Moderate Reduction in Dietary Net Energy Level Enhances Intestinal Health in Tunchang Pigs via Gut Microbiota Modulation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals, Diets, and Experimental Design
2.3. Sample Collection
2.4. Intestinal Histomorphology
2.5. Antioxidant Index Detection
2.6. Determination of Specific Gene Expression Using Quantitative Real-Time PCR
2.7. Intestinal Microbiota Analysis
2.8. Determination of Short-Chain Fatty Acids in Colon Contents
2.9. Statistical Analysis
3. Results
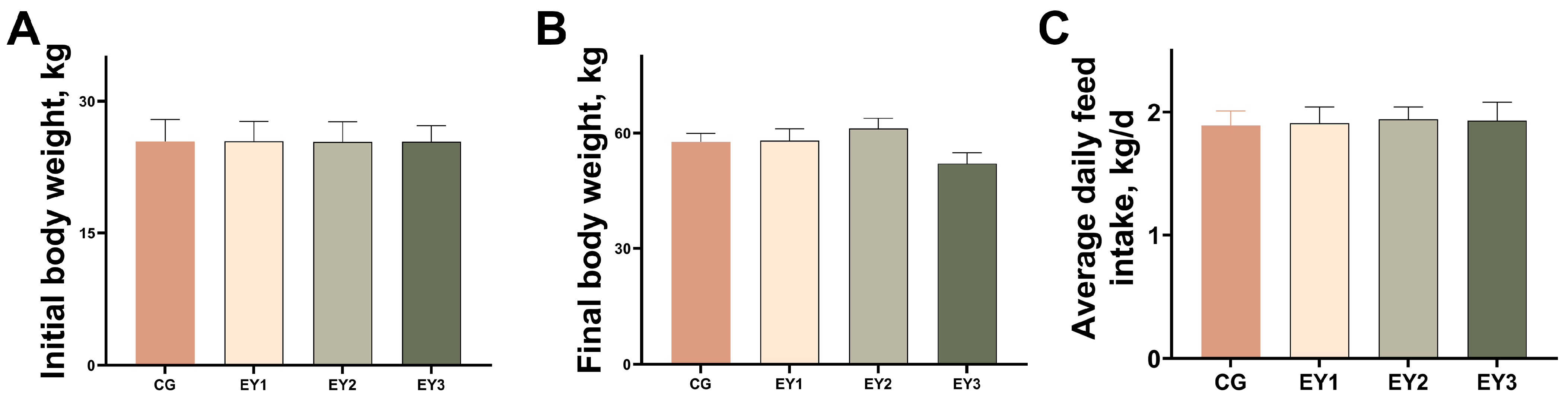
3.1. Effects of Low Net-Energy Diets on the Growth Performance of Tunchang Pigs
