Dietary Bile Acid Influences the Physiological, Morphological, Lipid Metabolism-Related Responses, and Transcriptomic Profile of Hepatopancreas in High-Fat Diet-Fed Juvenile Gibel Carp (Carassius auratus gibelio)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Feed
2.3. Fish Management and Feeding Experiment
2.4. Sample Collection
2.5. Serum Biochemical Indexes
2.6. Slice Preparation and Histological Observation
2.7. RNA Extraction, Library Sequencing, and Transcriptome Assembly
2.8. Identification and Functional Annotation of Differentially Expressed Genes (DEGs)
2.9. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
2.10. Statistical Analysis of Experimental Data
3. Results
3.1. Serum Biochemical Indices in Gibel Carp Fed with Various Levels of Dietary BA
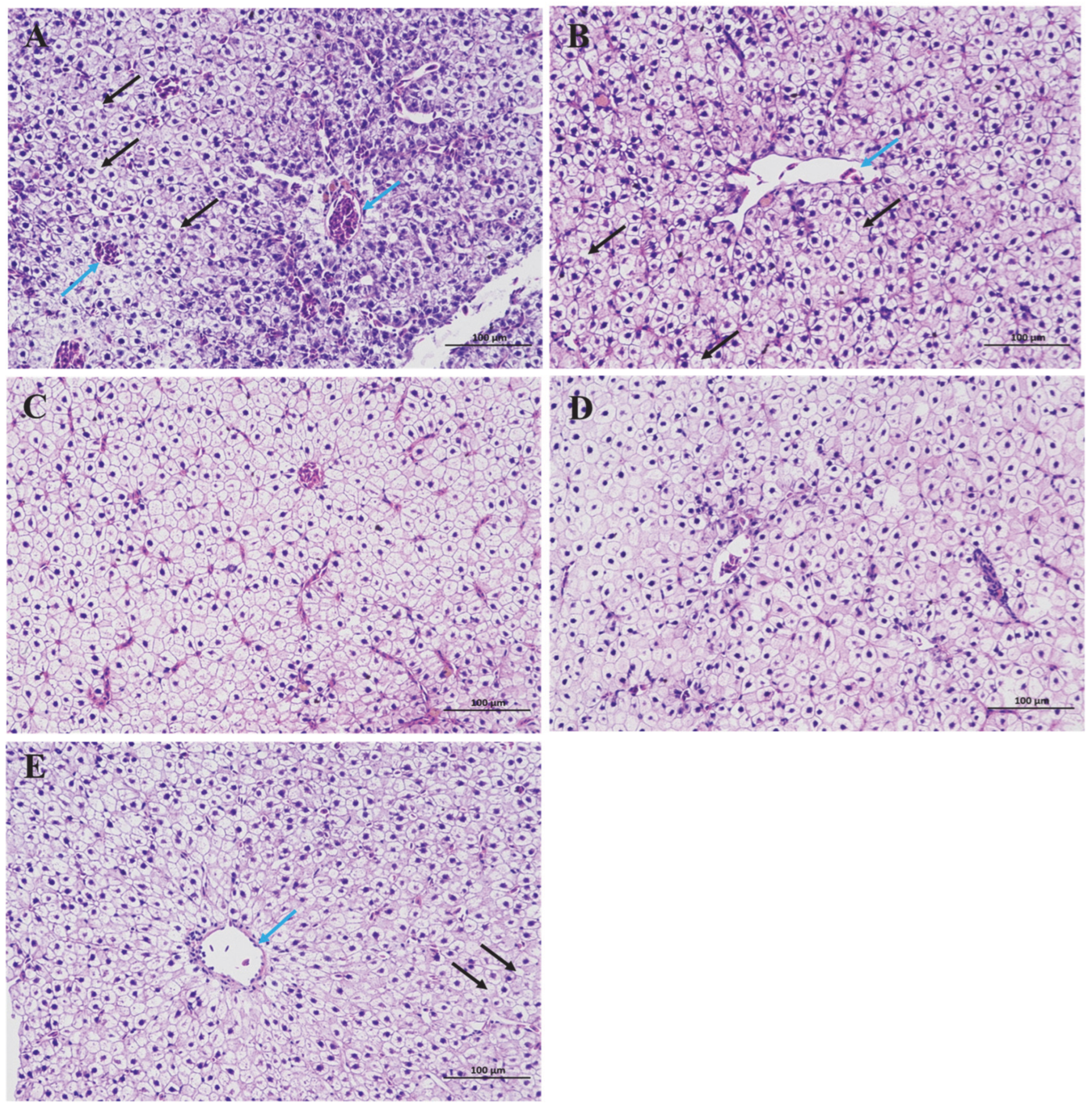
3.2. Histopathological Observation of the Hepatopancreas in Gibel Carp Fed with Various Levels of Dietary BA
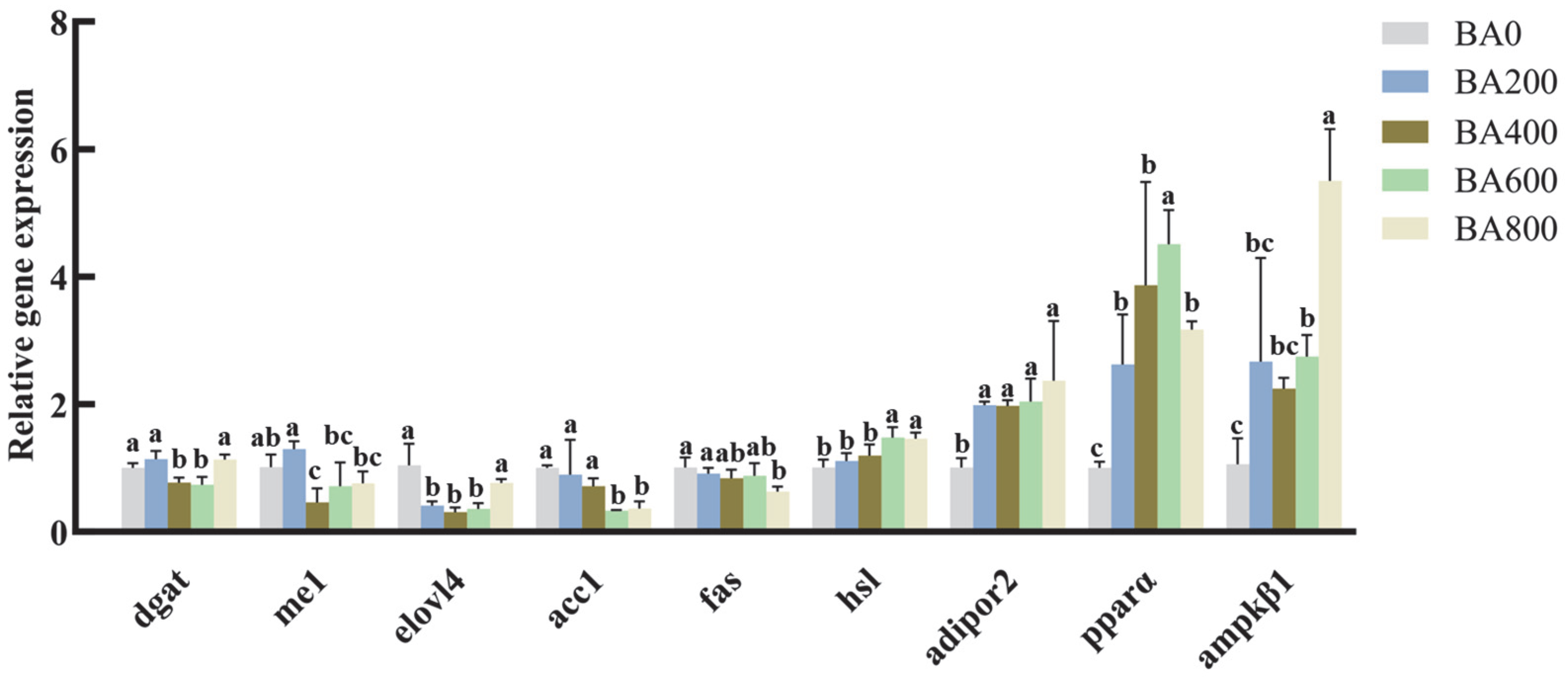
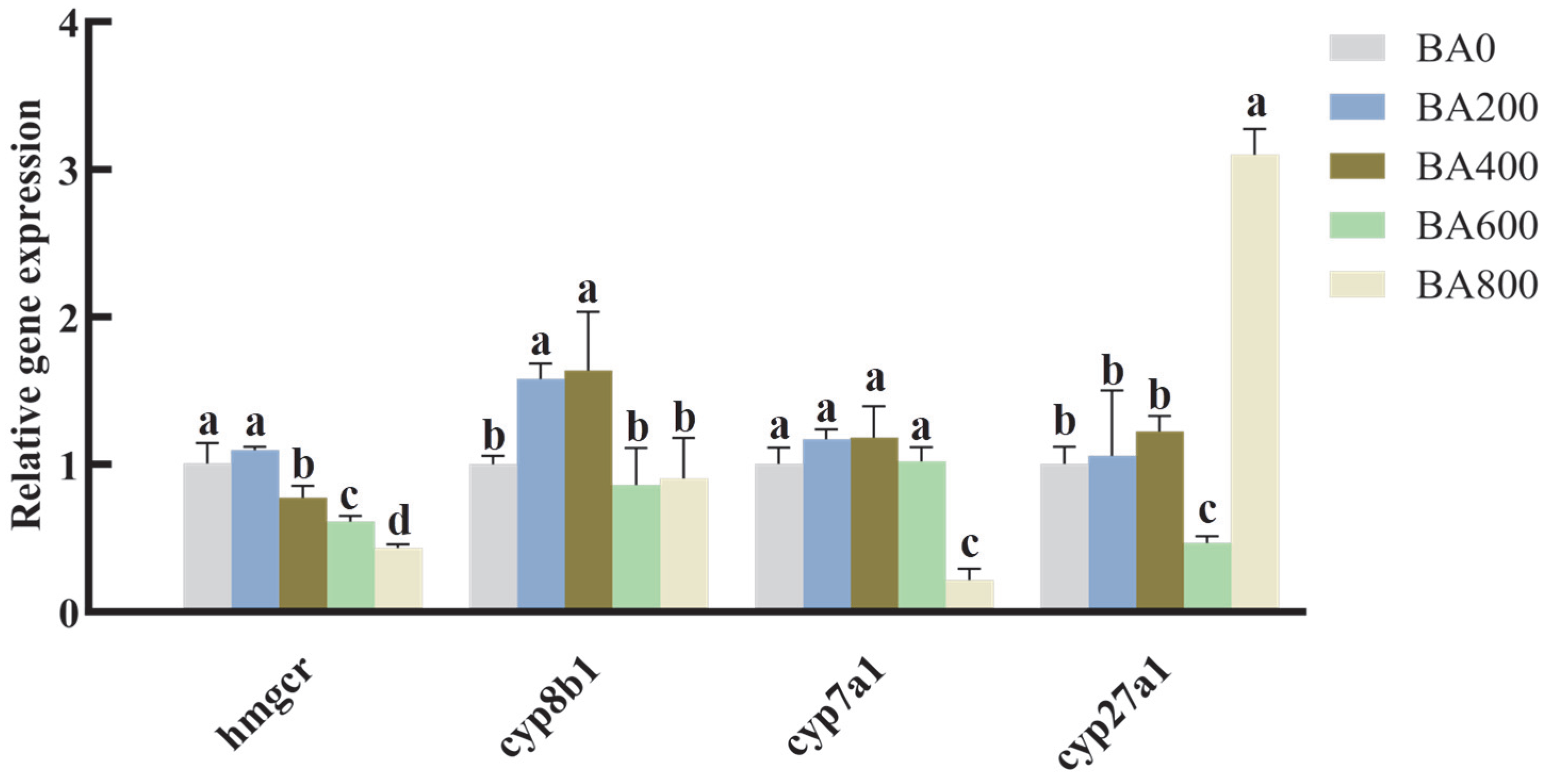
3.3. Relative Expression of Lipid Metabolism-Related Genes in the Hepatopancreas of Gibel Carp Fed with Various Levels of Dietary BA
3.4. Assembly of Transcriptome Sequencing and Identification of Differentially Expressed Genes (DEGs)
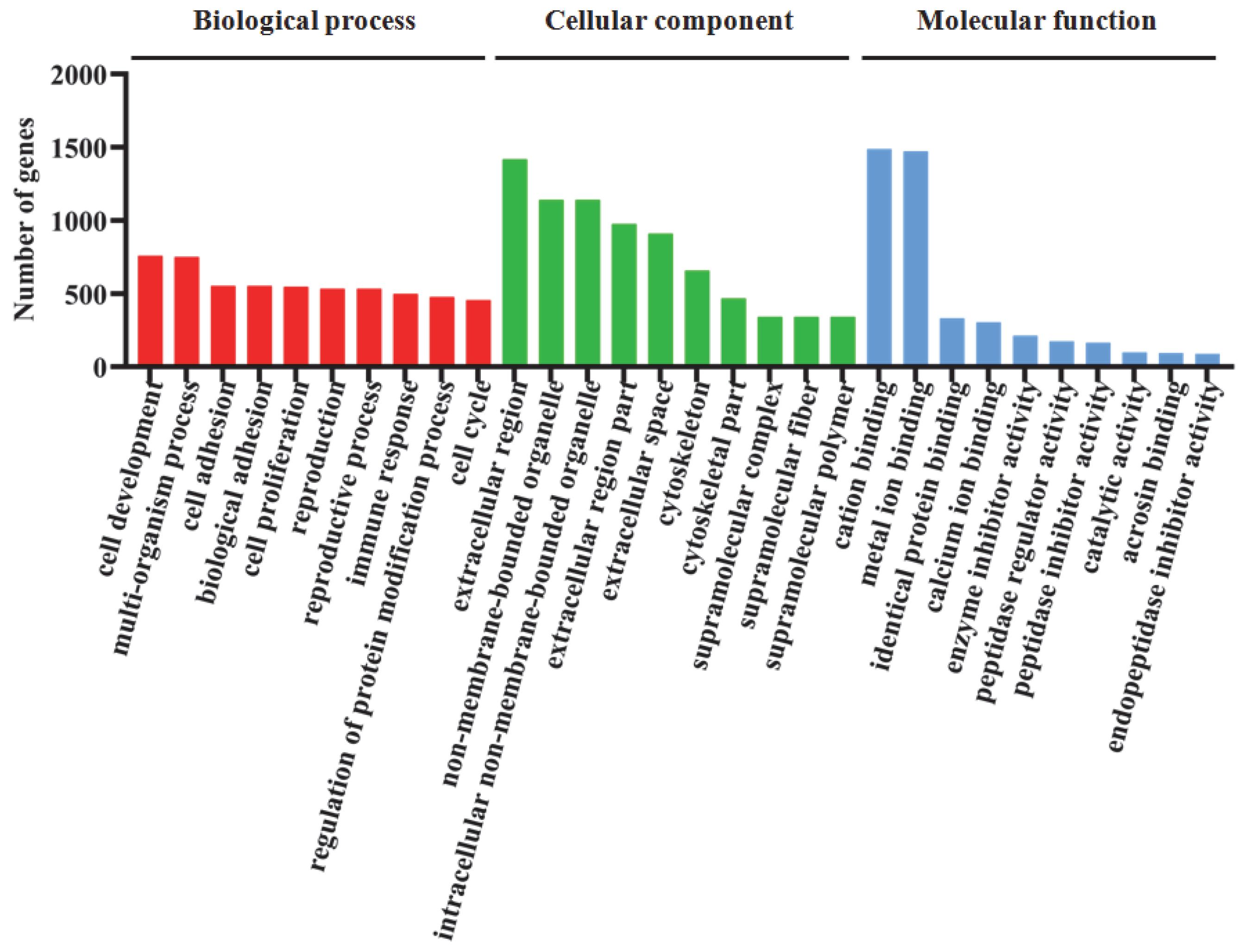
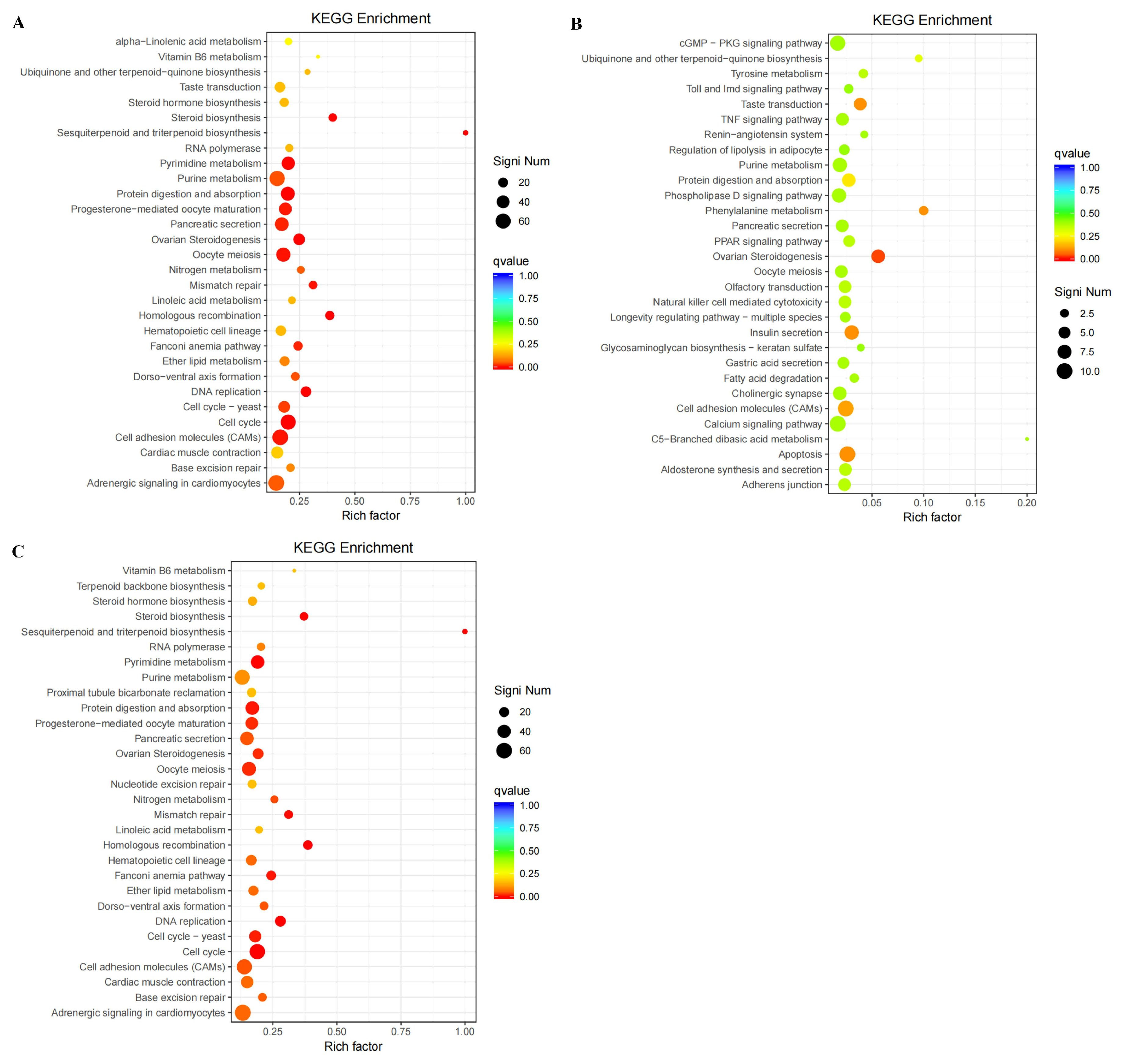
3.5. Functional Classification and Enrichment of DEGs Using GO and KEGG Analysis
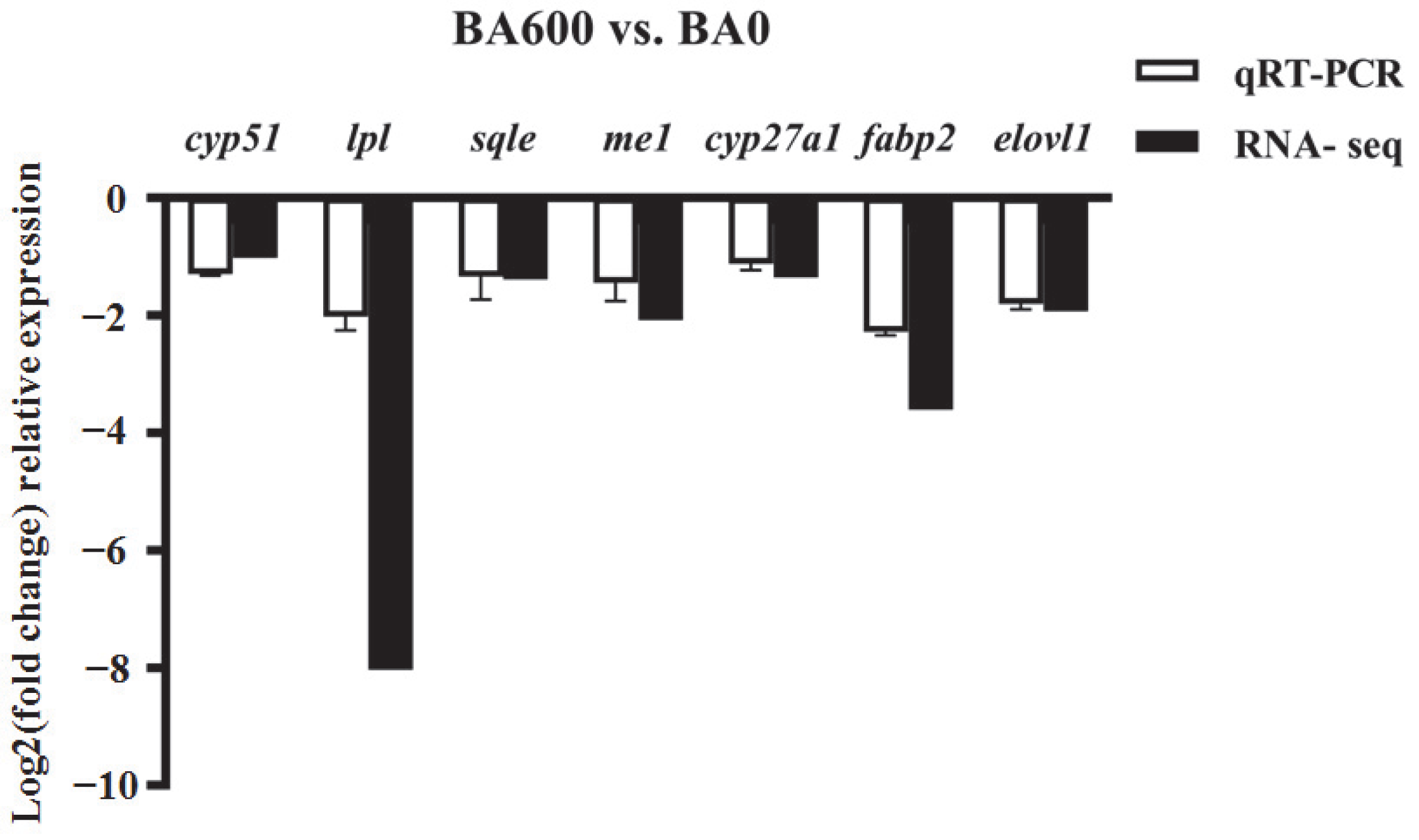
3.6. qRT-PCR Validation of DEGs
4. Discussion
4.1. Effects of Dietary BA Supplementation on Serum Biochemical Parameters in Juvenile Gibel Carp Fed HFD
4.2. Effects of Dietary BA Supplementation on Hepatopancreas Histology in Juvenile Gibel Carp Fed HFD
4.3. Effects of Dietary BA Supplementation on Gene Expression Related to Lipid Metabolism in Juvenile Gibel Carp Fed HFD
4.4. Effects of Dietary BA Supplementation on Hepatopancreas Transcriptomic Profiles in Juvenile Gibel Carp Fed HFD
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Zhi, X.; Song, Z.; Wang, G.; Zhao, X.; Chi, S.; Tan, B. Flesh Quality of Hybrid Grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) Fed with Hydrolyzed Porcine Mucosa-Supplemented Low Fishmeal Diet. Anim. Nutr. 2022, 8, 114–124. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhang, W.; Zheng, J.; Tang, Z.; Li, X.; Cao, X.; Zhang, L.; Xu, W.; Mai, K.; Ai, Q. Effects of Enzymatic Hydrolysis Chicken By-product in High Plant-based Protein Diet on Growth Performance, Digestive Capacity, Antioxidant Capacity and Non-specific Immunity of Juvenile Turbot (Scophthalmus maximus L.). Aquac. Nutr. 2021, 27, 1578–1589. [Google Scholar] [CrossRef]
- Ayisi, C.L.; Yamei, C.; Zhao, J.L. Genes, Transcription Factors and Enzymes Involved in Lipid Metabolism in Fin Fish. Agri Gene 2018, 7, 7–14. [Google Scholar] [CrossRef]
