Ultrasonographic Assessment of Caudal Vena Cava Collapsibility Index, Caudal Vena Cava-to-Aorta, and Femoral Vein-to-Artery Ratios in Healthy Sedated Adult Horses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
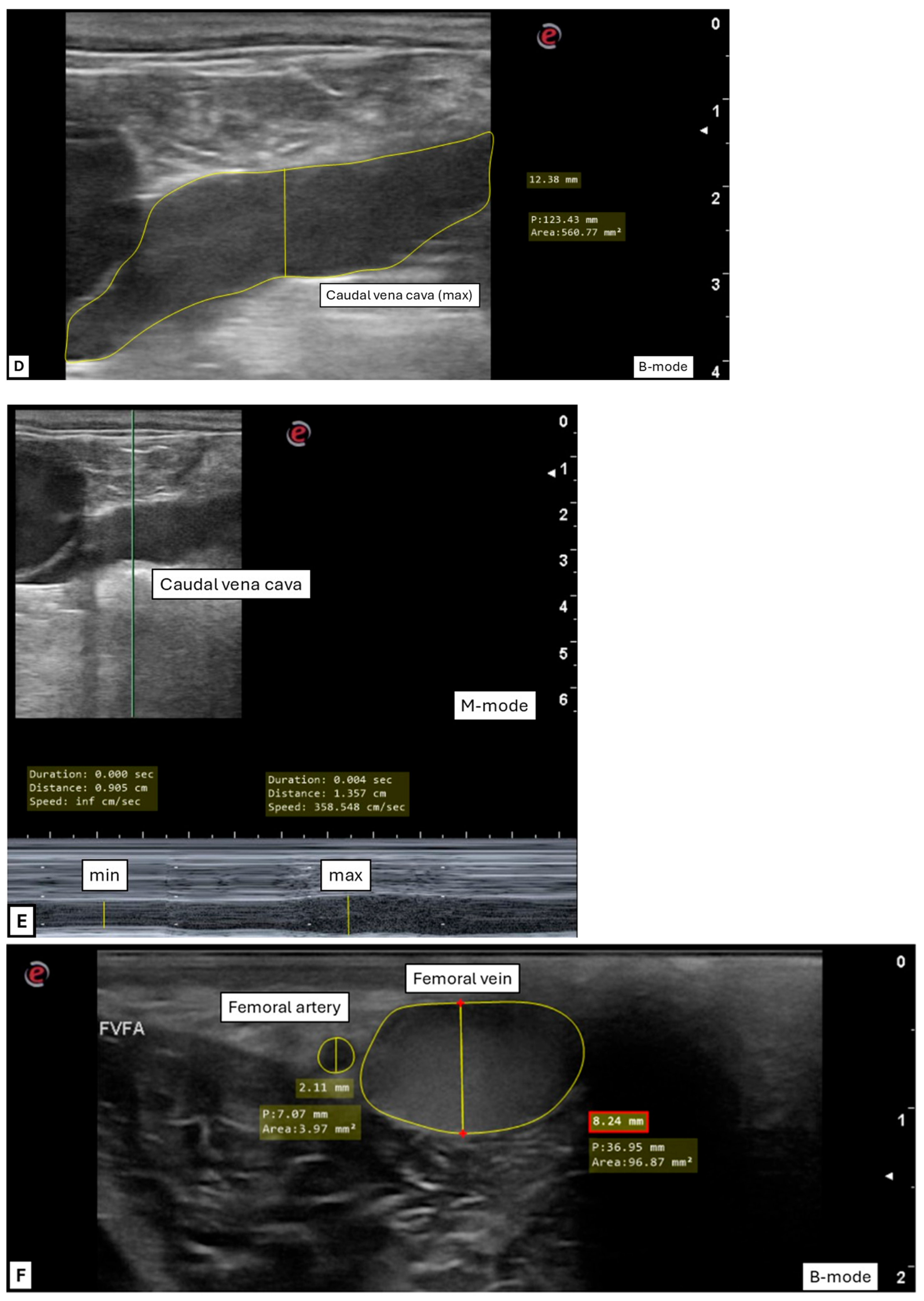
2.2. Ultrasonographic Technique and Image Acquisition
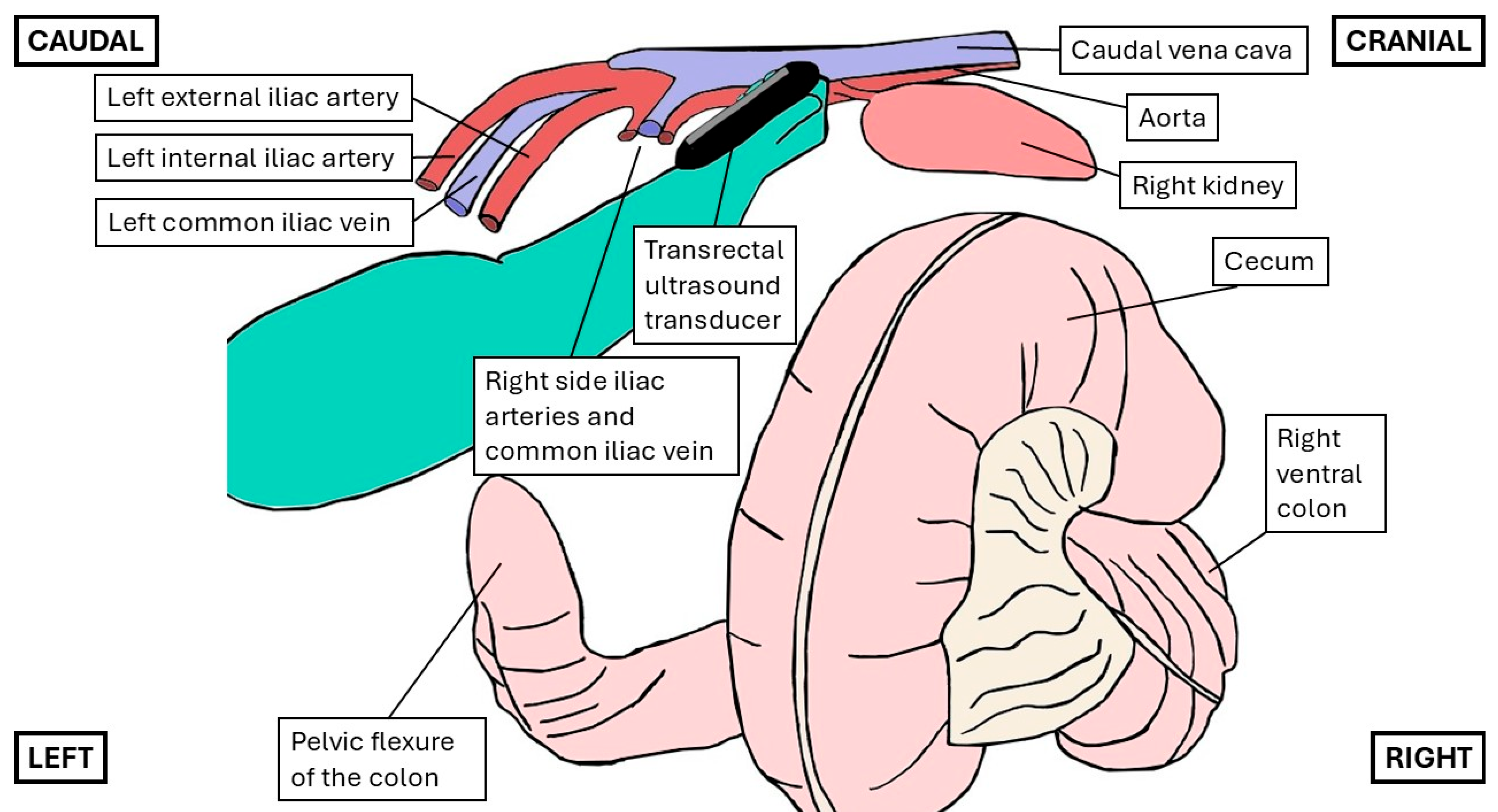
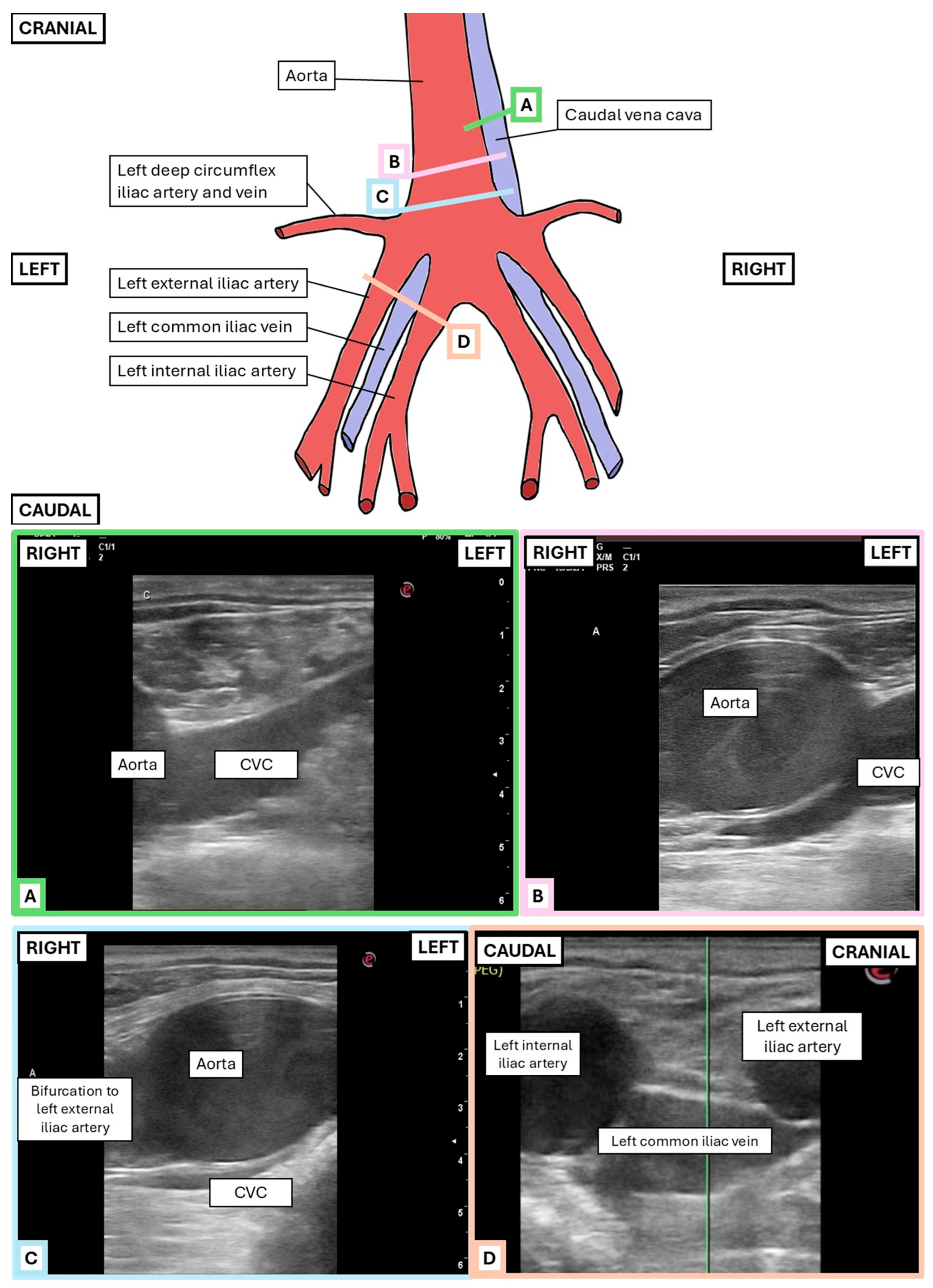
2.2.1. Transrectal Ultrasonographic Technique
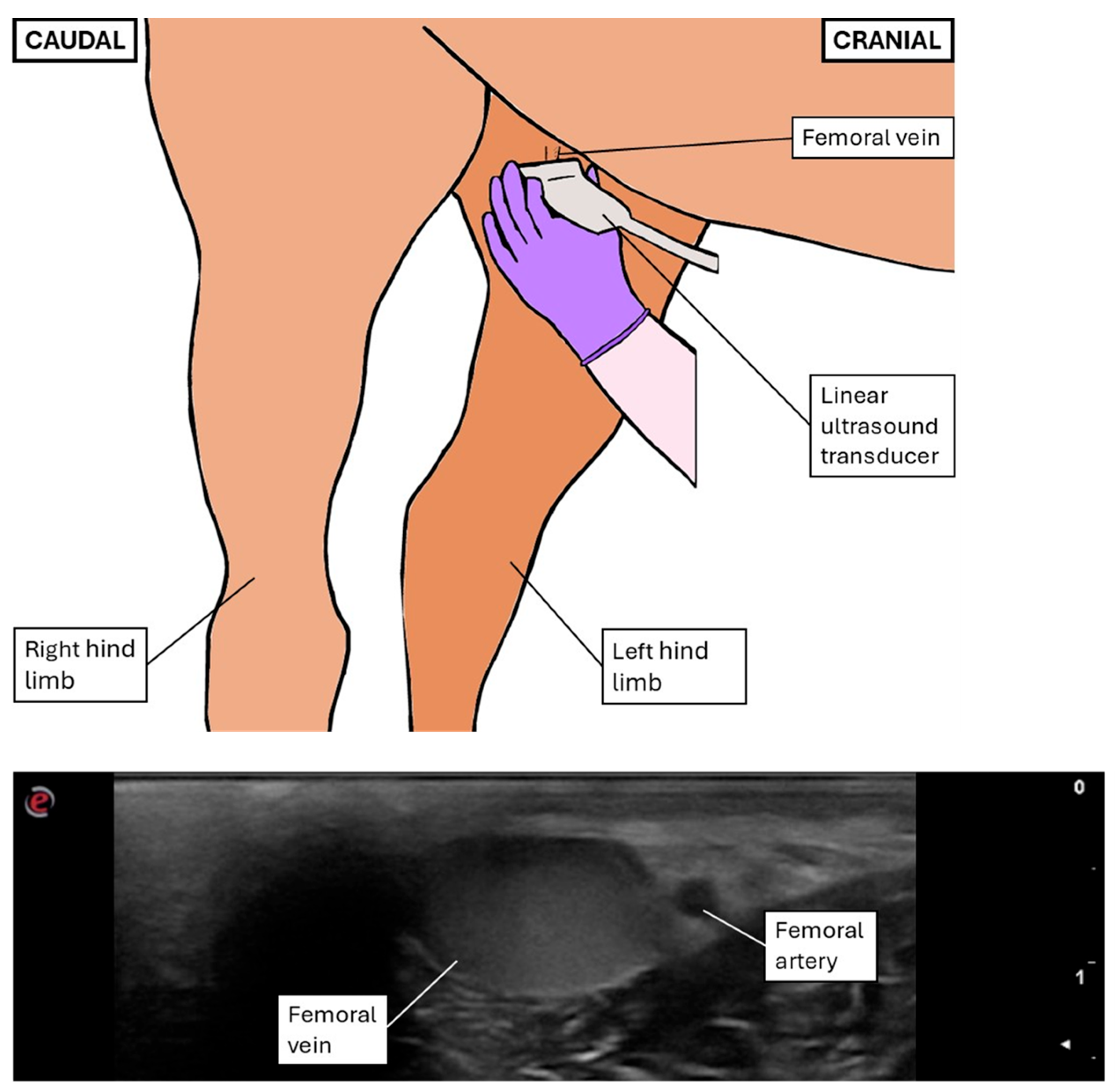
2.2.2. Transcutaneous Inguinal Ultrasonographic Technique
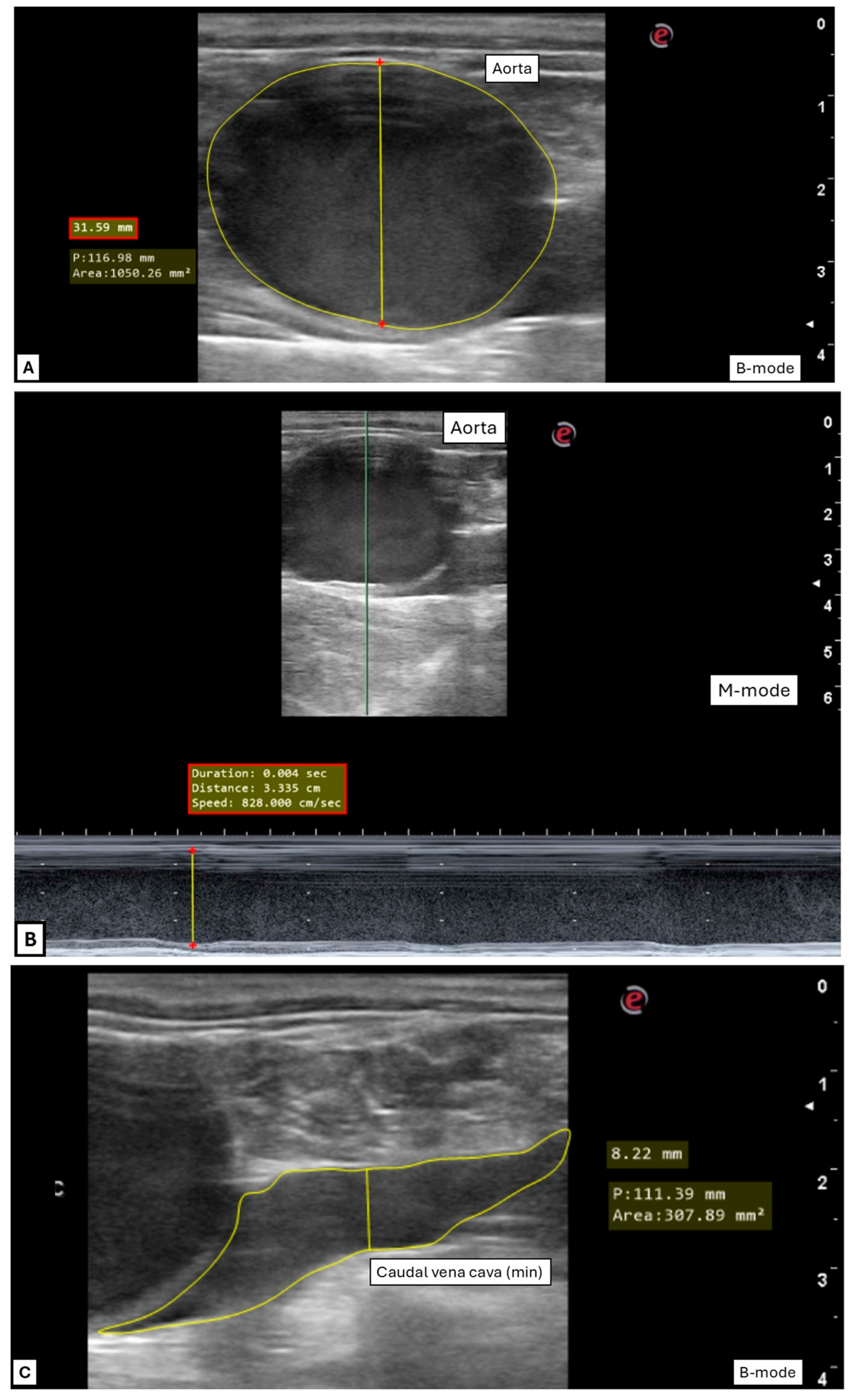
2.3. Ultrasonographic Measurements and Indexes Performed After Image Acquisition
2.3.1. General Variables
2.3.2. Transrectal Variables
2.3.3. Inguinal Variables
2.4. Statistical Analysis
3. Results
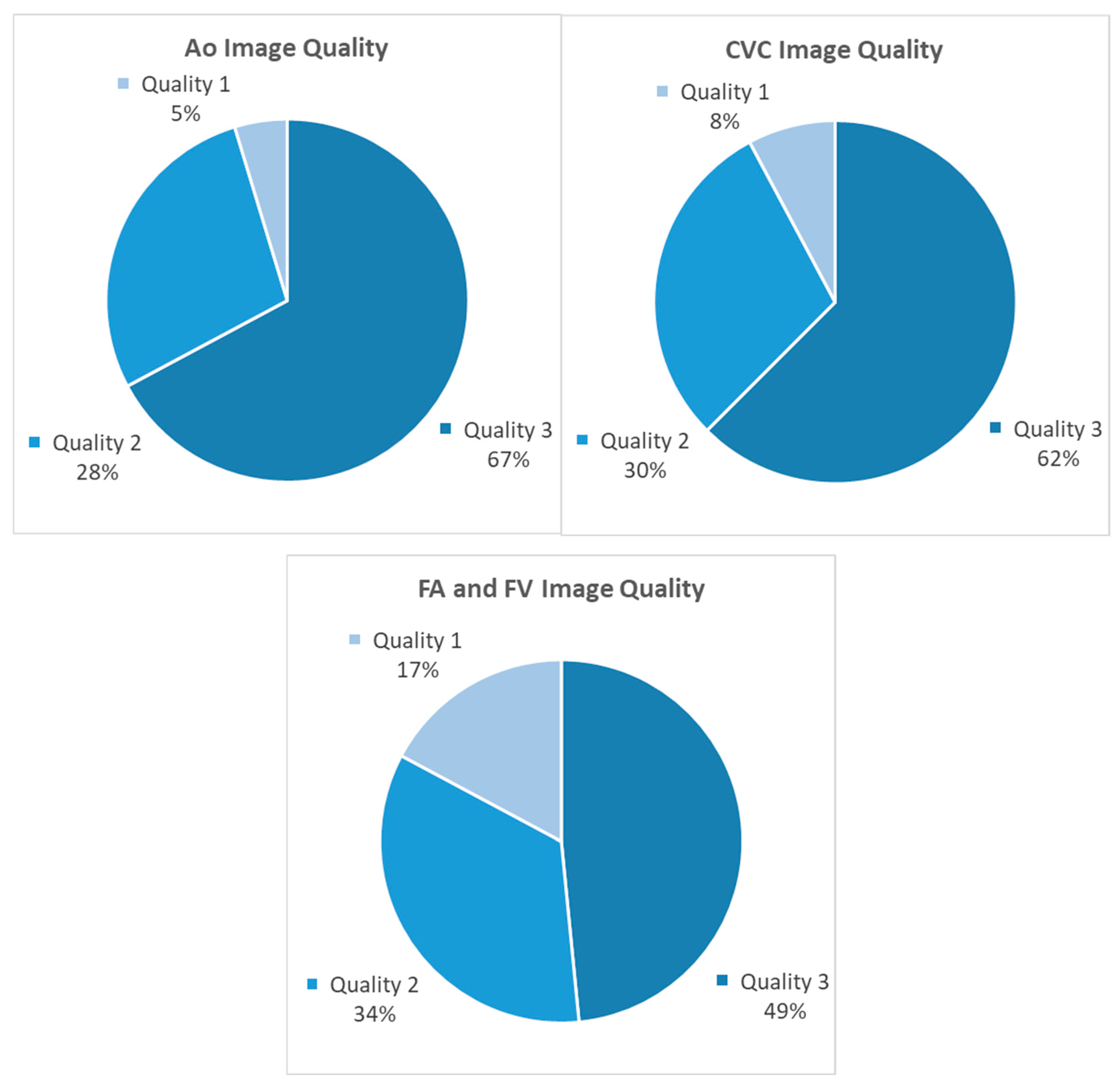
3.1. Ultrasonography Technique
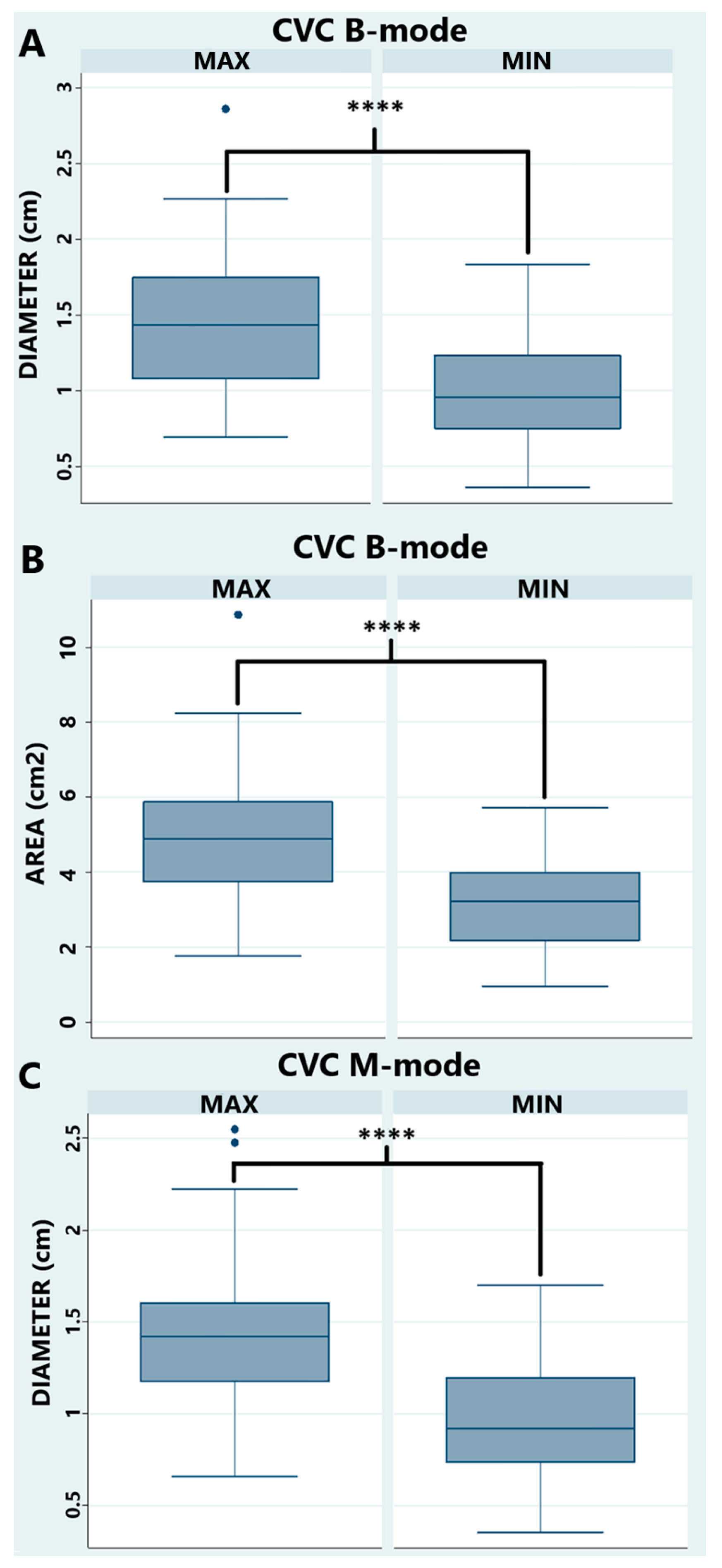
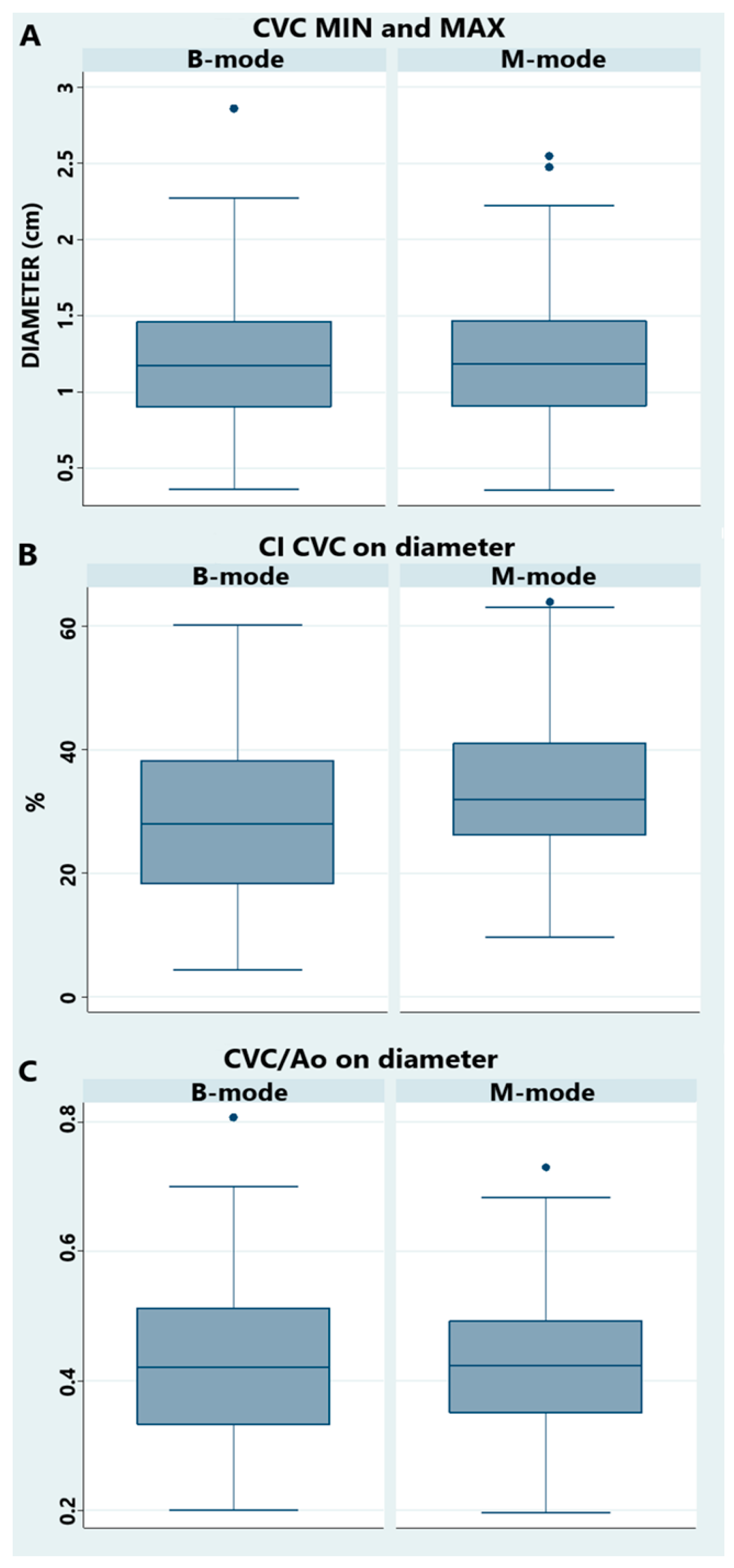
3.2. Transrectal Variables
3.3. Inguinal Variables
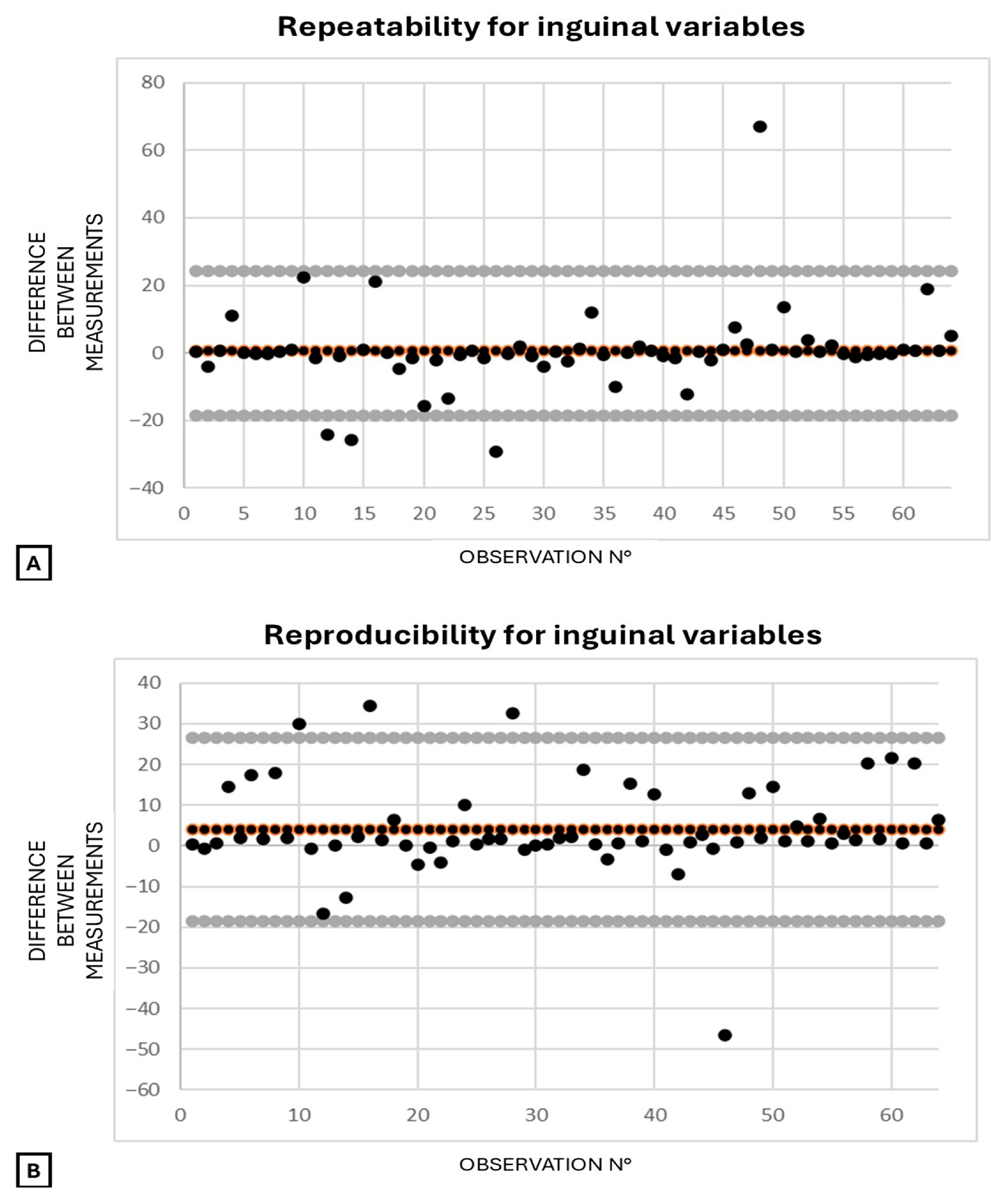
3.4. Agreement for Respiratory Indexes and Vein-to-Artery Ratios
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ao | Aorta |
| CI | Collapsibility Index |
| CI CVC | Collapsibility Index of the Caudal Vena Cava |
| CVC | Caudal Vena Cava |
| CVC/Ao | Caudal Vena Cava-to-Aorta ratio |
| FA | Femoral Artery |
| FV | Femoral Vein |
| FV/FA | Femoral Vein to Femoral Artery ratio |
| IQR | Interquartile Range |
| IVC | Inferior Vena Cava |
| IVC/Ao | Inferior Vena Cava to Aorta ratio |
| max | Maximum measurement |
| min | Minimum measurement |
| OP1 | Operator 1 |
| OP2 | Operator 2 |
| PCV | Packed Cell Volume |
| SD | Standard Deviation |
| SE | Standard Error |
References
- Reed, S.M.; Bayly, W.M.; Sellon, D.C. Cardiovascular Critical Care. In Equine Internal Medicine, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 173–174. [Google Scholar]
