencing and relative expression of genes directly and indirectly related to reproduction. PeerJ 2021, 9, e10694. [Google Scholar] [CrossRef]
- Zmora, N.; Trant, J.; Chang, S.; Chung, J. Vitellogenin and its messenger RNA during ovarian development in the female blue crab, Callinectes sapidus: Gene expression, synthesis, transport, and cleavage. Biol. Reprod. 2007, 77, 138–146. [Google Scholar] [CrossRef]
- Jimenez-Gutierrez, L.R. Female reproduction-specific proteins, origins in marine species, and their evolution in the animal kingdom. J. Bioinform. Comput. Biol. 2022, 20, 2240001. [Google Scholar] [CrossRef]
- Castañeda-Fernández de Lara, V.; Gómez-Rojo, C.; Castro-Salgado, J.; García-Borbón, J. Buenas prácticas de pesca de jaiba guerrera Callinectes bellicosus en Baja California Sur, México. Cienc. Pesq. 2015, 23, 53–64. [Google Scholar]
- Ramírez-Félix, E.; Singh-Cabanillas, J.; Gil, H.; Sarmiento, S.; Salazar, I.; Montemayor, G.; García, J.; Rodríguez, G.; Castañeda, N. La Pesquería de Jaiba (Callinectes spp.) en el Pacífico Mexicano: Diagnóstico y Propuesta de Regulación; Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación: Ciudad de México, Mexico, 2003; p. 55.
- Thongda, W.; Chung, J.S.; Tsutsui, N.; Zmora, N.; Katenta, A. Seasonal variations in reproductive activity of the blue crab, Callinectes sapidus: Vitellogenin expression and levels of vitellogenin in the hemolymph during ovarian development. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 179, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Wei, H.; Conradt, B. Caenorhabditis elegans ced-3 caspase is required for asymmetric divisions that generate cells programmed to die. Genetics 2018, 210, 983–998. [Google Scholar] [CrossRef]
- Yu, Z.; Geng, Y.; Huang, A.; Wang, K.; Huang, X.; Chen, D.; Ou, Y.; Wang, J. Molecular characterization of a p38 mitogen-activated protein kinase gene from Scylla paramamosain and its expression profiles during pathogenic challenge. J. Invertebr. Pathol. 2017, 144, 32–36. [Google Scholar] [CrossRef]
- Benzhen, L.; Shucheng, S.; Chenchang, B.; Zhaoxia, C.; Yanan, Y. Transcriptome analysis elucidates mating affects the expression of intra-/extra-ovarian factors, thereby influencing ovarian development in the mud crab Scylla paramamosain. Comp. Biochem. Physiol. D Genom. Proteom. 2024, 52, 101334. [Google Scholar] [CrossRef]
- Hansen, K.; Varvas, K.; Järving, I.; Samel, N. Novel membrane-associated prostaglandin E synthase-2 from crustacean arthropods. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 174, 45–52. [Google Scholar] [CrossRef]