3.2. Effects of Low Net-Energy Diets on the Intestinal Morphology of Tunchang Pigs
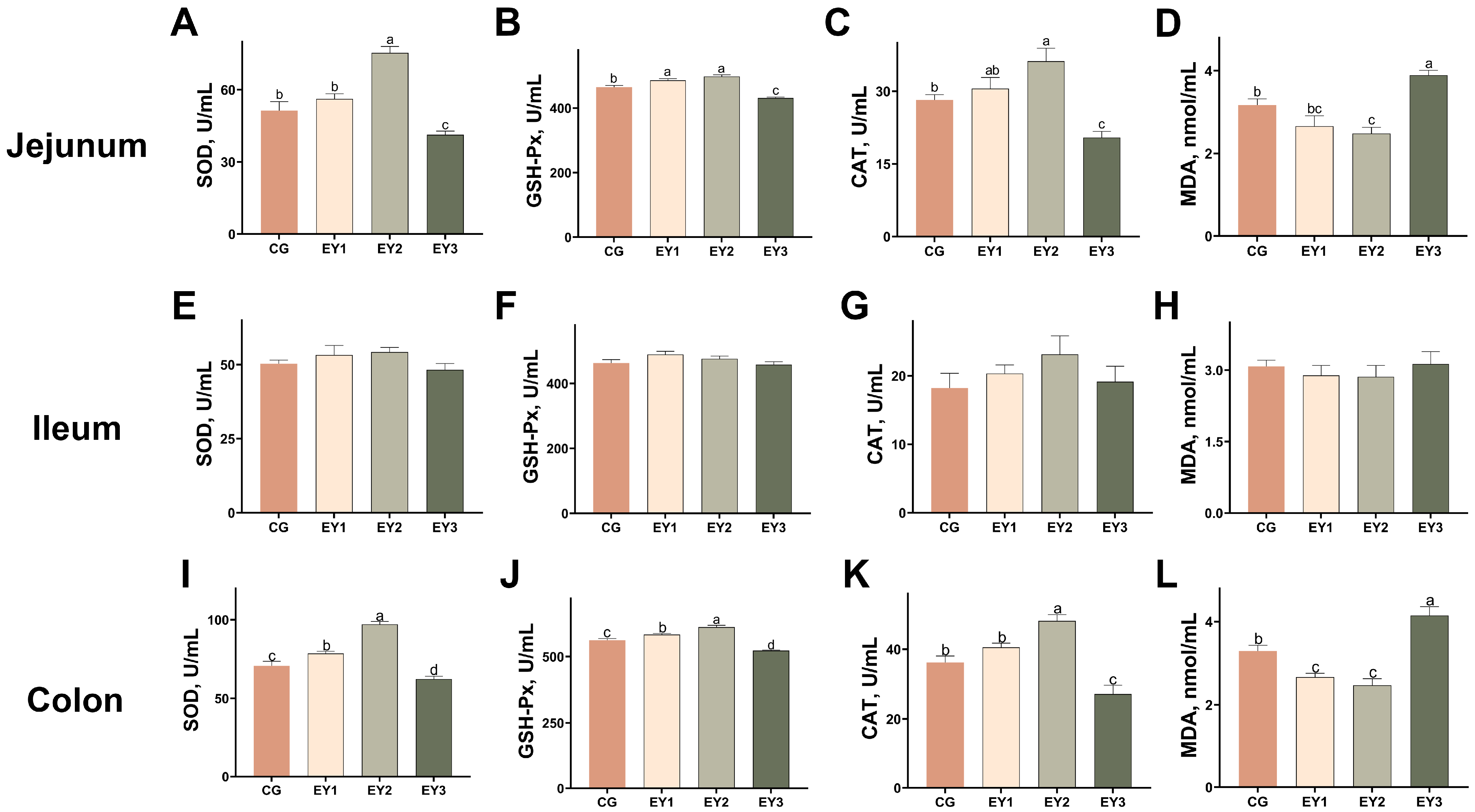
3.3. Effects of Low Net-Energy Diets on the Intestinal Antioxidant Capacity of Tunchang Pigs
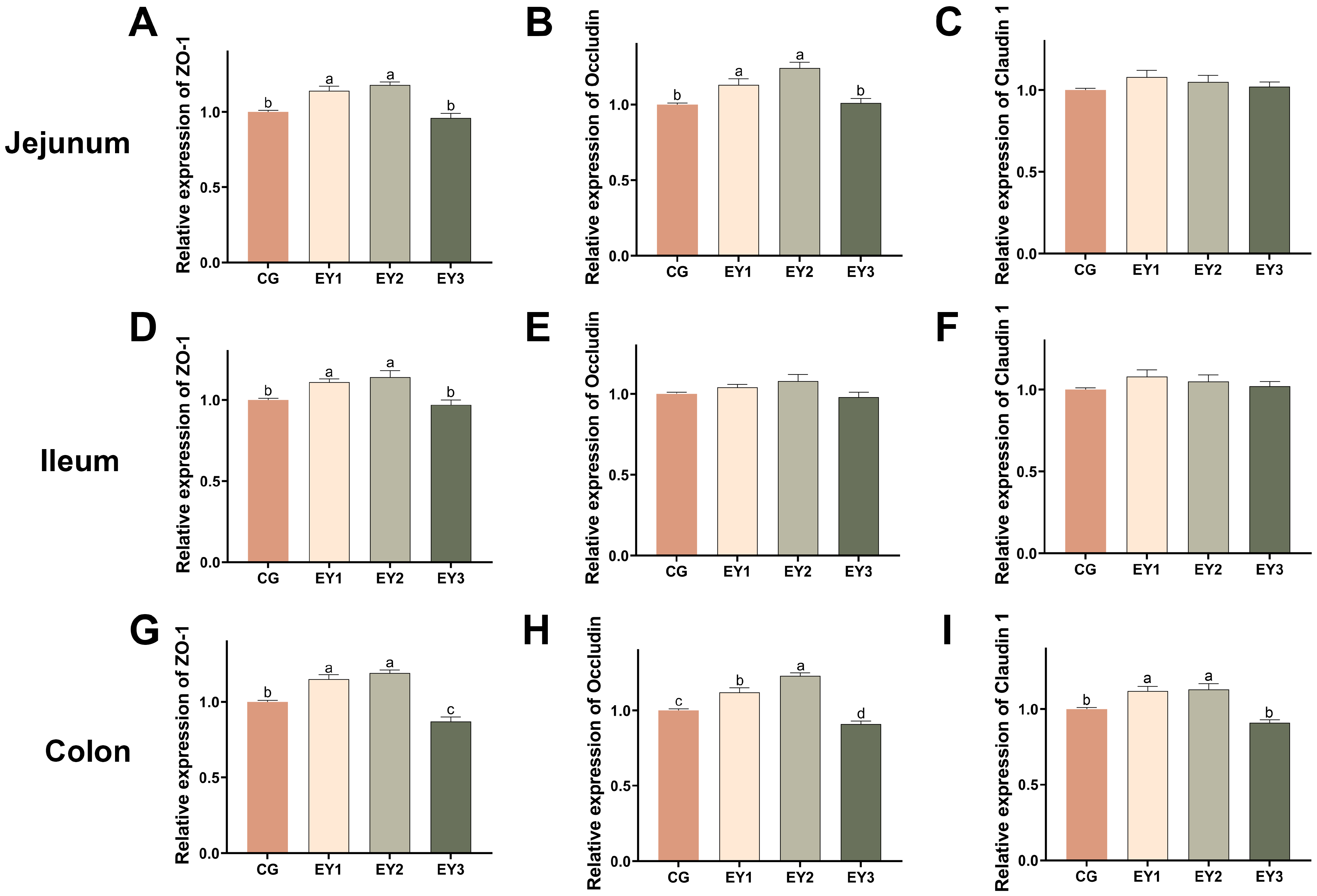
3.4. Effects of Low Net-Energy Diets on the Intestinal Barrier of Tunchang Pigs
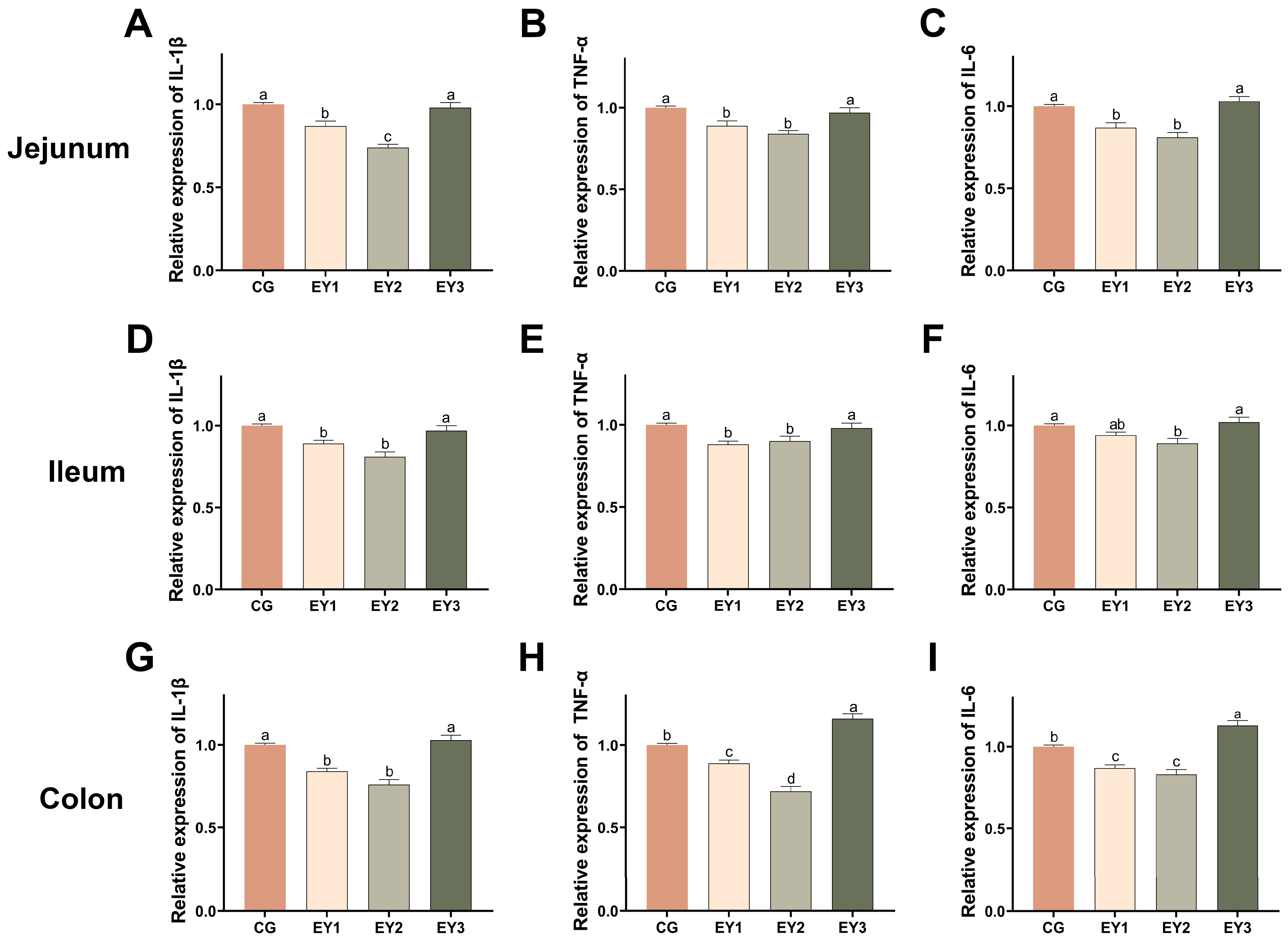
3.5. Effects of Low Net-Energy Diets on the Intestinal Inflammatory Response of Tunchang Pigs
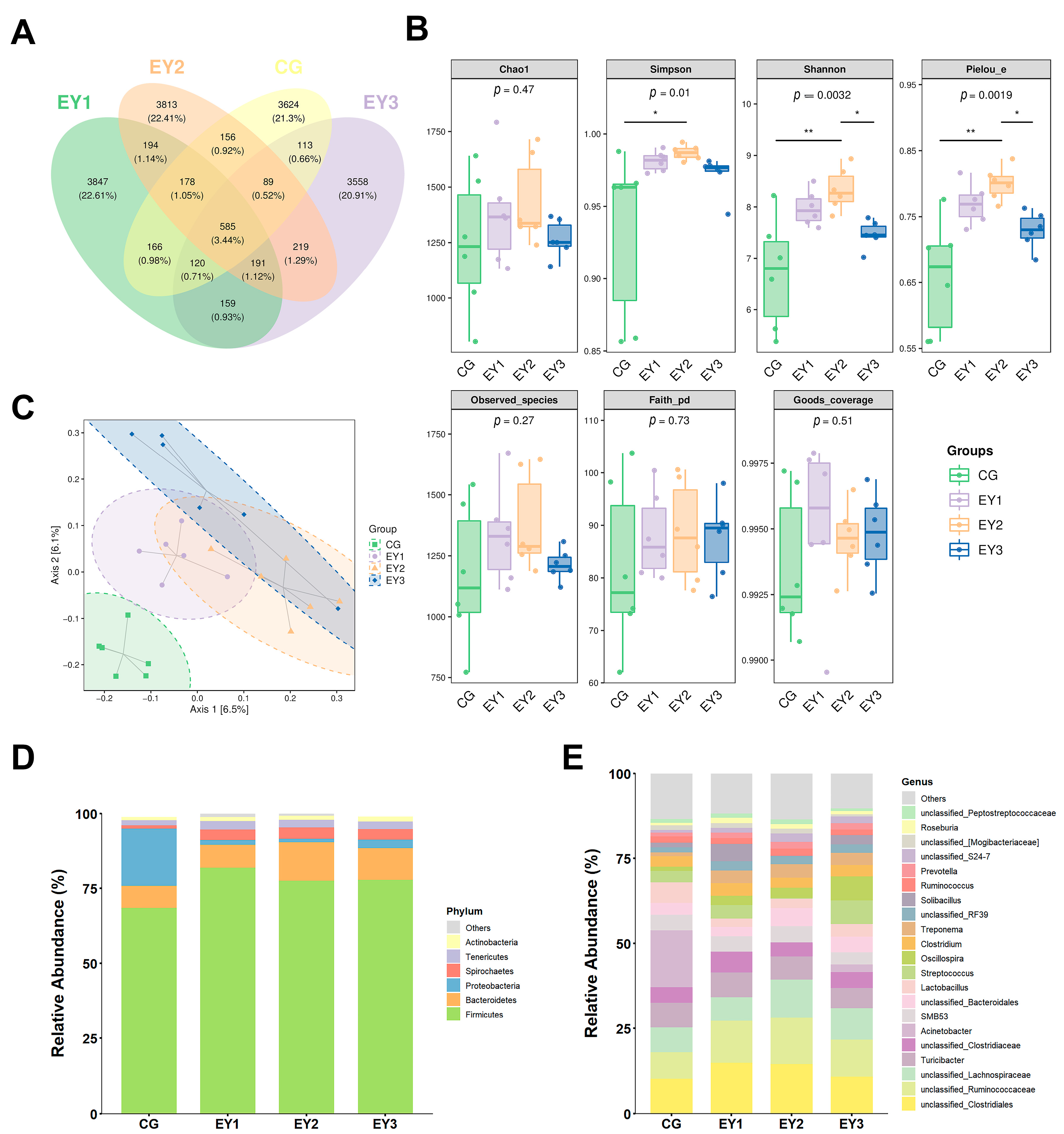
3.6. Diversity Changes in Gut Microbiota Under Low Net-Energy Diets
3.7. Compositional Changes in Gut Microbiota Under Low Net-Energy Diets