- Chen, X.; Qu, L.; Li, H.; Cui, X.; Zhang, J.; Guo, X.; Xia, T.; Wei, C.; Ding, Z.; Xu, J.; et al. Optimization of Soybean Oil Content in a Soybean Oil-Based Aquafeed for Grass Carp (Ctenopharyngodon idella) to Achieve Optimal Growth Performance, Proximate and Fatty Acid Compositions, and Lipid Metabolism. Aquac. Rep. 2024, 34, 101916. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiang, X.; Chen, Q.; Du, J.; Zhu, S.; Xu, X.; Shen, Y.; Wen, S.; Li, Y.; Xu, W.; et al. Sterol Regulatory Element Binding Protein 1: A Mediator for High-Fat Diet–Induced Hepatic Gluconeogenesis and Glucose Intolerance in Fish. J. Nutr. 2024, 154, 1505–1516. [Google Scholar] [CrossRef]
- Zhong, X.; Gu, J.; Zhang, S.; Chen, X.; Zhang, J.; Miao, J.; Ding, Z.; Xu, J.; Cheng, H. Dynamic Transcriptome Analysis of the Muscles in High-Fat Diet-Induced Obese Zebrafish (Danio rerio) under 5-HT Treatment. Gene 2022, 819, 146265. [Google Scholar] [CrossRef]
- Zhou, Y.-L.; Guo, J.-L.; Tang, R.-J.; Ma, H.-J.; Chen, Y.-J.; Lin, S.-M. High Dietary Lipid Level Alters the Growth, Hepatic Metabolism Enzyme, and Anti-Oxidative Capacity in Juvenile Largemouth Bass Micropterus Salmoides. Fish Physiol. Biochem. 2020, 46, 125–134. [Google Scholar] [CrossRef]
- Jia, R.; Hou, Y.; Zhou, L.; Zhang, L.; Li, B.; Zhu, J. Comparative Transcriptome Analysis Reveals the Impact of a High-Fat Diet on Hepatic Metabolic Function in Tilapia (Oreochromis niloticus). Animals 2024, 14, 3204. [Google Scholar] [CrossRef]
- Ou, Z.; Yue, Y.; Feng, X.; Qiu, Y.; Yu, H.; Yu, Y. Strategically Using Yupingfeng Polysaccharides to Improve the Growth Performance, Antioxidant Capacity, Liver Health, and Lipid Metabolism of Largemouth Bass (Micropterus salmoides) under a High-Lipid Diet. Aquac. Rep. 2024, 39, 102473. [Google Scholar] [CrossRef]
- Yin, P.; Xie, S.; Zhuang, Z.; He, X.; Tang, X.; Tian, L.; Liu, Y.; Niu, J. Dietary Supplementation of Bile Acid Attenuate Adverse Effects of High-Fat Diet on Growth Performance, Antioxidant Ability, Lipid Accumulation and Intestinal Health in Juvenile Largemouth Bass (Micropterus salmoides). Aquaculture 2021, 531, 735864. [Google Scholar] [CrossRef]
- Alrefai, W.A.; Gill, R.K. Bile Acid Transporters: Structure, Function, Regulation and Pathophysiological Implications. Pharm. Res. 2007, 24, 1803–1823. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; El-Dahan, S.; Elsaadawy, S.; Noreldin, A.E.; Sewilam, H. Effects of Dietary Bile Acid on the Growth Performance, Intestinal Health, Blood Biochemistry, and Antioxidative Response of Nile Tilapia (Oreochromus niloticus) Fed High-Fat Diets. Aquac. Rep. 2025, 40, 102605. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Li, J.; Yang, Y.; Liu, H.; Zhang, P. Effectiveness of Bile Acids as a Feed Supplement to Improve Growth Performance, Feed Utilization, Lipid Metabolism, Digestive Enzymes, and Hepatic Antioxidant Status in Aquaculture Animals: A Meta-Analysis. Aquac. Rep. 2024, 36, 102121. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Yao, X.; Xie, S.; Chi, S.; Zhang, S.; Cao, J.; Tan, B. Protective Effects of Bile Acids against Hepatic Lipid Accumulation in Hybrid Grouper Fed a High-Lipid Diet. Front. Nutr. 2022, 9, 813249. [Google Scholar] [CrossRef]