- Zheng, J.; Cheng, S.; Jia, Y.; Gu, Z.; Li, F.; Chi, M.; Liu, S.; Jiang, W. Molecular identification and expression profiles of four splice variants of Sex-lethal gene in Cherax quadricarinatus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 234, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Wan, H.; Zhang, Z.; Lin, J.; Wang, Y. Genome-wide identification and expression profile of the sox gene family in different tissues and during embryogenesis in the Pacific white shrimp (Litopenaeus vannamei). Gene 2020, 763, 144956. [Google Scholar] [CrossRef]
- Lan, H.; Wang, X.; Jiang, L.; Wu, J.; Wan, X.; Zeng, L.; Zhang, D.; Lin, Y.; Hou, C.; Wu, S.; et al. An extracellular matrix protein promotes anillin-dependent processes in the Caenorhabditis elegans germline. Life Sci. Alliance 2019, 2, e201800152. [Google Scholar] [CrossRef] [PubMed]
- Akerberg, B.N.; Pu, W.T. Genetic and Epigenetic Control of Heart Development. Cold Spring Harb. Perspect. Biol. 2020, 12, a036756. [Google Scholar] [CrossRef]
- Dearden, P.K. Origin and evolution of the enhancer of split complex. BMC Genom. 2015, 16, 712. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, J.; Angerer, L.M.; Inaba, K.; Yaguchi, S. Zinc finger homeobox is required for the differentiation of serotonergic neurons in the sea urchin embryo. Dev. Biol. 2012, 363, 74–83. [Google Scholar] [CrossRef]
- Neves, S.R.; Ram, P.T.; Iyengar, R. G protein pathways. Science 2002, 296, 1636–1639. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Ryan, L.W.; Nocillado, J.; Le Groumellec, M.; Elizur, A.; Ventura, T. Transcriptomic changes across vitellogenesis in the black tiger prawn (Penaeus monodon), neuropeptides and G protein-coupled receptors repertoire curation. Gen. Comp. Endocrinol. 2020, 298, 113585. [Google Scholar] [CrossRef]
- Jayasankar, V.; Tsutsui, N.; Jasmani, S.; Saido-Sakanaka, H.; Yang, W.J.; Okuno, A.; Hien, T.T.T.; Aida, K.; Wilder, M.N. Dynamics of vitellogenin mRNA expression and changes in hemolymph vitellogenin levels during ovarian maturation in the giant freshwater prawn Macrobrachium rosenbergii. J. Exp. Zool. 2002, 293, 675–682. [Google Scholar] [CrossRef]
- Auttarat, J.; Phiriyangkul, P.; Utarabhand, P. Characterization of vitellin from the ovaries of the banana shrimp Litopenaeus merguiensis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 143, 27–36. [Google Scholar] [CrossRef]