- Crabtree, N.E.; Epstein, K.L. Current Concepts in Fluid Therapy in Horses. Front. Vet. Sci. 2021, 8, 648774. [Google Scholar] [CrossRef] [PubMed]
- Henderson, I.S.F. Diagnostic and prognostic use of L-lactate measurement in equine practice. Equine Vet. Educ. 2013, 25, 468–475. [Google Scholar] [CrossRef]
- Pachtinger, G.E.; Drobatz, K. Assessment and Treatment of Hypovolemic States. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 629–643. [Google Scholar] [CrossRef]
- Boysen, S.R.; Gommeren, K. Assessment of Volume Status and Fluid Responsiveness in Small Animals. Front. Vet. Sci. 2021, 8, 630643. [Google Scholar] [CrossRef]
- Donati, P.A.; Guevara, J.M.; Ardiles, V.; Guillemi, E.C.; Londoño, L.; Dubin, A. Caudal vena cava collapsibility index as a tool to predict fluid responsiveness in dogs. J. Vet. Emerg. Crit. Care 2020, 30, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Argaiz, E.R.; Koratala, A.; Reisinger, N. Comprehensive Assessment of Fluid Status by Point-of-Care Ultrasonography. Kidney360 2021, 2, 1326–1338. [Google Scholar] [CrossRef]
- Kearney, D.; Reisinger, N.; Lohani, S. Integrative Volume Status Assessment. POCUS J. 2022, 7, 65–77. [Google Scholar] [CrossRef]
- Di Nicolò, P.; Tavazzi, G.; Nannoni, L.; Corradi, F. Inferior Vena Cava Ultrasonography for Volume Status Evaluation: An Intriguing Promise Never Fulfilled. J. Clin. Med. 2023, 12, 2217. [Google Scholar] [CrossRef]
- Rahman, N.H.N.; Ahmad, R.; Kareem, M.M.; Mohammed, M.I. Ultrasonographic assessment of inferior vena cava/abdominal aorta diameter index: A new approach of assessing hypovolemic shock class 1. Int. J. Emerg. Med. 2016, 9, 8. [Google Scholar] [CrossRef]