- Su, C.; Liu, X.; Li, J.; Zhang, M.; Pan, L.; Lu, Y.; Wang, Y.; Ding, Y. Effects of Bile Acids on the Growth Performance, Lipid Metabolism, Non-specific Immunity and Intestinal Microbiota of Pacific White Shrimp (Litopenaeus vannamei). Aquac. Nutr. 2021, 27, 2029–2041. [Google Scholar] [CrossRef]
- Wang, L.; Sagada, G.; Wang, C.; Liu, R.; Li, Q.; Zhang, C.; Yan, Y. Exogenous Bile Acids Regulate Energy Metabolism and Improve the Health Condition of Farmed Fish. Aquaculture 2023, 562, 738852. [Google Scholar] [CrossRef]
- Yao, T.; Gu, X.; Liang, X.; Fall, F.N.; Cao, A.; Zhang, S.; Guan, Y.; Sun, B.; Xue, M. Tolerance Assessment of Dietary Bile Acids in Common Carp (Cyprinus carpio L.) Fed a High Plant Protein Diet. Aquaculture 2021, 543, 737012. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Ren, M.; Liang, H.; Mi, H.; Huang, D. A Study on the Function of Arginine in the Growth, Immunity, Antioxidant Activity, and Oxygen Carrying-Capacity of Juvenile Gibel Carp (Carassius auratus gibelio). BioTech 2024, 13, 56. [Google Scholar] [CrossRef]
- Lei, X.; Wang, X.; Lai, Q.; Gao, P.; Cao, X.; Li, Y. Transcriptome Analysis of the Immunoprotective Effect of Bacillus Licheniformis on the Intestinal Tract of Carassius auratus Gibelio Infected with Aeromonas Hydrophila. Aquac. Int. 2024, 32, 1213–1234. [Google Scholar] [CrossRef]
- Wu, D.; Li, J.; Fan, Z.; Sun, Z.; Zheng, X.; Zhang, H.; Xu, H.; Wang, L. Dietary Lycium Barbarum Polysaccharide Modulates Growth Performance, Antioxidant Capacity, and Lipid Metabolism in Common Carp (Cyprinus carpio) Fed with High-Fat Diet. Antioxidants 2024, 13, 540. [Google Scholar] [CrossRef]
- He, Y.; Tang, Y.; Xu, N.; Yao, C.; Gong, Y.; Yin, Z.; Li, Q.; Zhang, Y.; Lai, W.; Liu, Y.; et al. Effects of Supplemental Phytosterol on Growth Performance, Body Composition, Serum Biochemical Indexes and Lipid Metabolism of Juvenile Large Yellow Croaker (Larimichthys crocea) Fed with High Lipid Diet. Aquaculture 2022, 551, 737889. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Qu, L.; Xia, T.; Ding, H.; Xu, Y.; Xu, J.; Cheng, H. Effect of Bile Acids in High-Lipid Diet on Growth Performance and Muscle Fatty Acid Composition of Gibel Carp (Carassius auratus Gibelio). Feed Res. 2023, 46, 41–45. [Google Scholar] [CrossRef]
- Gui, J.-F. Chinese Wisdom and Modern Innovation of Aquaculture. Water Biol. Secur. 2024, 3, 100271. [Google Scholar] [CrossRef]
- Zhou, J.S.; Chen, H.J.; Ji, H.; Shi, X.C.; Li, X.X.; Chen, L.Q.; Du, Z.Y.; Yu, H.B. Effect of Dietary Bile Acids on Growth, Body Composition, Lipid Metabolism and Microbiota in Grass Carp (Ctenopharyngodon idella). Aquac. Nutr. 2018, 24, 802–813. [Google Scholar] [CrossRef]
