Transcriptome Analysis of Potential Genes Involved in Innate Immunity in Mudflat Crab (Helice tientsinensis)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Samples
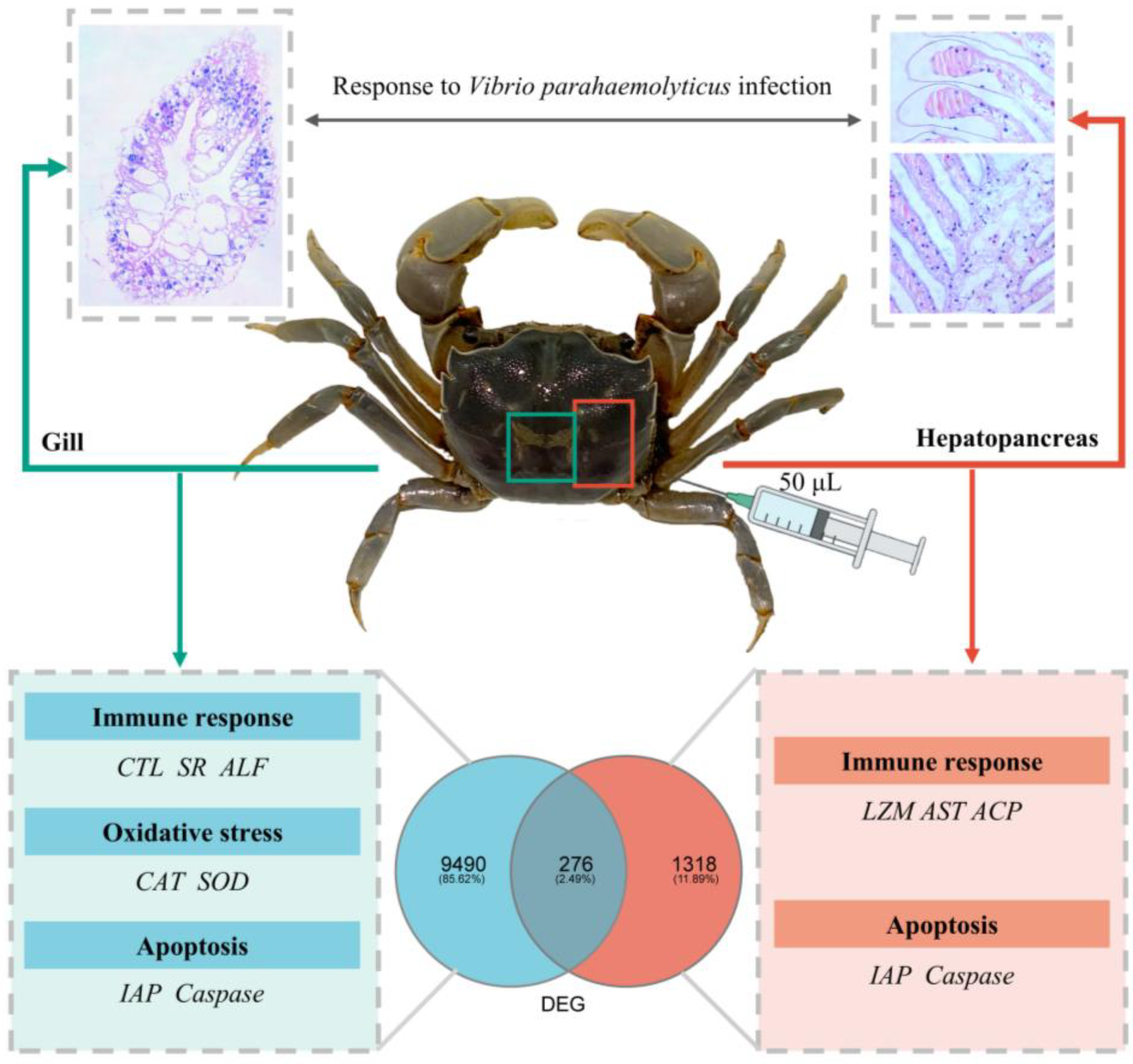
2.2. V. parahaemolyticus Infection and Sample Collection
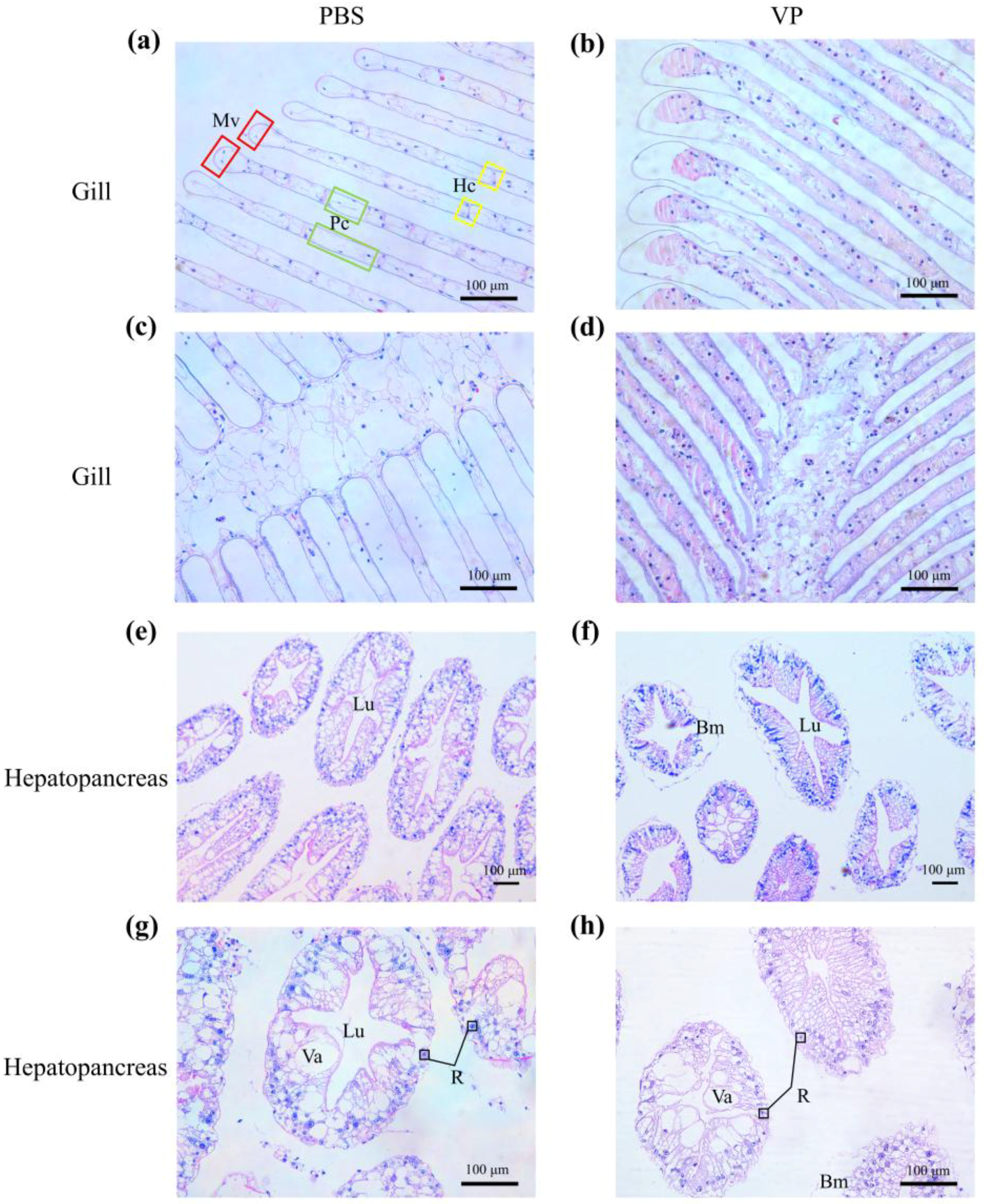
2.3. Histopathological Investigation
2.4. RNA Extraction, cDNA Library Construction, and Sequencing
2.5. Transcriptome Assembly and Functional Annotation
2.6. Analysis of DEGs
2.7. qRT-PCR Validation
3. Results
3.1. Histopathological Evaluation
3.2. De Novo Assembly and Annotation of Unigenes
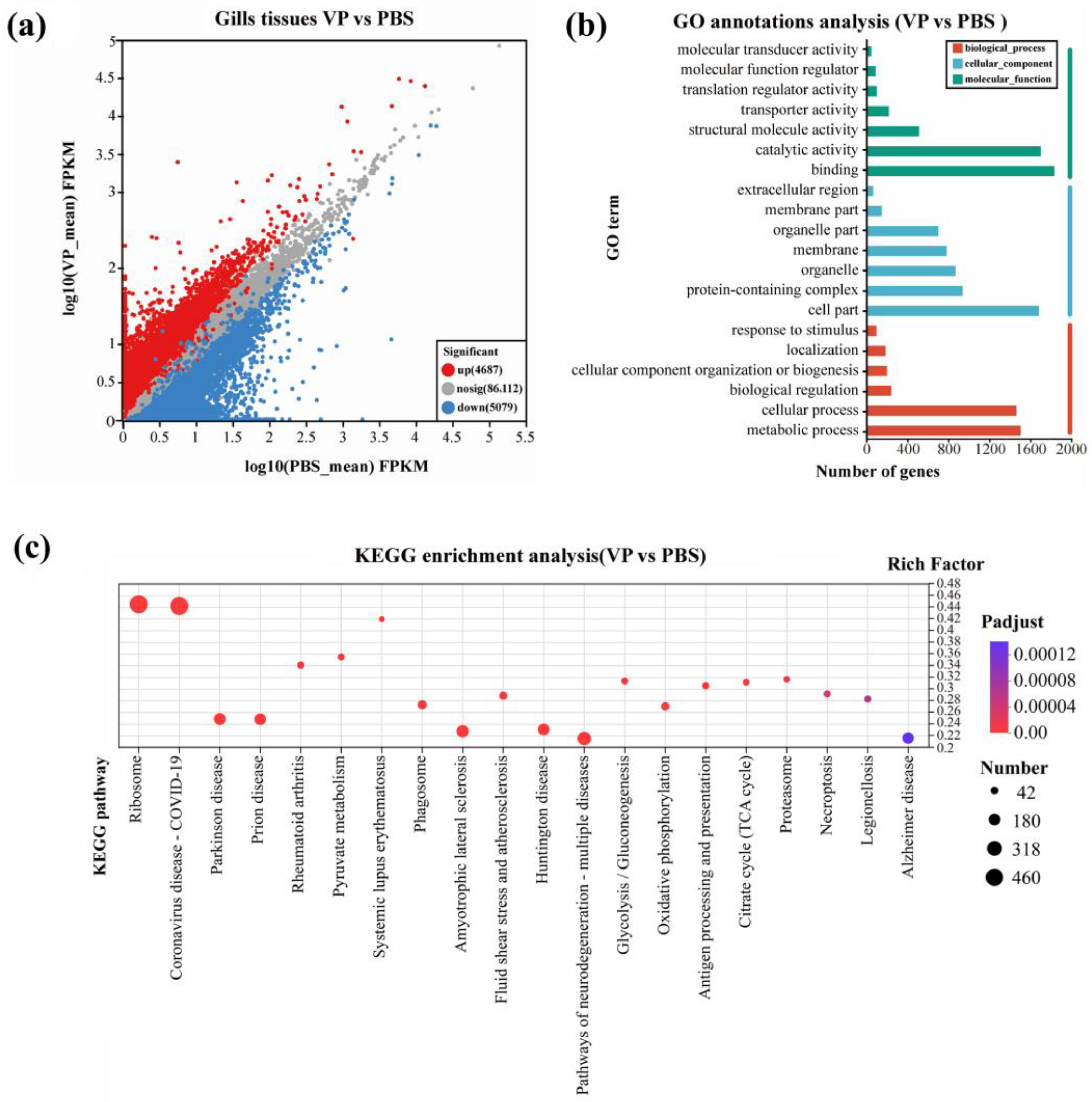
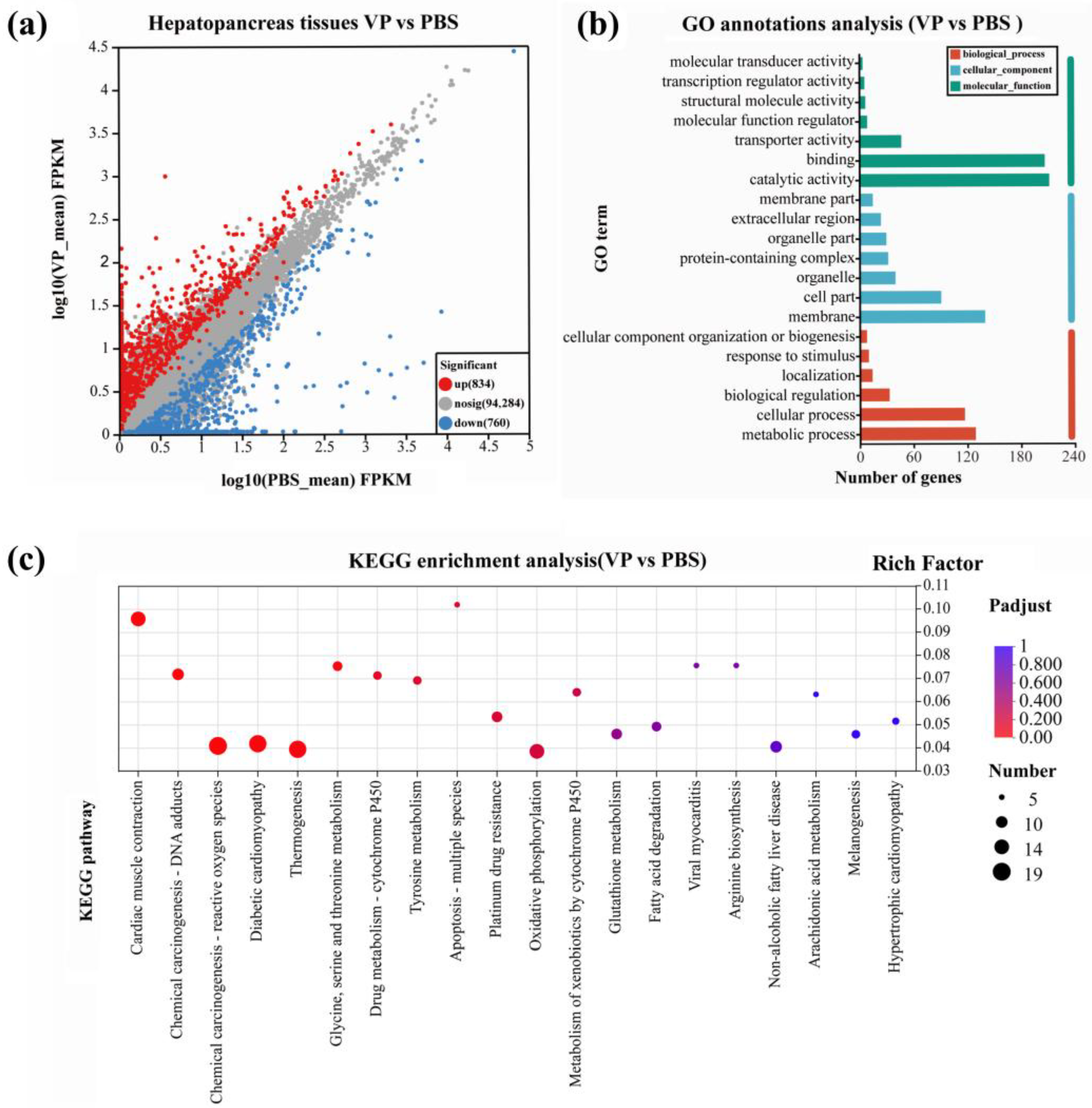
3.3. Classification and Analysis of DEGs
3.4. GO and KEGG Analysis of DEGs
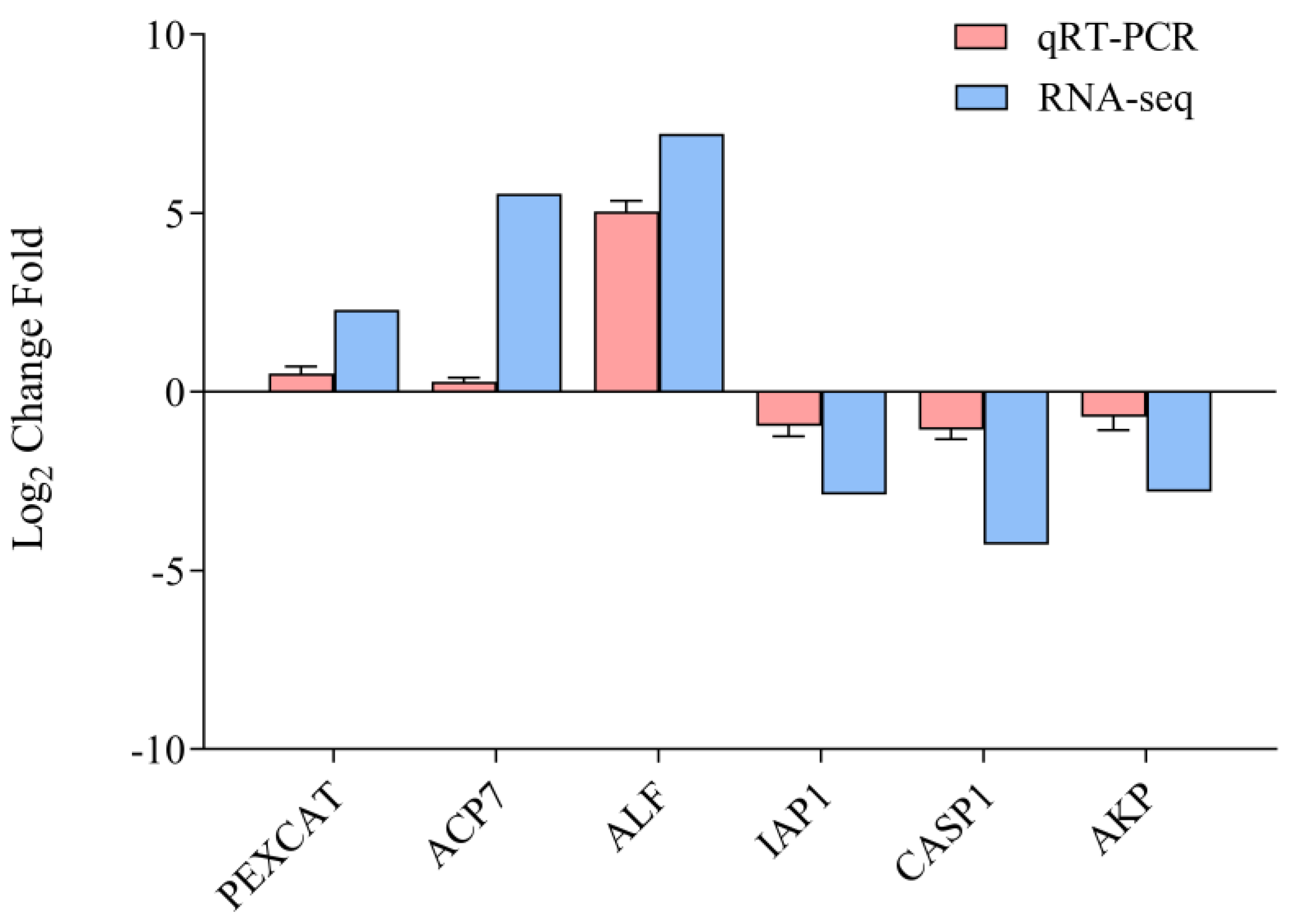
3.5. Validation of RNA-Seq Data by qRT-PCR
4. Discussion
4.1. Immune-Related Genes
4.2. Oxidative Stress-Related Genes
4.3. Genes Related to Apoptosis and Pyroptosis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, D.; Ding, G.; Zhang, H.; Tang, B. Isolation and characterization of 10 microsatellite markers in Helice tientsinensis (Brachyura: Varunidae). Conserv. Genet. Resour. 2009, 1, 321–323. [Google Scholar] [CrossRef]
- Zhang, L.; Lan, S.; Angelini, C.; Yi, H.; Zhao, L.; Chen, L.; Han, G. Interactive effects of crab herbivory and spring drought on a Phragmites australis-dominated salt marsh in the Yellow River Delta. Sci. Total Environ. 2021, 766, 144254. [Google Scholar] [CrossRef]
- Qiu, D.; Yan, J.; Ma, X.; Luo, M.; Wang, Q.; Cui, B. Microtopographical modification by a herbivore facilitates the growth of a coastal saltmarsh plant. Mar. Pollut. Bull. 2019, 140, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yan, C.; Gao, C.; Xu, H.; Hua, E.; Liu, X. Seasonal Distribution of Meiofaunal Assemblages in the Mangrove Tidal Flat of Futian, Shenzhen, China. J. Ocean Univ. China 2022, 21, 955–964. [Google Scholar] [CrossRef]
- Chen, Z.; Li, B.; Zhong, Y.; Chen, J. Local competitive effects of introduced Spartina alterniflora on Scirpus mariqueter at Dongtan of Chongming Island, the Yangtze River estuary and their potential ecological consequences. Hydrobiologia 2004, 528, 99–106. [Google Scholar] [CrossRef]
- Elmer, W.H. A Tripartite Interaction Between Spartina alterniflora, Fusarium palustre, and the Purple Marsh Crab (Sesarma reticulatum) Contributes to Sudden Vegetation Dieback of Salt Marshes in New England. Phytopathology 2014, 104, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.Q.; Zhang, L.W.; Yi, H.P.; Xu, C.L.; Lu, F.; Feng, G.H.; Han, G.X. Food source and feeding habit of Helice tientsinensis from the common reed vegetation in high marsh of Yellow River Delta, China. Chin. J. Appl. Ecol. 2020, 31, 319–325. [Google Scholar] [CrossRef]
- Reinsel, K.A. Impact of fiddler crab foraging and tidal inundation on an intertidal sandflat: Season-dependent effects in one tidal cycle. J. Exp. Mar. Biol. Ecol. 2004, 313, 1–17. [Google Scholar] [CrossRef]