- Jia, X.; Chen, Y.; Zou, Z.; Lin, P.; Wang, Y.; Zhang, Z. Characterization and expression profile of Vitellogenin gene from Scylla paramamosain. Gene 2013, 520, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Kung, S.Y.; Chan, S.M.; Hui, J.H.L.; Tsang, W.S.; Mak, A.; He, J.G. Vitellogenesis in the Sand Shrimp, Metapenaeus ensis: The Contribution from the Hepatopancreas-Specific Vitellogenin Gene (MeVg2). Biol. Reprod. 2004, 71, 863–870. [Google Scholar] [CrossRef]
- Ferré, L.E.; Medesani, D.A.; García, C.F.; Grodzielski, M.; Rodríguez, E.M. Vitellogenin levels in hemolymph, ovary and hepatopancreas of the freshwater crayfish Cherax quadricarinatus (Decapoda: Parastacidae) during the reproductive cycle. Rev. Biol. Trop. 2012, 60, 253–261. [Google Scholar] [CrossRef]
- Bembe, S.; Zmora, N.; Williams, E.; Place, A.; Liang, D.; Chung, J. Effects of temperature and photoperiod on hemolymph vitellogenin levels during spawning events of the blue crab, Callinectes sapidus, in captivity. Aquac. Res. 2018, 49, 2201–2209. [Google Scholar] [CrossRef]
- Bai, H.; Qiao, H.; Li, F.; Fu, H.; Sun, S.; Zhang, W.; Jin, S.; Gong, Y.; Jiang, S.; Xiong, Y. Molecular characterization and developmental expression of vitellogenin in the oriental river prawn Macrobrachium nipponense and the effects of RNA interference and eyestalk ablation on ovarian maturation. Gene 2015, 562, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Phiriyangkul, P.; Puengyam, P.; Jakobsen, I.B.; Utarabhand, P. Dynamics of vitellogenin mRNA expression during vitellogenesis in the banana shrimp Penaeus (Fenneropenaeus) merguiensis using real-time PCR. Mol. Reprod. Dev. 2007, 74, 1198–1207. [Google Scholar] [CrossRef]
- Perdichizzi, A.; Pirrera, L.; Micale, V.; Muglia, U.; Rinelli, P. A Histological study of ovarian development in the giant red shrimp Aristaeomorpha foliacea (Crustacea: Decapoda: Aristeidae) from the Southern Tyrrhenian Sea (Western Mediterranean). Sci. World J. 2012, 2012, 289608. [Google Scholar] [CrossRef]
- Pérez-Ferro, D.G.; Paramo-Granados, J.E. Maturity stages of pink shrimp Farfantepenaeus notialis (Penaeidae) in the colombian Caribbean. Acta Biol. Colomb. 2014, 19, 185–194. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Rotllant, G.E.; Cummins, S.F.; Elizur, A.; Tomer, T. Insights into sexual maturation and reproduction in the Norway lobster (Nephrops norvegicus) via in silico prediction and characterization of neuropeptides and G protein-coupled receptors. Front. Endocrinol. 2018, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Shamsudin, M.I.; Kang, Y.; Lili, Z.; Tan, T.T.; Kwong, Q.B.; Liu, H.; Zhang, G.; Othman, R.Y.; Bhassu, S. In-depth transcriptomic analysis on giant freshwater prawns. PLoS ONE 2013, 8, e60839. [Google Scholar] [CrossRef] [PubMed]