- Cui, X.; Huang, X.; Chen, X.; Li, H.; Wu, Y.; Yang, Z.; Liu, Z.; Feng, R.; Xu, J.; Wei, C.; et al. Influence of Starvation on Biochemical, Physiological, Morphological, and Transcriptional Responses Associated with Glucose and Lipid Metabolism in the Liver of Javelin Goby (Synechogobius hasta). Animals 2024, 14, 2734. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, X.; Han, Z.; Qu, L.; Xia, T.; Chen, X.; Xu, J.; Ding, Z.; Wei, C.; Cheng, H. Comparative Analysis of Hepatopancreas RNA-Seq of Juvenile Grass Carp (Ctenopharyngodon idella) Fed Different Starch Diets. Fishes 2023, 8, 495. [Google Scholar] [CrossRef]
- Maulu, S.; Nawanzi, K.; Abdel-Tawwab, M.; Khalil, H.S. Fish Nutritional Value as an Approach to Children’s Nutrition. Front. Nutr. 2021, 8, 780844. [Google Scholar] [CrossRef]
- Pakhira, C.; Nagesh, T.S.; Abraham, T.J.; Dash, G.; Behera, S. Stress Responses in Rohu, Labeo Rohita Transported at Different Densities. Aquac. Rep. 2015, 2, 39–45. [Google Scholar] [CrossRef]
- Adam, A.H.; Verdegem, M.; Soliman, A.A.; Zaki, M.; Khalil, R.H.; Nour, A.-E.M.; Khaled, A.A.; El Basuini, M.F.; Khalil, H.S. Effect of Dietary Bile Acids: Growth Performance, Immune Response, Genes Expression of Fatty Acid Metabolism, Intestinal, and Liver Morphology of Striped Catfish (Pangasianodon hypophthalmus). Aquac. Rep. 2023, 29, 101510. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Abdel-Latif, H.M.R.; El Basuini, M.F.; El-Nokrashy, A.M.; Khaled, A.A.; Kord, M.; Soliman, A.A.; Zaki, M.; Nour, A.-E.; Labib, E.M.H.; et al. Effects of Exogenous Bile Acids (BAs) on Growth, Lipid Profile, Digestive Enzymes, and Immune Responses of Thinlip Mullet, Liza Ramada. Sci. Rep. 2023, 13, 22875. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, H.; Liang, X.-F.; He, S.; Lan, J.; Li, L. Dietary Bile Acids Reduce Liver Lipid Deposition via Activating Farnesoid X Receptor, and Improve Gut Health by Regulating Gut Microbiota in Chinese Perch (Siniperca chuatsi). Fish Shellfish Immunol. 2022, 121, 265–275. [Google Scholar] [CrossRef]
- Jin, M.; Pan, T.; Cheng, X.; Zhu, T.T.; Sun, P.; Zhou, F.; Ding, X.; Zhou, Q. Effects of Supplemental Dietary L-Carnitine and Bile Acids on Growth Performance, Antioxidant and Immune Ability, Histopathological Changes and Inflammatory Response in Juvenile Black Seabream (Acanthopagrus schlegelii) Fed High-Fat Diet. Aquaculture 2019, 504, 199–209. [Google Scholar] [CrossRef]
- Hori, S.; Abe, T.; Lee, D.G.; Fukiya, S.; Yokota, A.; Aso, N.; Shirouchi, B.; Sato, M.; Ishizuka, S. Association between 12α-Hydroxylated Bile Acids and Hepatic Steatosis in Rats Fed a High-Fat Diet. J. Nutr. Biochem. 2020, 83, 108412. [Google Scholar] [CrossRef]
- Naiel, M.A.E.; Negm, S.S.; Ghazanfar, S.; Shukry, M.; Abdelnour, S.A. The Risk Assessment of High-fat Diet in Farmed Fish and Its Mitigation Approaches: A Review. J. Anim. Physiol. Anim. Nutr. 2023, 107, 948–969. [Google Scholar] [CrossRef]
- Liao, Z.; Sun, B.; Zhang, Q.; Jia, L.; Wei, Y.; Liang, M.; Xu, H. Dietary Bile Acids Regulate the Hepatic Lipid Homeostasis in Tiger Puffer Fed Normal or High-Lipid Diets. Aquaculture 2020, 519, 734935. [Google Scholar] [CrossRef]