- Ma, Z.; Gai, J.; Sun, Y.; Bai, Y.; Cai, H.; Wu, L.; Sun, L.; Liu, J.; Xue, L.; Liu, B. Measuring the ratio of femoral vein diameter to femoral artery diameter by ultrasound to estimate volume status. BMC Cardiovasc. Disord. 2021, 21, 506. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, M.; Kaçmaz, M. Correlation of internal jugular vein, common carotid artery, femoral artery and femoral vein diameters with central venous pressure. Medicine 2022, 101, E31207. [Google Scholar] [CrossRef]
- Darnis, E.; Boysen, S.; Merveille, A.C.; Desquilbet, L.; Chalhoub, S.; Gommeren, K. Establishment of reference values of the caudal vena cava by fast-ultrasonography through different views in healthy dogs. J. Vet. Intern. Med. 2018, 32, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Darnis, E.; Merveille, A.C.; Desquilbet, L.; Boysen, S.; Gommeren, K. Interobserver agreement between non-cardiologist veterinarians and a cardiologist after a 6-hour training course for echographic evaluation of basic echocardiographic parameters and caudal vena cava diameter in 15 healthy Beagles. J. Vet. Emerg. Crit. Care 2019, 29, 495–504. [Google Scholar] [CrossRef]
- Rabozzi, R.; Oricco, S.; Meneghini, C.; Bucci, M.; Franci, P. Evaluation of the caudal vena cava diameter to abdominal aortic diameter ratio and the caudal vena cava respiratory collapsibility for predicting fluid responsiveness in a heterogeneous population of hospitalized conscious dogs. J. Vet. Med. Sci. 2020, 82, 337–344. [Google Scholar] [CrossRef]
- Fujioka, T.; Nakamura, K.; Minamoto, T.; Tsuzuki, N.; Yamaguchi, J.; Hidaka, Y. Ultrasonographic evaluation of the caudal vena cava in dogs with right-sided heart disease. J. Vet. Cardiol. 2021, 34, 80–92. [Google Scholar] [CrossRef]
- Barthélemy, A.; Combet-Curt, J.; Dupanloup, A.; Gillet, B.; Cambournac, M.; Bonnet-Garin, J.-M.; Goy-Thollot, I.; Pouzot-Nevoret, C. Establishment of Reference Intervals for Caudal Vena Cava-to-Aorta Ratio Measured Ultrasonographically in Healthy Nonsedated Dogs. Top. Companion Anim. Med. 2023, 56–57, 100822. [Google Scholar] [CrossRef]