3.8. Impact of Low Net-Energy Diets on Gut Microbiota via LEfSe Analysis
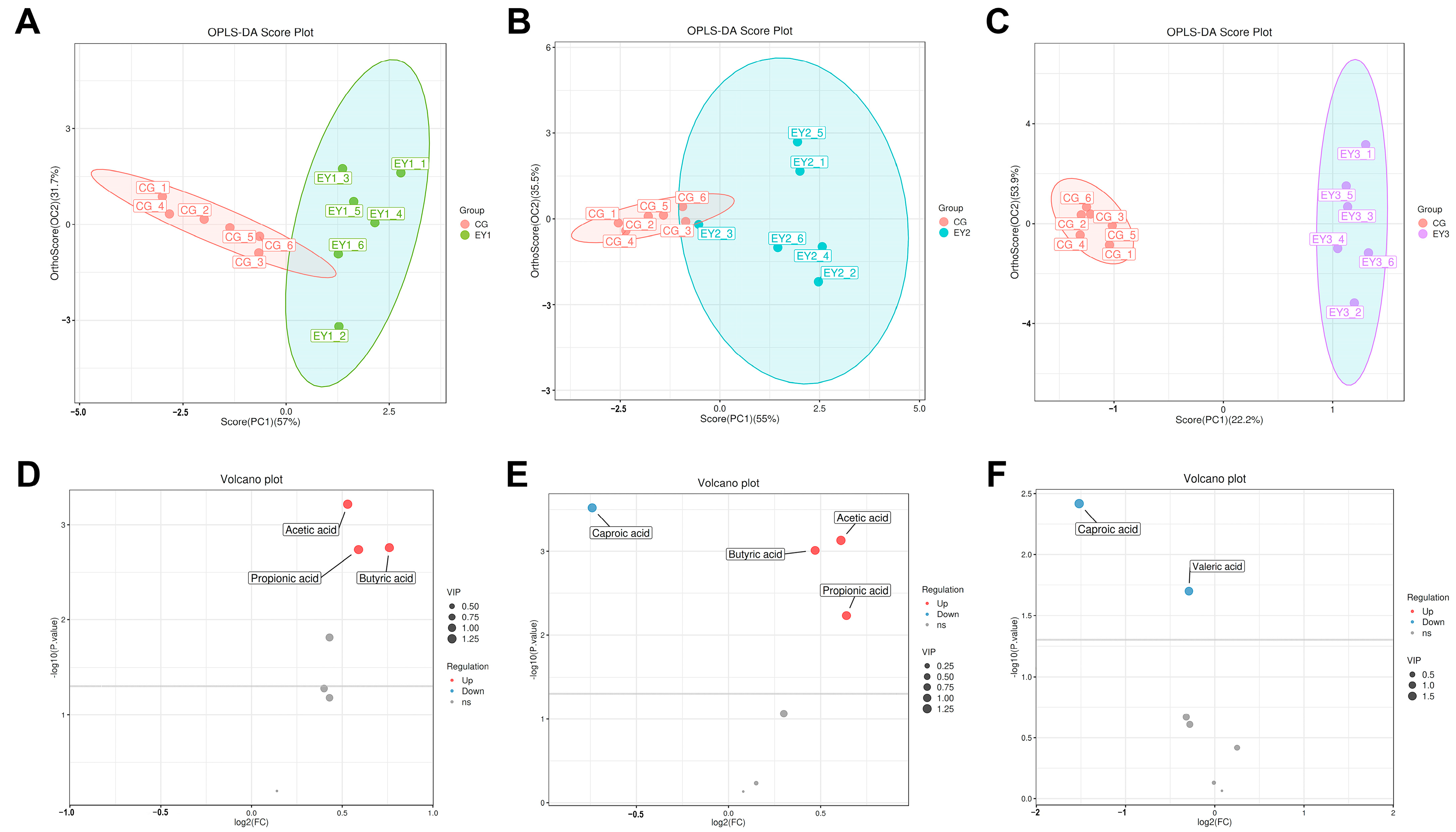
3.9. Changes in Gut Short-Chain Fatty Acids
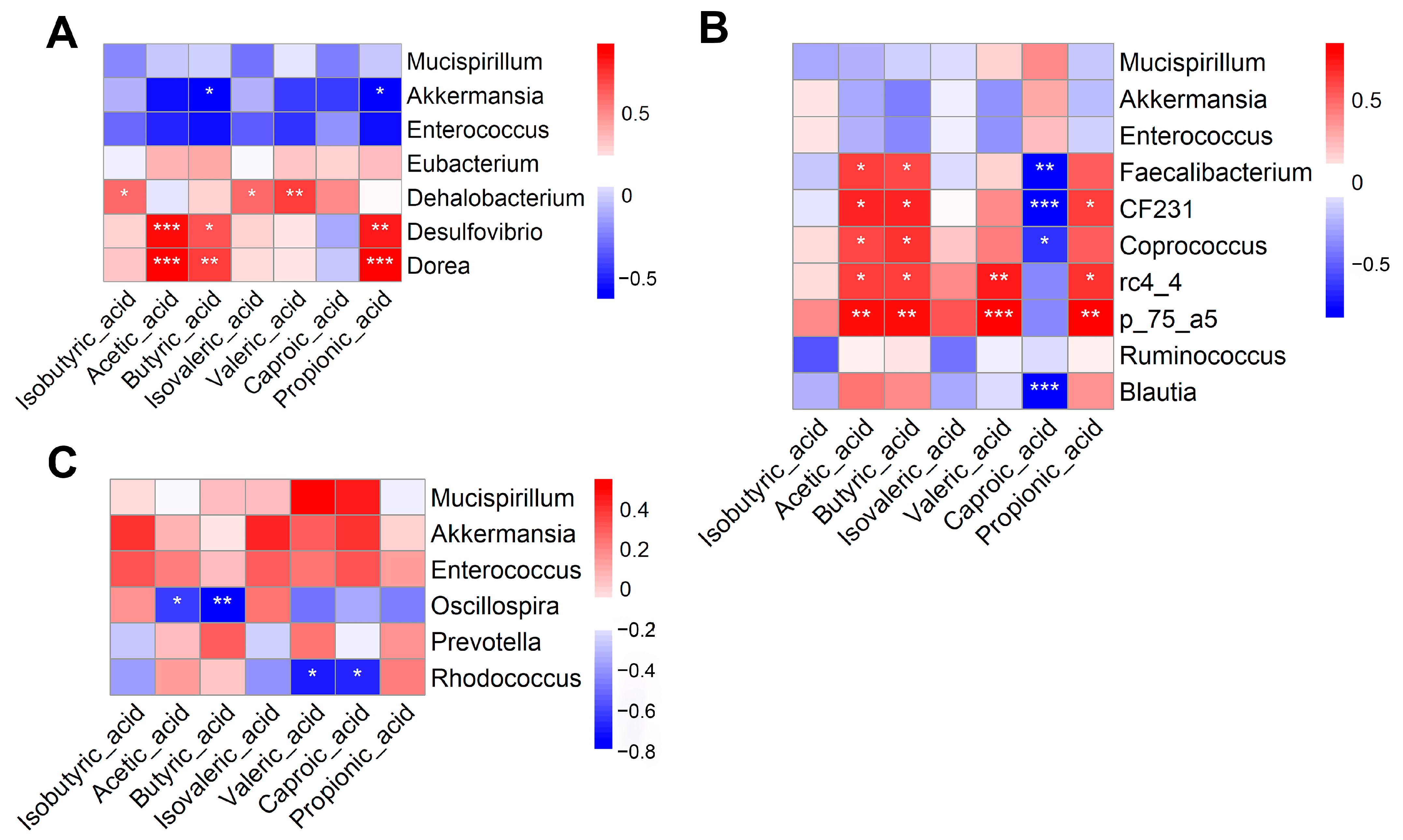
3.10. Correlation Analysis Between Differentially Abundant Microbial Genera and Short-Chain Fatty Acids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, H.; Zhan, W.; Du, Z.; Hong, S.; Dong, T.; She, J.; Min, C. Pork primal cuts recognition method via computer vision. Meat Sci. 2022, 192, 108898. [Google Scholar] [CrossRef]
- Noblet, J.; Wu, S.-B.; Choct, M. Methodologies for energy evaluation of pig and poultry feeds: A review. Anim. Nutr. 2022, 8, 185–203. [Google Scholar] [CrossRef]