- He, A.Y.; Ning, L.J.; Chen, L.Q.; Chen, Y.L.; Xing, Q.; Li, J.M.; Qiao, F.; Li, D.L.; Zhang, M.L.; Du, Z.Y. Systemic Adaptation of Lipid Metabolism in Response to Low- and High-Fat Diet in Nile Tilapia (Oreochromis niloticus). Physiol. Rep. 2015, 3, e12485. [Google Scholar] [CrossRef] [PubMed]
- Li, R.X.; Qian, Y.F.; Zhou, W.H.; Wang, J.X.; Zhang, Y.Y.; Luo, Y.; Qiao, F.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. The Adaptive Characteristics of Cholesterol and Bile Acid Metabolism in Nile Tilapia Fed a High-Fat Diet. Aquac. Nutr. 2022, 2022, 8016616. [Google Scholar] [CrossRef]
- Peng, X.-R.; Feng, L.; Jiang, W.-D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Zhou, X.-Q. Supplementation Exogenous Bile Acid Improved Growth and Intestinal Immune Function Associated with NF-ΚB and TOR Signalling Pathways in on-Growing Grass Carp (Ctenopharyngodon idella): Enhancement the Effect of Protein-Sparing by Dietary Lipid. Fish Shellfish Immunol. 2019, 92, 552–569. [Google Scholar] [CrossRef] [PubMed]
- Marrero, M.; Monroig, Ó.; Navarro, J.C.; Ribes-Navarro, A.; Pérez, J.A.; Galindo, A.; Rodríguez, C. Metabolic and Molecular Evidence for Long-Chain PUFA Biosynthesis Capacity in the Grass Carp Ctenopharyngodon Idella. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2022, 270, 111232. [Google Scholar] [CrossRef] [PubMed]
- Monroig, Ó.; Shu-Chien, A.C.; Kabeya, N.; Tocher, D.R.; Castro, L.F.C. Desaturases and Elongases Involved in Long-Chain Polyunsaturated Fatty Acid Biosynthesis in Aquatic Animals: From Genes to Functions. Prog. Lipid Res. 2022, 86, 101157. [Google Scholar] [CrossRef]
- Du, J.; Xiang, X.; Xu, D.; Zhang, J.; Fang, W.; Xu, W.; Mai, K.; Ai, Q. FXR, a Key Regulator of Lipid Metabolism, Is Inhibited by ER Stress-Mediated Activation of JNK and P38 MAPK in Large Yellow Croakers (Larimichthys crocea) Fed High Fat Diets. Nutrients 2021, 13, 4343. [Google Scholar] [CrossRef]
- Zheng, H.; Xu, Y.-C.; Zhao, T.; Luo, Z.; Zhang, D.-G.; Song, C.-C.; Yu, A.-G.; Tan, X. Dietary Chenodeoxycholic Acid Attenuates High-Fat Diet-Induced Growth Retardation, Lipid Accumulation and Bile Acid Metabolism Disorder in the Liver of Yellow Catfish Pelteobagrus Fulvidraco. Br. J. Nutr. 2024, 131, 921–934. [Google Scholar] [CrossRef]
- Xiang, X.; Ji, R.; Han, S.; Xu, X.; Zhu, S.; Li, Y.; Du, J.; Mai, K.; Ai, Q. Differences in Diacylglycerol Acyltransferases Expression Patterns and Regulation Cause Distinct Hepatic Triglyceride Deposition in Fish. Commun. Biol. 2024, 7, 480. [Google Scholar] [CrossRef]
- Villanueva, C.J.; Monetti, M.; Shih, M.; Zhou, P.; Watkins, S.M.; Bhanot, S.; Farese, R.V. Specific Role for Acyl CoA. Hepatology 2009, 50, 434–442. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, L.; Chen, P.; Liang, X.; Cao, A.; Han, J.; Wu, X.; Zheng, Y.; Qin, Y.; Xue, M. Dietary Bile Acids Enhance Growth, and Alleviate Hepatic Fibrosis Induced by a High Starch Diet via AKT/FOXO1 and CAMP/AMPK/SREBP1 Pathway in Micropterus Salmoides. Front. Physiol. 2019, 10, 1430. [Google Scholar] [CrossRef]