- Webb, A.P.; Eyre, B.D. The effect of natural populations of the burrowing and grazing soldier crab (Mictyris longicarpus) on sediment irrigation, benthic metabolism and nitrogen fluxes. J. Exp. Mar. Biol. Ecol. 2004, 309, 1–19. [Google Scholar] [CrossRef]
- Meziane, T.; Dagata, F.; Lee, S. Fate of mangrove organic matter along a subtropical estuary: Small-scale exportation and contribution to the food of crab communtities. Mar. Ecol. Prog Ser. 2006, 312, 15–27. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Chen, L.; Lloyd, H.; Zhang, Z.W. Burrow ambient temperature influences Helice crab activity and availability for migratory Red-crowned cranes Grus japonensis. Ecol. Evol. 2020, 10, 11523–11534. [Google Scholar] [CrossRef]
- Huang, Z.S.; Aweya, J.J.; Zhu, C.H.; Tran, N.T.; Hong, Y.J.; Li, S.K.; Yao, D.F.; Zhang, Y.L. Modulation of crustacean innate immune response by amino acids and their metabolites: Inferences from other species. Front. Immunol. 2020, 11, 574721. [Google Scholar] [CrossRef]
- Fajardo, C.; Martinez-Rodriguez, G.; Costas, B.; Mancera, J.M.; Fernandez-Boo, S.; Rodulfo, H.; De Donato, M. Shrimp immune response: A transcriptomic perspective. Rev. Aquacult. 2022, 14, 1136–1149. [Google Scholar] [CrossRef]
- Goulden, E.F.; Hall, M.R.; Bourne, D.G.; Pereg, L.L.; Hoj, L. Pathogenicity and Infection Cycle of Vibrio owensii in larviculture of the Ornate Spiny Lobster (Panulirus ornatus). Appl. Environ. Microbiol. 2012, 78, 2841–2849. [Google Scholar] [CrossRef]
- Lafferty, K.D.; Harvell, C.D.; Conrad, J.M.; Friedman, C.S.; Kent, M.L.; Kuris, A.M.; Powell, E.N.; Rondeau, D.; Saksida, S.M. Infectious Diseases Affect Marine Fisheries and Aquaculture Economics. Annu. Rev. Mar. Sci. 2015, 7, 471–496. [Google Scholar] [CrossRef]
- Ma, S.; Kim, A.; Lee, W.; Kim, S.; Lee, S.; Yoon, D.; Bae, J.-S.; Park, C.-I.; Kim, S. Vibrio harveyi Infection Significantly Alters Amino Acid and Carbohydrate Metabolism in Whiteleg Shrimp, Litopenaeus vannamei. Metabolites 2020, 10, 265. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.F.; Han, Z.R.; Chen, F.; Lv, A.J.; Hu, X.C.; Sun, X.L.; Qi, H.L.; Guo, Y.J. Vibrio parahaemolyticus alters the community composition and function of intestinal microbiota in Pacific white shrimp, Penaeus vannamei. Aquaculture 2021, 544, 737061. [Google Scholar] [CrossRef]
- Han, J.E.; Tang, K.F.J.; Tran, L.H.; Lightner, D.V. Photorhabdus insect-related (Pir) toxin-like genes in a plasmid of Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease (AHPND) of shrimp. Dis. Aquat. Org. 2015, 113, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.F.; Dai, T.M.; Zhong, S.Q.; Jin, M.; Sun, P.; Zhou, Q.C. Vibrio parahaemolyticus infection impaired intestinal barrier function and nutrient absorption in Litopenaeus vannamei. Fish Shellfish Immunol. 2020, 99, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.D.; Xin, Y.T.; Teng, J.; Zhao, X.D.; Lu, J.B.; Li, Y.B.; Wang, H. Comparative transcriptomic and molecular biology analyses to explore potential immune responses to Vibrio parahaemolyticus challenge in Eriocheir sinensis. Front. Cell. Infect. Microbiol. 