- Shen, X.; Qiu, C. Research on the mechanism of corn price formation in China based on the PLS-SEM model. Foods 2024, 13, 875. [Google Scholar] [CrossRef]
- Tappenden, K.A. Inflammation and Intestinal Function. Where does it start and what does it mean? J. Parenter. Enteral Nutr. 2008, 32, 648–650. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Fan, S.; Wang, J.; Zhou, W.; Zhou, Z.; Liu, Y.; Wang, Q.; Liu, W.; Dai, X. Chitosan oligosaccharide improves intestinal homeostasis to achieve the protection for the epithelial barrier of female Drosophila melanogaster via regulating intestinal microflora. Microbiol. Spectr. 2024, 12, e03639-23. [Google Scholar] [CrossRef] [PubMed]
- Adebowale, T.; Jiang, Q.; Yao, K. Dietary fat and high energy density diet: Influence on intestinal health, oxidative stress and performance of weaned piglets. J. Anim. Physiol. Anim. Nutr. 2024, 108, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Kok, D.E.G.; Rusli, F.; van der Lugt, B.; Lute, C.; Laghi, L.; Salvioli, S.; Picone, G.; Franceschi, C.; Smidt, H.; Vervoort, J.; et al. Lifelong calorie restriction affects indicators of colonic health in aging C57Bl/6J mice. J. Nutr. Biochem. 