- Du, Y.; Wang, G.; Yu, E.; Xie, J.; Xia, Y.; Li, H.; Zhang, K.; Gong, W.; Li, Z.; Xie, W.; et al. Dietary Deoxycholic Acid Decreases Fat Accumulation by Activating Liver Farnesoid X Receptor in Grass Crap (Ctenopharyngodon idella). Aquaculture 2024, 578, 740123. [Google Scholar] [CrossRef]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef]
- Ji, R.; Xu, X.; Turchini, G.M.; Mai, K.; Ai, Q. Adiponectin’s Roles in Lipid and Glucose Metabolism Modulation in Fish: Mechanisms and Perspectives. Rev. Aquac. 2021, 13, 2305–2321. [Google Scholar] [CrossRef]
- Pi, D.; Wang, J.; Zhao, M.; Liu, M.; Zhang, Y.; Qin, C.; Yang, L.; Yan, X.; Nie, G. Adiponectin and Adiponectin Receptors in Common Carp (Cyprinus carpio): Tissue Distribution and Their Expressions in Response to High-Carbohydrate and High-Lipid Diets. Aquac. Rep. 2022, 27, 101341. [Google Scholar] [CrossRef]
- Wu, W.; Sun, J.; Ji, H.; Yu, H.; Zhou, J. AMP-Activated Protein Kinase in the Grass Carp Ctenopharyngodon Idellus: Molecular Characterization, Tissue Distribution and MRNA Expression in Response to Overwinter Starvation Stress. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 246–247, 110457. [Google Scholar] [CrossRef]
- Zhi, S.; Wang, J.; Wang, Y.; Li, Y.; Zhao, M.; Yang, L.; Qin, C.; Yan, X.; Nie, G. Molecular Characterization of AMP-Activated Protein Kinase (AMPK) A1/A2 from Cyprinus Carpio and Its Roles in Glucolipid Metabolism and Immune Response. Int. J. Biol. Macromol. 2025, 303, 140736. [Google Scholar] [CrossRef]
- Nilsson, L.-M.; Abrahamsson, A.; Sahlin, S.; Gustafsson, U.; Angelin, B.; Parini, P.; Einarsson, C. Bile Acids and Lipoprotein Metabolism: Effects of Cholestyramine and Chenodeoxycholic Acid on Human Hepatic MRNA Expression. Biochem. Biophys. Res. Commun. 2007, 357, 707–711. [Google Scholar] [CrossRef]
- Guo, J.-L.; Kuang, W.-M.; Zhong, Y.-F.; Zhou, Y.-L.; Chen, Y.-J.; Lin, S.-M. Effects of Supplemental Dietary Bile Acids on Growth, Liver Function and Immunity of Juvenile Largemouth Bass (Micropterus salmoides) fed High-Starch Diet. Fish Shellfish Immunol. 2020, 97, 602–607. [Google Scholar] [CrossRef]
- Kong, Y.; Li, M.; Xia, C.; Liu, X.; Wang, G. A Novel Model Construction of Lithocholic Acid-Induced Cholestasis and Transcriptome Analysis in Snakehead Fish (Channa argus). Aquaculture 2021, 543, 737014. [Google Scholar] [CrossRef]
- Xiong, F.; Wu, S.; Qin, L.; Shi, M.; Li, W.; Zou, H.; Li, M.; Wang, G. Transcriptome Analysis of Grass Carp Provides Insights into Disease-Related Genes and Novel Regulation Pattern of Bile Acid Feedback in Response to Lithocholic Acid. Aquaculture 2019, 500, 613–621. [Google Scholar] [CrossRef]
- Xu, J.; Shi, M.; Chen, L.; Chi, S.; Zhang, S.; Cao, J.; Tan, B.; Xie, S. Muscular Lipidomics and Transcriptomics Reveal the Effects of Bile Acids on Lipid Metabolism in High-Fat Diet-Fed Grouper. Fish Physiol. Biochem. 2024, 50, 127–143. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Wu, A.; Wang, J.; Zhang, J. Strategies of Targeting CYP51 for IFIs Therapy: Emerging Prospects, Opportunities and Challenges. Eur. J. Med. Chem. 2023, 259, 115658. [Google Scholar] [CrossRef]