2024, 14, 1456130. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.L.; Feng, Y.; Duan, H.M.; Zhao, L.; Yang, C.; Geng, Y.; Ouyang, P.; Chen, D.F.; Yin, L.Z.; Yang, S.Y. Evaluation of pathology and environmental variables contributing to hepatopancreatic necrosis syndrome of chinese mitten crab, Eriocheir sinensis. Ecotox. Environ. Saf. 2021, 215, 112157. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.R.; Song, C.W.; Ning, J.H.; Liu, Y.; Cui, Z.X. Identification, functional characterization and the potential role of variable lymphocyte receptor EsVLRA from Eriocheir sinensis in response to secondary challenge after Vibrio parahaemolyticus vaccine. Fish Shellfish Immunol. 2020, 98, 201–209. [Google Scholar] [CrossRef]
- Sun, D.; Lv, J.; Li, Y.; Wu, J.; Liu, P.; Gao, B. Comparative Transcriptome Analysis of the Response to Vibrio parahaemolyticus and Low-Salinity Stress in the Swimming Crab Portunus trituberculatus. Biology 2023, 12, 1518. [Google Scholar] [CrossRef]
- Cheng, C.H.; Liu, X.Z.; Ma, H.L.; Liu, G.X.; Deng, Y.Q.; Feng, J.; Jie, Y.K.; Guo, Z.X. The role of caspase 3 in the mud crab (Scylla paramamosain) after Vibrio parahaemolyticus infection. Fish Shellfish Immunol. 2021, 118, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Letchumanan, V.; Yin, W.F.; Lee, L.H.; Chan, K.G. Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticus isolated from retail shrimps in Malaysia. Front. Microbiol. 2015, 6, 33. [Google Scholar] [CrossRef]
- Lopez-Joven, C.; de Blas, I.; Furones, M.D.; Roque, A. Prevalences of pathogenic and non-pathogenic Vibrio parahaemolyticus in mollusks from the Spanish Mediterranean Coast. Front. Microbiol. 2015, 6, 736. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Cao, L.P.; Du, J.L.; Gao, J.C.; Zhang, Y.N.; Sun, Y.; Li, Q.J.; Nie, Z.J.; Xu, G.H. Effects of Prometryn Exposure on Hepatopancreas Oxidative Stress and Intestinal Flora in Eriocheir sinensis (Crustacea: Decapoda). Antioxidants 2023, 12, 1548. [Google Scholar] [CrossRef]
- Cervellione, F.; McGurk, C.; Silva, P.; Owen, M.A.G.; Van den Broeck, W. Optimization of fixation methods for image analysis of the hepatopancreas in whiteleg shrimp, Penaeus vannamei (Boone). J. Fish Dis. 2016, 40, 517–527. [Google Scholar] [CrossRef]
- Ching, T.; Huang, S.; Garmire, L.X. Power analysis and sample size estimation for RNA-Seq differential expression. RNA 2014, 20, 1684–1696. [Google Scholar] [CrossRef]
- Chen, L.; Hua, Y.; Ji, W.; Wang, J.; Zhao, H.; Wang, Z. Cloning, characterization, and expression analysis of the CHITINASE gene family in Helice tientsinensis. PeerJ 2023, 11, 15045. [Google Scholar] [CrossRef]
- Persch, T.S.P.; Weimer, R.N.; Freitas, B.S.; Oliveira, G.T. Metabolic parameters and oxidative balance in juvenile Rhamdia quelen exposed to rice paddy herbicides: Roundup, primoleo, and facet. Chemosphere 2017, 174, 98–109. [Google Scholar] [CrossRef]