- Avena-Soto, J.A.; Montes-Dominguez, A.L. Expresión de la Vtg, RPro, PDO y FaMet en los Estadios Gonadales del Camarón Blanco Penaeus vannamei (Boone, 1931) en Medio Silvestre y Cultivo. College Thesis, Universidad Autonoma de Sinaloa, Culiacán, Mexico, 2022. [Google Scholar]
- Gao, J.; Wang, X.; Zou, Z.; Jia, X.; Wang, Y.; Zhang, Z. Transcriptome analysis of the differences in gene expression between testis and ovary in green mud crab (Scylla paramamosain). BMC Genom. 2014, 15, 585. [Google Scholar] [CrossRef] [PubMed]



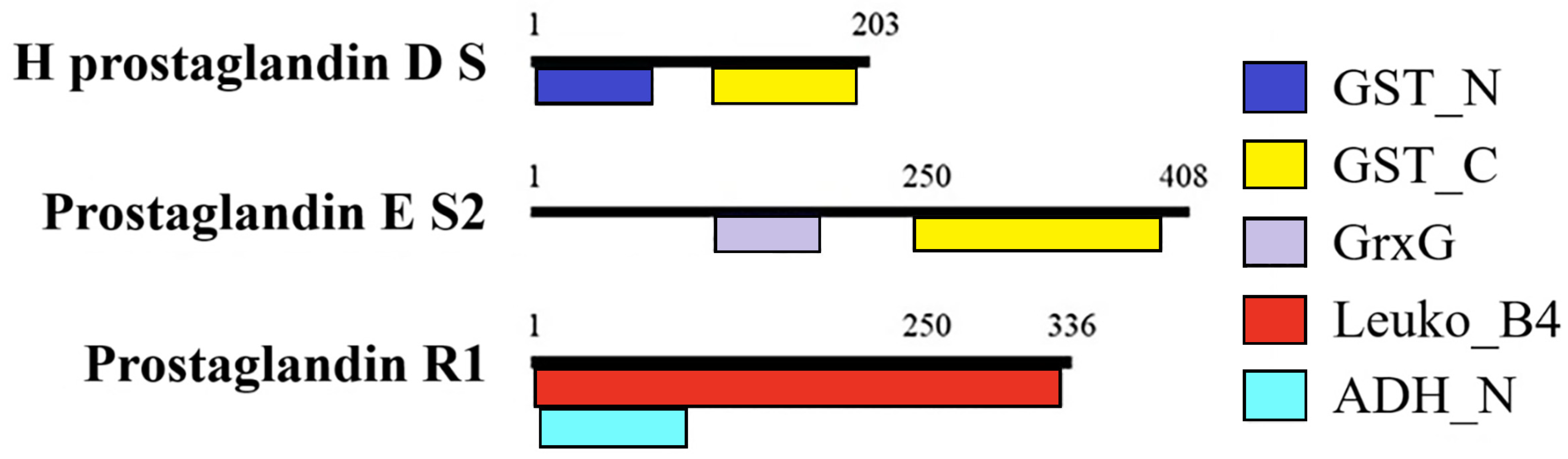
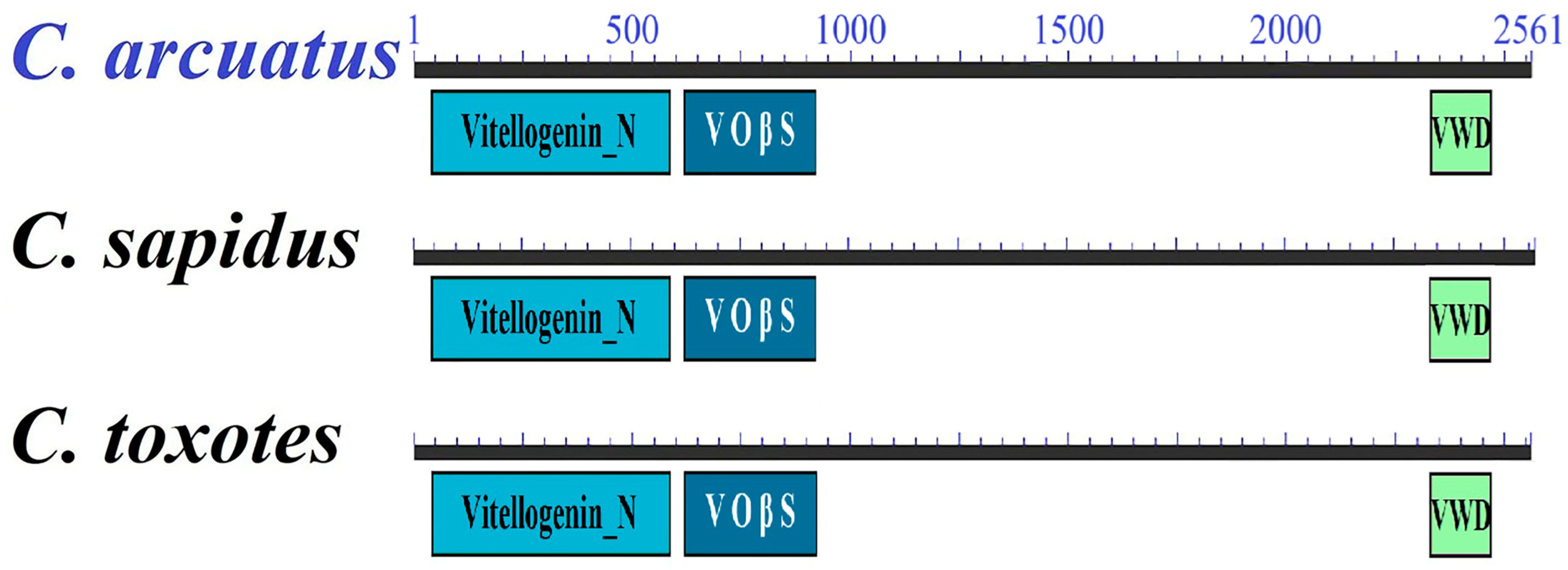
| Maturity | Vtg Relative Expression | |||||
|---|---|---|---|---|---|---|
| Stage | GSI (%) | Ovaries | Hepatopancreas | |||
| I | 0.933 | ±0.38 a | 1.006 | ±0.133 a | 1.153 | ±0.707 a |
| II | 3.074 | ±0.17 b | 0.000 | ±0.000 b | 4.2 × 107 | ±8.2 × 106 b |
| III | 5.934 | ±0.51 c | 0.060 | ±0.006 b | 1.8 × 108 | ±7.3 × 107 b |
| IV | 8.201 | ±0.31 d | 0.010 | ±0.002 b | 1.03 × 103 | ±318 × 18 c |
| V | 11.142 | ±1.02 e | 0.000 | ±0.000 b | 6.5 × 1013 | ±1.5 × 1012 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes-Dominguez, A.L.; Avena-Soto, J.A.; Borrego, M.I.; Jimenez-Gutierrez, L.R. Characterization of Maturation-Associated Genes in Ovary–Hepatopancreas Transcriptome and Vitellogenin Expression in Pacific Blue Swimming Crab Callinectes arcuatus During Gonadal Maturity Stages. Animals 2025, 15, 2860. https://doi.org/10.3390/ani15192860
Montes-Dominguez AL, Avena-Soto JA, Borrego MI, Jimenez-Gutierrez LR. Characterization of Maturation-Associated Genes in Ovary–Hepatopancreas Transcriptome and Vitellogenin Expression in Pacific Blue Swimming Crab Callinectes arcuatus During Gonadal Maturity Stages. Animals. 2025; 15(19):2860. https://doi.org/10.3390/ani15192860
Chicago/Turabian StyleMontes-Dominguez, Araceli Lorena, Jesus Arian Avena-Soto, Martin Ignacio Borrego, and Laura Rebeca Jimenez-Gutierrez. 2025. "Characterization of Maturation-Associated Genes in Ovary–Hepatopancreas Transcriptome and Vitellogenin Expression in Pacific Blue Swimming Crab Callinectes arcuatus During Gonadal Maturity Stages" Animals 15, no. 19: 2860. https://doi.org/10.3390/ani15192860
APA StyleMontes-Dominguez, A. L., Avena-Soto, J. A., Borrego, M. I., & Jimenez-Gutierrez, L. R. (2025). Characterization of Maturation-Associated Genes in Ovary–Hepatopancreas Transcriptome and Vitellogenin Expression in Pacific Blue Swimming Crab Callinectes arcuatus During Gonadal Maturity Stages. Animals, 15(19), 2860. https://doi.org/10.3390/ani15192860