- Song, Z.; Xiong, H.; Meng, X.; Ma, Q.; Wei, Y.; Li, Y.; Liu, J.; Liang, M.; Xu, H. Dietary Cholesterol Supplementation Inhibits the Steroid Biosynthesis but Does Not Affect the Cholesterol Transport in Two Marine Teleosts: A Hepatic Transcriptome Study. Aquac. Nutr. 2023, 2023, 2308669. [Google Scholar] [CrossRef]
- Skubic, C.; Trček, H.; Nassib, P.; Kreft, T.; Walakira, A.; Pohar, K.; Petek, S.; Režen, T.; Ihan, A.; Rozman, D. Knockouts of CYP51A1, DHCR24, or SC5D from Cholesterol Synthesis Reveal Pathways Modulated by Sterol Intermediates. iScience 2024, 27, 110651. [Google Scholar] [CrossRef]
- Urlep, Ž.; Lorbek, G.; Perše, M.; Jeruc, J.; Juvan, P.; Matz-Soja, M.; Gebhardt, R.; Björkhem, I.; Hall, J.A.; Bonneau, R.; et al. Disrupting Hepatocyte Cyp51 from Cholesterol Synthesis Leads to Progressive Liver Injury in the Developing Mouse and Decreases RORC Signalling. Sci. Rep. 2017, 7, 40775. [Google Scholar] [CrossRef]


| Ingredient | Group | ||||
|---|---|---|---|---|---|
| BA0 | BA200 | BA400 | BA600 | BA800 | |
| Defatted fish meal | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 |
| Soybean meal | 23.00 | 23.00 | 23.00 | 23.00 | 23.00 |
| Cottonseed meal | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 |
| Canola meal | 11.80 | 11.80 | 11.80 | 11.80 | 11.80 |
| Flour | 17.00 | 16.98 | 16.96 | 16.94 | 16.92 |
| Ca(H2PO4)2 | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 |
| Lys | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Met | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Choline | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Bile acid | 0.00 | 0.02 | 0.04 | 0.06 | 0.08 |
| Soybean oil | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 |
| Vitamin premix | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Mineral premix | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutrient levels (%) | |||||
| Crude protein | 37.57 ± 0.17 | 37.34 ± 0.18 | 37.80 ± 0.22 | 37.75 ± 0.01 | 37.98 ± 0.49 |
| Crude fat | 12.50 ± 0.12 | 12.37 ± 0.21 | 12.20 ± 0.20 | 12.29 ± 0.18 | 12.37 ± 0.22 |
| Crude ash | 7.99 ± 0.60 | 7.97 ± 0.70 | 8.00 ± 0.73 | 8.07 ± 0.14 | 8.15 ± 0.93 |
| Wet | 10.48 ± 0.24 | 10.28 ± 0.06 | 10.06 ± 0.20 | 10.31 ± 0.23 | 10.70 ± 0.16 |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Size (bp) |
|---|---|---|---|
| ef1a | CGCCAGTGTTGCCTTCGT | CGCTCAATCTTCCATCCCTT | 98 |
| rpl13a | CTTCTGGAGGACAGTAAGAGGTATGC | GGAGGAGGGATGCCATCAAAGAC | 96 |
| acc1 | AAATGTTTCGCAATGAACGAG | ATCTTGATATACTCTGCGTTGG | 85 |
| adipor2 | CGAAAAGGAGGAGAAAACA | CTTCAGCCAATCAGGGAG | 172 |
| ampkβ1 | TGGAGCTCGACCCAAAATCC | AACACAGTGGGCCTTTCCTC | 141 |
| cyp7a1 | ACCTCGGTTGTGCTCTTCAG | GGACATACTGCCCAGCAATC | 192 |
| cyp8b1 | CGACCGTTTTCTCACACC | GTTCCTGCTCCCCAAG | 96 |
| cyp27a1 | CCCACTGGTGATCTGGTCTC | GTCCAGTTTGGCAGGAACAC | 170 |
| cyp51 | CGGAGAAACACGACGACA | GCCAGGAAGAAGCCCA | 160 |