- Henry, R.P.; Lucu, C.; Onken, H.; Weihrauch, D. Multiple functions of the crustacean gill: Osmotic/ionic regulation, acid-base balance, ammonia excretion, and bioaccumulation of toxic metals. Front. Physiol. 2012, 3, 431. [Google Scholar] [CrossRef]
- Tang, D.; Shi, X.L.; Guo, H.Y.; Bai, Y.Z.; Shen, C.C.; Zhang, Y.P.; Wang, Z.F. Comparative transcriptome analysis of the gills of Procambarus clarkii provides novel insights into the immune-related mechanism of copper stress tolerance. Fish Shellfish Immunol. 2020, 96, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Huang, J.J.; Dahms, H.U.; Zhen, J.J.; Ying, X.P. Cell damage and apoptosis in the hepatopancreas of Eriocheir sinensis induced by cadmium. Aquat. Toxicol. 2017, 190, 190–198. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.Y.; Kholodkevich, S.; Sharov, A.; Feng, Y.J.; Ren, N.Q.; Sun, K. Cadmium-induced oxidative stress, histopathology, and transcriptome changes in the hepatopancreas of freshwater crayfish (Procambarus clarkii). Sci. Total Environ. 2019, 666, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Ma, H.L.; Deng, Y.Q.; Feng, J.; Jie, Y.K.; Guo, Z.X. Effects of Vibrio parahaemolyticus infection on physiological response, histopathology and transcriptome changes in the mud crab (Scylla paramamosain). Fish Shellfish Immunol. 2020, 106, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.J.; Xiao, L.; Mo, A.J.; Yuan, Y.C.; Yuan, J.F.; Gu, Z.M.; Wang, J.H. Effect of dietary bacillus subtilis supplement on cd toxicokinetics and cd-induced immune and antioxidant impairment of Procambarus clarkii. Environ. Sci. Pollut. Res. 2023, 30, 43914–43926. [Google Scholar] [CrossRef]
- Fu, L.L.; Zhou, G.; Pan, J.L.; Li, Y.H.; Lu, Q.P.; Zhou, J.; Li, X.G. Effects of Astragalus polysaccharides on antioxidant abilities and non-specific immune responses of chinese mitten crab, Eriocheir sinensis. Aquacult. Int. 2017, 25, 1333–1343. [Google Scholar] [CrossRef]
- Peters, C.B.; Urich, K.; Pollwein, R.; Grzeschik, K.H.; Sippel, A. The human lysozyme gene. Sequence organization and chromosomal localization. Eur. J. Biochem. 1989, 182, 507–516. [Google Scholar] [CrossRef]
- Tassanakajon, A.; Somboonwiwat, K.; Supungul, P.; Tang, S. Discovery of immune molecules and their crucial functions in shrimp immunity. Fish Shellfish Immunol. 2013, 34, 954–967. [Google Scholar] [CrossRef]
- Liu, H.P.; Jiravanichpaisal, P.; Soderhall, I.; Cerenius, L.; Soderhall, K. Antilipopolysaccharide Factor Interferes with White Spot Syndrome Virus Replication In Vitro and In Vivo in the Crayfish Pacifastacus leniusculus. J. Virol. 2006, 80, 10365–10371. [Google Scholar] [CrossRef]
- Sun, B.; Wang, Z.; Zhu, F. The crustin-like peptide plays opposite role in shrimp immune response to Vibrio alginolyticus and white spot syndrome virus (WSSV) infection. Fish Shellfish Immunol. 2017, 66, 487–496. [Google Scholar] [CrossRef]