- Tuplin, M.C.; Romero, A.E.; Boysen, S.R. Influence of the Respiratory Cycle on Caudal Vena Cava Diameter Measured by Sonography in Healthy Foals: A Pilot Study. J. Vet. Intern. Med. 2017, 31, 1556–1562. [Google Scholar] [CrossRef]
- Casalta, H.; Busoni, V.; Eppe, J.; Grulke, S.; Merveille, A.-C.; Moula, N.; Gommeren, K. Ultrasonographical Assessment of Caudal Vena Cava Size through Different Views in Healthy Calves: A Pilot Study. Vet. Sci. 2022, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, C.; Freccero, F.; Lanci, A.; Hallowell, G.D.; Bullone, C.; Castagnetti, C.; Pasolini, M.P. Transabdominal ultrasonographic measurement of caudal vena cava to aorta derived ratios in clinically healthy neonatal foals. Vet. Med. Sci. 2021, 7, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.A.; Thomovsky, E.J.; Brooks, A.C.; Johnson, P.A.; Kin Lim, C.; Gan Heng, H. Article Ultrasound measurements of the caudal vena cava before and after blood donation in 9 greyhound dogs. Can. Vet. J. 2018, 59, 973–980. [Google Scholar]
- Cambournac, M.; Goy-Thollot, I.; Violé, A.; Boisvineau, C.; Pouzot-Nevoret, C.; Barthélemy, A. Sonographic assessment of volaemia: Development and validation of a new method in dogs. J. Small Anim. Pract. 2018, 59, 174–182. [Google Scholar] [CrossRef]
- Kwak, J.; Yoon, H.; Kim, J.; Kim, M.; Eom, K. Ultrasonographic measurement of caudal vena cava to aorta ratios for determination of volume depletion in normal beagle dogs. Vet. Radiol. Ultrasound 2018, 59, 203–211. [Google Scholar] [CrossRef]
- Balıkçı, C.; Gülersoy, E.; Şahan, A.; Günal, I. Efficacy of ultrasonographic caudal vena cava to aorta ratios for quantifying canine parvoviral enteritis rehydration. Vet. Radiol. Ultrasound 2023, 64, 930–935. [Google Scholar] [CrossRef]