| dgat | CTCAGTTAGCCGTGTTCTTC | TCTGTGCCATCATTCCC | 105 |
| elovl1 | CCTTCTTCTTCGTGCTGT | CTGTTGGGTGTTCTTTGG | 80 |
| elovl4 | GGCTTCTAATCTACTCTCCT | GTTCATTGGTTCCTTGTG | 104 |
| fabp2 | CCTTTGACTATTCTCTGGC | TTTCCGTTGTCCTTGC | 100 |
| fas | GGCCAAGAGAATCTACTGCAC | TGATGGGAATGTCACCCCTT | 95 |
| hmgcr | ATTCCCAGAGCCCACG | TGCTTTCCATCCAATAACAG | 144 |
| hsl | GAAGAGTGTTTCTATGCCTACT | CCGTGAGACATTGCCCTCAT | 140 |
| lpl | AGTACGCAGATGCCCAAAG | CTGGCCTCTGAATCCCAATAC | 111 |
| me1 | TTGTGCTCTTCCACTTCTG | TGGCTGGGTTTCCGAC | 224 |
| pparα | AAGAACCGAAACAAATGCCAA | AACCTCAGCTTCTCCGACT | 110 |
| sqle | CAAACTCTTGACTACATTCCC | CCCTCTTTCGCTTTACATC | 104 |
| Items | BA0-1 | BA0-2 | BA0-3 | BA600-1 | BA600-2 | BA600-3 |
|---|---|---|---|---|---|---|
| Raw reads | 47,142,592 | 49,939,382 | 51,110,188 | 56,172,768 | 41,170,334 | 62,836,908 |
| Clean reads | 45,190,484 | 48,071,420 | 49,105,916 | 54,077,826 | 39,506,686 | 60,591,842 |
| Clean bases | 6,165,204,891 | 6,325,647,847 | 6,778,769,014 | 7,329,021,946 | 5,427,859,827 | 8,268,273,878 |
| Q20 (%) | 97.00 | 97.47 | 97.16 | 97.24 | 97.13 | 97.39 |
| Q30 (%) | 90.45 | 91.83 | 90.89 | 91.20 | 90.83 | 91.65 |
| GC (%) | 48.63 | 48.15 | 48.01 | 48.71 | 48.04 | 48.62 |
| Total mapping ratio (%) | 87.94 | 87.88 | 88.02 | 88.32 | 87.98 | 88.56 |
| Uniquely mapping ratio (%) | 79.44 | 79.32 | 80.30 | 80.01 | 80.15 | 80.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Yang, Z.; Chen, X.; Zhang, J.; Wu, Y.; Li, H.; Yuan, H.; Feng, R.; Wei, C.; Ding, Z.; et al. Dietary Bile Acid Influences the Physiological, Morphological, Lipid Metabolism-Related Responses, and Transcriptomic Profile of Hepatopancreas in High-Fat Diet-Fed Juvenile Gibel Carp (Carassius auratus gibelio). Animals 2025, 15, 2853. https://doi.org/10.3390/ani15192853
Huang X, Yang Z, Chen X, Zhang J, Wu Y, Li H, Yuan H, Feng R, Wei C, Ding Z, et al. Dietary Bile Acid Influences the Physiological, Morphological, Lipid Metabolism-Related Responses, and Transcriptomic Profile of Hepatopancreas in High-Fat Diet-Fed Juvenile Gibel Carp (Carassius auratus gibelio). Animals. 2025; 15(19):2853. https://doi.org/10.3390/ani15192853
Chicago/Turabian StyleHuang, Xiaoyang, Zikui Yang, Xiangning Chen, Jingjing Zhang, Yanru Wu, Huiqing Li, Haiming Yuan, Rui Feng, Chaoqing Wei, Zhujin Ding, and et al. 2025. "Dietary Bile Acid Influences the Physiological, Morphological, Lipid Metabolism-Related Responses, and Transcriptomic Profile of Hepatopancreas in High-Fat Diet-Fed Juvenile Gibel Carp (Carassius auratus gibelio)" Animals 15, no. 19: 2853. https://doi.org/10.3390/ani15192853
APA StyleHuang, X., Yang, Z., Chen, X., Zhang, J., Wu, Y., Li, H., Yuan, H., Feng, R., Wei, C., Ding, Z., Xu, J., & Cheng, H. (2025). Dietary Bile Acid Influences the Physiological, Morphological, Lipid Metabolism-Related Responses, and Transcriptomic Profile of Hepatopancreas in High-Fat Diet-Fed Juvenile Gibel Carp (Carassius auratus gibelio). Animals, 15(19), 2853. https://doi.org/10.3390/ani15192853