- Hamilton, A.; Harrington, D.; Sutcliffe, I.C. Characterization of Acid Phosphatase Activities in the Equine Pathogen Streptococcus equi. Syst. Appl. Microbiol. 2000, 23, 325–329. [Google Scholar] [CrossRef]
- Huang, H.T.; Hu, Y.F.; Lee, B.H.; Huang, C.Y.; Lin, Y.R.; Huang, S.N.; Chen, Y.Y.; Chang, J.J.; Nan, F.H. Dietary of Lactobacillus paracasei and Bifidobacterium longum improve nonspecific immune responses, growth performance, and resistance against Vibrio parahaemolyticus in Penaeus vannamei. Fish Shellfish Immunol. 2022, 128, 307–315. [Google Scholar] [CrossRef]
- Guan, T.Y.; Feng, J.B.; Zhu, Q.Q.; Wang, L.; Xie, P.; Wang, H.; Li, J.L. Effects of abamectin on nonspecific immunity, antioxidation, and apoptosis in red swamp crayfish (Procambarus clarkii). Fish Shellfish Immunol. 2023, 142, 109137. [Google Scholar] [CrossRef]
- Stara, A.; Zuskova, E.; Kouba, A.; Velisek, J. Effects of terbuthylazine-desethyl, a terbuthylazine degradation product, on red swamp crayfish (Procambarus clarkii). Sci. Total Environ. 2016, 566–567, 733–740. [Google Scholar] [CrossRef]
- Wei, K.; Yang, J. Oxidative damage of hepatopancreas induced by pollution depresses humoral immunity response in the freshwater crayfish Procambarus clarkii. Fish Shellfish Immunol. 2015, 43, 510–519. [Google Scholar] [CrossRef]
- Wang, C.; Li, P.F.; Hu, D.G.; Wang, H. Effect of Clostridium butyricum on intestinal microbiota and resistance to Vibrio alginolyticus of Penaeus vannamei. Fish Shellfish Immunol. 2023, 138, 108790. [Google Scholar] [CrossRef] [PubMed]
- Gough, P.J.; Siamon, G. The role of scavenger receptors in the innate immune system. Microbes. Infect. 2000, 2, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.L. Inflammation, atherosclerosis, and arterial thrombosis: Role of the scavenger receptor CD36. Clev. Clin. J. Med. 2009, 76, S27–S30. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, S.O.; Zhou, G.; Silverstein, R.L. Vav protein guanine nucleotide exchange factor regulates CD36 protein-mediated macrophage foam cell formation via calcium and dynamin-dependent processes. J. Biol. Chem. 2011, 286, 36011–36019. [Google Scholar] [CrossRef]
- Dambuza, I.M.; Brown, G.D. C-type lectins in immunity: Recent developments. Curr. Opin. Immunol. 2015, 32, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.X.; Li, X.J.; Yang, L.; Wang, Q.; Li, W.W. A class B scavenger receptor mediates antimicrobial peptide secretion and phagocytosis in Chinese mitten crab (Eriocheir sinensis). Dev. Comp. Immunol. 2020, 103, 103496. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Yang, L.; Li, X.J.; Li, L.; Wang, Q.; Li, W.W. A class B scavenger receptor from Eriocheir sinensis (EsSR-B1) restricts bacteria proliferation by promoting phagocytosis. Fish Shellfish Immunol. 2017, 70, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, X.; Wang, Z.; Tan, J.M.; Hui, K.M.; Wang, W.; Ren, Q. Function of two novel single-CRD containing C-type lectins in innate immunity from Eriocheir sinensis. Fish Shellfish Immunol. 2014, 37, 313–321. [Google Scholar] [CrossRef]