- Combet-Curt, J.; Pouzot-Nevoret, C.; Cambournac, M.; Magnin, M.; Nectoux, A.; Bonnet-Garin, J.M.; Goy-Thollot, I.; Barthélemy, A. Ultrasonographic measurement of caudal vena cava to aorta ratio during fluid resuscitation of dogs with spontaneous circulatory shock. J. Small Anim. Pract. 2023, 64, 669–679. [Google Scholar] [CrossRef]
- Meneghini, C.; Rabozzi, R.; Franci, P. Correlation of the ratio of caudal vena cava diameter and aorta diameter with systolic pressure variation in anesthetized dogs. Am. J. Vet. Res. 2016, 77, 137–143. [Google Scholar] [CrossRef]
- Henneke, D.R.; Potter, G.D.; Kreider, J.L.; Yeates, B.F. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet. J. 1983, 15, 371–372. [Google Scholar] [CrossRef]
- Moore, J.; Barton, M.; White, N.; Buchanan, F.; Melton, T.; Jackson, J.; Gilleland, B.; Smith, M.; Lockerman, K. The Glass Horse Equine Colic; The University of Georgia: Athens, GA, USA, 2007. [Google Scholar]
- Denoix, J.M. Ventral aspect of the equine pelvis: Vessels and nerves. In Essentials of Clinical Anatomy of the Equine Locomotor System, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019; p. 182. [Google Scholar]
- Watson, P.F.; Petrie, A. Method agreement analysis: A review of correct methodology. Theriogenology 2010, 73, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.M. Airway Physiology and Clinical Function Testing. Vet. Clin. N. Am. Small Anim. Pract. 2007, 37, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.; Hudson, J.; Bao, Y.; Gaillard, P. Aortic to caudal vena cava ratio measurements using abdominal ultrasound are increased in dogs with confirmed systemic hypertension. Vet. Radiol. Ultrasound 2020, 61, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Kaminecki, I.; Huang, D.M.; Shipman, P.C.; Gibson, R.W. Point-of-Care Ultrasonography for the Assessment of Dehydration in Children A Systematic Review. Pediatr. Emerg. Care 2023, 39, 786–796. [Google Scholar] [CrossRef]
- Mannarino, S.; Bulzomì, P.; Codazzi, A.C.; Rispoli, G.A.; Tinelli, C.; De Silvestri, A.; Manzoni, F.; Chiapedi, S. Inferior vena cava, abdominal aorta, and IVC-to-aorta ratio in healthy Caucasian children: Ultrasound Z-scores according to BSA and age. J. Cardiol. 2019, 74, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Fielding, C.L.; Magdesian, K.G.; Edman, J.E. Determination of body water compartments in neonatal foals by use of indicator dilution techniques and multifrequency bioelectrical impedance analysis. Am. J. Vet. Res. 2011, 72, 1390–1396. [Google Scholar] [CrossRef]
- Cardillo, J.H.; Zersen, K.M.; Cavanagh, A.A. Point of care ultrasound measurement of paralumbar caudal vena cava diameter and caudal vena cava to aortic ratio in hypovolemic dogs. Front. Vet. Sci. 2024, 11, 1467043. [Google Scholar] [CrossRef]
- Oh, J.H. Noninvasive and simple, but accurate? Meta-analysis of evidence-based point-of-care ultrasound for assessing dehydration in children. Clin. Exp. Pediatr. 2023, 66, 475. [Google Scholar] [CrossRef]
- Mele, D.; Pedini, I.; Alboni, P.; Levine, R.A. Anatomic M-mode: A new technique for quantitative assessment of left ventricular size and function. Am. J. Cardiol. 1998, 81, 82G–85G. [Google Scholar] [CrossRef]
- American College of Emergency Physicians. Emergency ultrasound guidelines. Ann. Emerg. Med. 2009, 53, 550–570. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.W.; Frost, C. Reliability, repeatability and reproducibility: Analysis of measurement errors in continuous variables. Ultrasound Obstet. Gynecol. 2008, 31, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Muir, W.W.; Hubbell, J.A.E. Equine Anesthesia: Monitoring and Emergency Therapy, 2nd ed.; Saunders: Philadelphia, PA, USA, 2014. [Google Scholar]