- Li, S.; Jia, Z.R.; Li, X.J.; Geng, X.Y.; Sun, J.S. Identification and expression analysis of lipopolysaccharide-induced TNF-alpha factor gene in Chinese mitten crab Eriocheir sinensis. Fish Shellfish Immunol. 2014, 38, 190–195. [Google Scholar] [CrossRef]
- Yang, D.; Wei, X.; Yang, J.; Yang, J.; Xu, J.; Fang, J.; Wang, S.; Liu, X. Identification of a LPS-induced tnf-α factor (LITAF) from mollusk Solen grandis and its expression pattern towards PAMPS stimulation. Fish Shellfish Immunol. 2013, 35, 1325–1328. [Google Scholar] [CrossRef]
- Wang, P.H.; Wan, D.H.; Pang, L.R.; Gu, Z.H.; Qiu, W.; Weng, S.P.; Yu, X.Q.; He, J.G. Molecular cloning, characterization and expression analysis of the tumor necrosis factor (TNF) superfamily gene, TNF receptor superfamily gene and lipopolysaccharide-induced tnf-α factor (LITAF) gene from Litopenaeus vannamei. Dev. Comp. Immunol. 2012, 36, 39–50. [Google Scholar] [CrossRef]
- Jiao, L.; Dai, T.; Zhong, S.; Jin, M.; Sun, P.; Zhou, Q. Vibrio parahaemolyticus Infection Influenced Trace Element Homeostasis, Impaired Antioxidant Function, and Induced Inflammation Response in Litopenaeus vannamei. Biol. Trace Elem. Res. 2020, 199, 329–337. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Xu, H. Autophagy-lysosome pathway in insulin & glucagon homeostasis. Front. Endocrinol. 2025, 16, 1541794. [Google Scholar] [CrossRef]
- Subash, P.; Chrisolite, B.; Sivasankar, P.; Rosalind George, M.; Vijay Amirtharaj, K.S.; Padmavathy, P.; Rani, V.; Sankar Sri Balaje, R.; Gowtham, S.; Mageshkumar, P. White feces syndrome in Penaeus vannamei is potentially an Enterocytozoon hepatopenaei (EHP) associated pathobiome origin of Vibrio spp. J. Invertebr. Pathol. 2023, 198, 107932. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2019, 54, 287–293. [Google Scholar] [CrossRef]
- Wang, H.; Li, E.; Huang, Q.; Liu, J.; Miao, Y.; Wang, X.; Qin, C.; Qin, J.; Chen, L.; Xu, H. Growth and Hepatopancreas Health of Juvenile Chinese Mitten Crab (Eriocheir sinensis) Fed Different Levels of Black Soldier Fly (Hermetia illucens) Larvae Meal for Fish Meal Replacement. Aquac. Nutr. 2024, 2024, 6625061. [Google Scholar] [CrossRef]
- Hung, M.N.; Shiomi, R.; Nozaki, R.; Kondo, H.; Hirono, I. Identification of novel copper/zinc superoxide dismutase (Cu/ZnSOD) genes in kuruma shrimp Marsupenaeus japonicus. Fish Shellfish Immunol. 2014, 40, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Qu, R.J.; Feng, M.B.; Sun, P.; Wang, Z.Y. A Comparative Study on Antioxidant Status Combined with Integrated Biomarker Response in Carassius auratus Fish Exposed to Nine Phthalates. Environ. Toxicol. 2015, 30, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Atli, G.; Canli, E.G.; Eroglu, A.; Canli, M. Characterization of antioxidant system parameters in four freshwater fish species. Ecotox. Environ. Saf. 2016, 126, 30–37. [Google Scholar] [CrossRef]