- Yamashita, K.; Tsubakishita, S.; Futaoka, S.; Ueda, I.; Hamaguchi, H.; Seno, T.; Katoh, S.; Izumisawa, Y.; Kotani, T.; Muir, W.W. Cardiovascular Effects of Medetomidine, Detomidine and Xylazine in Horses. J. Vet. Med. Sci. 2000, 62, 1025–1032. [Google Scholar] [CrossRef]
- Herreria-Bustillo, V.J.; Fitzgerald, E.; Humm, K.R. Caval-aortic ratio and caudal vena cava diameter in dogs before and after blood donation. J. Vet. Emerg. Crit. Care 2019, 29, 643–646. [Google Scholar] [CrossRef]

| B-Mode | M-Mode | |
|---|---|---|
| Transrectal examination | ||
| Measured variables | ||
| Ao | Diam (cm) | Diam (cm) |
| Area (cm2) | ||
| CVC min | Diam (cm) | Diam (cm) |
| Area (cm2) | ||
| CVC max | Diam (cm) | Diam (cm) |
| Area (cm2) | ||
| Calculated variables | ||
| CI CVC | =(CVC diam max -CVC diam min)/(CVC diam max) | =(CVC diam max -CVC diam min)/(CVC diam max) |
| = (CVC area max -CVC area min)/(CVC area max) | ||
| CVC/Ao | =(CVC diam max/Ao diam) | =(CVC diam max/Ao diam) |
| =(CVC area max/Ao area) | ||
| Inguinal examination | ||
| Measured variables | ||
| FA | Diam (cm) | n/a |
| Area (cm2) | ||
| FV | Diam (cm) | n/a |
| Area (cm2) | ||
| Calculated variables | ||
| FV/FA | =(FV diam max/FA diam) | n/a |
| =(FV area max/FA area) |
| B-Mode | M-Mode | |||
|---|---|---|---|---|
| Vessel | Mean ± SD | 95% Confidence Interval (Lower-Upper) | Mean ± SD | 95% CI (Lower-Upper) |
| Ao diam (cm) | 3.3 ± 0.3 | (3.23–3.40) | 3.3 ± 0.3 | (3.25–3.42) |
| Ao area (cm2) | 8.7 ± 1.7 | (8.27–9.14) | n/a | n/a |
| CVC diam min (cm) | 1.0 ± 0.3 | (0.91–1.07) | 1.0 ± 0.3 | (0.88–1.04) |
| CVC diam max (cm) | 1.4 ± 0.4 | (1.32–1.53) | 1.4 ± 0.4 | (1.34–1.54) |
| CVC area min (cm2) | 3.2 ± 1.2 | (2.86–3.46) | n/a | n/a |
| CVC area max (cm2) | 5.0 ± 1.7 | (4.56–5.43) | n/a | n/a |
| B-Mode | M-Mode | |||||
|---|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | 95% CI (Lower-Upper) | Mean ± SD | Median (IQR) | 95% CI (Lower-Upper) | |
| CI CVC on diam | 0.30 ± 0.13 | n/a | (0.27–0.33) | 0.33 ± 0.12 | n/a | (0.30–0.36) |