- Ren, X.Y.; Yu, X.; Gao, B.Q.; Liu, P.; Li, J. Characterization of three caspases and their pathogen-induced expression pattern in Portunus trituberculatus. Fish Shellfish Immunol. 2017, 66, 189–197. [Google Scholar] [CrossRef]
- Boatright, K.M.; Salvesen, G.S. Mechanisms of caspase activation. Curr. Opin. Cell Biol. 2003, 15, 725–731. [Google Scholar] [CrossRef]
- Reis, M.I.; Nascimento, D.S.; do Vale, A.; Silva, M.T.; dos Santos, N.M. Molecular cloning and characterisation of sea bass (Dicentrarchus labrax L.) caspase-3 gene. Mol. Immunol. 2007, 44, 774–783. [Google Scholar] [CrossRef]
- Hermann, S. Mechanisms and Genes of Cellular Suicide. Science 1995, 267, 1445–1449. [Google Scholar] [CrossRef]
- Kiss, T. Apoptosis and its functional significance in molluscs. Apoptosis 2010, 15, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Sun, J.J.; Xu, Q.S.; Lv, X.J.; Yang, W.; Wang, F.F.; Wang, Y.; Yi, Q.L.; Jia, Z.H.; Wang, L.L.; et al. An inhibitor of apoptosis protein (EsIAP1) from Chinese mitten crab Eriocheir sinensis regulates apoptosis through inhibiting the activity of Escaspase-3/7-1. Sci. Rep. 2019, 9, 20421. [Google Scholar] [CrossRef]
- Miao, E.A.; Alpuche-Aranda, C.M.; Dors, M.; Clark, A.E.; Bader, M.W.; Miller, S.I.; Aderem, A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat. Immunol. 2006, 7, 569–575. [Google Scholar] [CrossRef]
- Wang, Y.P.; Gao, W.Q.; Shi, X.Y.; Ding, J.J.; Liu, W.; He, H.B.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a Gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J.J.; Luo, T.; Zhang, X.B. White spot syndrome virus infection activates Caspase 1-mediated cell death in crustacean. Virology 2019, 528, 37–47. [Google Scholar] [CrossRef]
- Yazdi, A.S.; Guarda, G.; Riteau, N.; Drexler, S.K.; Tardivel, A.; Couillin, I.; Tschopp, J. Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1α and IL-1β. Proc. Natl. Acad. Sci. USA 2010, 107, 19449–19454. [Google Scholar] [CrossRef] [PubMed]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Kalkavan, H.; Chen, M.J.; Crawford, J.C.; Quarato, G.; Fitzgerald, P.; Tait, S.W.G.; Goding, C.R.; Green, D.R. Sublethal cytochrome c release generates drug-tolerant persister cells. Cell 2022, 185, 3356–3374. [Google Scholar] [CrossRef]
| Database | Exp_Unigene Number (Percent) | Exp_Transcript Number (Precent) | All_Unigene Number (Precent) | All_Transcript Number (Precent) |
|---|---|---|---|---|
| GO | 20,666 (22.03%) | 31,379 (21.74%) | 20,962 (21.86%) | 31,777 (21.61%) |
| KEGG | 19,386 (20.67%) | 30,061 (20.83%) | 19,668 (20.51%) | 30,445 (20.71%) |
| eggNOG | 23,773 (25.35%) | 37,263 (25.82%) | 24,074 (25.11%) | 37,679 (25.63%) |
| NR | 36,182 (38.58%) | 58,368 (40.45%) | 36,689 (38.27%) | 59,102 (40.2%) |
| Swiss-Prot | 19,161 (20.43%) | 30,194 (20.92%) | 19,375 (20.21%) | 30,501 (20.75%) |
| Pfam | 20,620 (21.99%) | 33,750 (23.39%) | 20,793 (21.69%) | 34,030 (23.15%) |
| Total_anno | 39,169 (41.76%) | 62,414 (43.25%) | 39,743 (41.45%) | 63,229 (43.01%) |
| Total | 93,791 (100%) | 144,311 (100%) | 95,878 (100%) | 147,021 (100%) |