| CI CVC on area | 0.36 ± 0.15 | n/a | (0.32–0.40) | n/a | n/a | n/a |
| CVC/Ao diam | 0.43 ± 0.15 | n/a | (0.40–0.46) | 0.43 ± 0.11 | n/a | (0.40–0.46) |
| CVC/Ao area * | n/a | 0.56 (0.53–0.64) | n/a | n/a | n/a | n/a |
| Vessel | Median (IQR) |
|---|---|
| FA diam (cm) * | 0.2 (0.22–0.25) |
| FA area (cm2) * | 0.04 (0.04–0.05) |
| FV diam (cm) * | 0.8 (0.82–0.97) |
| FV area (cm2) * | 1.0 (1.08–1.46) |
| Mean ± SD | Median (IQR) | 95% CI (Lower-Upper) | |
|---|---|---|---|
| FV/FA diam | 3.80 ± 1.02 | n/a | (3.54–4.05) |
| FV/FA area * | n/a | 26.74 (25.23–32.40) | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scala, E.; Durie, I.; Gommeren, K.; Saegerman, C.; van Galen, G. Ultrasonographic Assessment of Caudal Vena Cava Collapsibility Index, Caudal Vena Cava-to-Aorta, and Femoral Vein-to-Artery Ratios in Healthy Sedated Adult Horses. Animals 2025, 15, 2837. https://doi.org/10.3390/ani15192837
Scala E, Durie I, Gommeren K, Saegerman C, van Galen G. Ultrasonographic Assessment of Caudal Vena Cava Collapsibility Index, Caudal Vena Cava-to-Aorta, and Femoral Vein-to-Artery Ratios in Healthy Sedated Adult Horses. Animals. 2025; 15(19):2837. https://doi.org/10.3390/ani15192837
Chicago/Turabian StyleScala, Elisa, Inge Durie, Kris Gommeren, Claude Saegerman, and Gaby van Galen. 2025. "Ultrasonographic Assessment of Caudal Vena Cava Collapsibility Index, Caudal Vena Cava-to-Aorta, and Femoral Vein-to-Artery Ratios in Healthy Sedated Adult Horses" Animals 15, no. 19: 2837. https://doi.org/10.3390/ani15192837
APA StyleScala, E., Durie, I., Gommeren, K., Saegerman, C., & van Galen, G. (2025). Ultrasonographic Assessment of Caudal Vena Cava Collapsibility Index, Caudal Vena Cava-to-Aorta, and Femoral Vein-to-Artery Ratios in Healthy Sedated Adult Horses. Animals, 15(19), 2837. https://doi.org/10.3390/ani15192837