| Immune Gene | NR Description | Regulation | log2FC |
|---|---|---|---|
| XP_050707313.1 | baculoviral IAP repeat-containing protein 7-like | up | 2.11004846858 |
| XP_050713859.1 | caspase-like | up | 1.21144456479 |
| XP_055388818.1 | peroxisomal catalase-like | up | 2.29787720409 |
| AAP93637.2 | Cu/Zn superoxide dismutase | up | 2.39786254809 |
| XP_033114365.1 | superoxide dismutase [Mn], mitochondrial-like | up | 3.26009252810 |
| XP_043643659.1 | lipopolysaccharide-induced tumor necrosis factor-alpha factor homolog | up | 1.85199931240 |
| XP_050686027.1 | C-type lectin domain family 6 member A-like | up | 1.45539557671 |
| XP_052761447.1 | scavenger receptor class F member 1-like | up | 2.80249497366 |
| XP_050689758.1 | acid phosphatase type 7-like | up | 5.54549241063 |
| CAI8058558.1 | Probable GH family 25 lysozyme 2 | up | 1.80022048962 |
| XP_050727498.1 | anti-lipopolysaccharide factor-like | up | 7.21866970762 |
| XP_050727500.1 | anti-lipopolysaccharide factor-like | up | 6.53449003998 |
| XP_026462568.1 | alanine aminotransferase 1-like | up | 1.75217166318 |
| XP_034337692.1 | aspartate aminotransferase, mitochondrial | up | 2.89772210593 |
| XP_014247769.1 | aspartate aminotransferase, mitochondrial | up | 1.57787836676 |
| Immune Gene | NR Description | Regulation | log2FC |
|---|---|---|---|
| WAS29203.1 | inhibitor of apoptosis protein 1 | down | 2.87308466464 |
| XP_050738051.1 | caspase-1-like | down | 4.26808751308 |
| XP_045105030.1 | alkaline phosphatase-like | down | 2.79366226575 |
| XP_050689758.1 | acid phosphatase type 7-like | down | 6.61045995700 |
| XP_050716204.1 | lysozyme-like | down | 1.31142731712 |
| XP_050686298.1 | baculoviral IAP repeat-containing protein 7-like | down | 6.36488561800 |
| XP_050729782.1 | aspartate aminotransferase-like | up | 1.33082013560 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Wang, M.; Zhou, M.; Fang, Y.; Ji, T.; Xia, R.; Bai, M.; Wang, Z.; Shen, J. Transcriptome Analysis of Potential Genes Involved in Innate Immunity in Mudflat Crab (Helice tientsinensis). Animals 2025, 15, 2855. https://doi.org/10.3390/ani15192855
Chen L, Wang M, Zhou M, Fang Y, Ji T, Xia R, Bai M, Wang Z, Shen J. Transcriptome Analysis of Potential Genes Involved in Innate Immunity in Mudflat Crab (Helice tientsinensis). Animals. 2025; 15(19):2855. https://doi.org/10.3390/ani15192855
Chicago/Turabian StyleChen, Lulu, Ming Wang, Mengdi Zhou, Youkun Fang, Tingting Ji, Ruyang Xia, Menglu Bai, Zhengfei Wang, and Jiafei Shen. 2025. "Transcriptome Analysis of Potential Genes Involved in Innate Immunity in Mudflat Crab (Helice tientsinensis)" Animals 15, no. 19: 2855. https://doi.org/10.3390/ani15192855
APA StyleChen, L., Wang, M., Zhou, M., Fang, Y., Ji, T., Xia, R., Bai, M., Wang, Z., & Shen, J. (2025). Transcriptome Analysis of Potential Genes Involved in Innate Immunity in Mudflat Crab (Helice tientsinensis). Animals, 15(19), 2855. https://doi.org/10.3390/ani15192855