2018, 56, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; Lv, Z.; Zhou, L.; Guo, Y. Analysis of the effects of b-mannanase on immune function and intestinal flora in broilers fed the low energy diet based on 16S rRNA sequencing and metagenomic sequencing. Poult. Sci. 2024, 103, 103581. [Google Scholar] [CrossRef]
- Li, Z.; Pu, J.; Zeng, T.; Cai, J.; Jia, G.; Zhao, H.; Liu, G.; Zeng, Q.; Luo, Y.; Tian, G. Effects of betaine on growth performance and intestinal health of rabbits fed different digestible energy diets. J. Anim. Sci. 2024, 102, skae029. [Google Scholar] [CrossRef]
- Griela, E.; Paraskeuas, V.; Mountzouris, K.C. Effects of diet and phytogenic inclusion on the antioxidant capacity of the broiler chicken gut. Animals 2021, 11, 739. [Google Scholar] [CrossRef]
- China GB/T 39235-2020; Nutrient Requirements of Swine, China. Standards Press of China: Beijing, China, 2020.
- Zheng, L.; Duarte, M.E.; Sevarolli Loftus, A.; Kim, S.W. Intestinal Health of Pigs Upon Weaning: Challenges and Nutritional Intervention. Front. Vet. Sci. 2021, 8, 628258. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Y.; Zhou, T.; Feng, Y.; Li, L. Common factors and nutrients affecting intestinal villus height—A review. Anim. Biosci. 2025, 38, 1557–1569. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Piao, X. Dietary 25-hydroxycholecalciferol supplementation improves performance, immunity, antioxidant status, intestinal morphology, and bone quality in weaned piglets. J. Sci. Food Agric. 2021, 101, 2592–2600. [Google Scholar] [CrossRef]
- Zuo, L.; Kuo, W.-T.; Turner, J.R. Tight junctions as targets and effectors of mucosal immune homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 327–340. [Google Scholar] [CrossRef]
- Oladokun, S.; Koehler, A.; MacIsaac, J.; Ibeagha-Awemu, E.M.; Adewole, D.I. Bacillus subtilis delivery route: Effect on growth performance, intestinal morphology, cecal short-chain fatty acid concentration, and cecal microbiota in broiler chickens. Poult. Sci. 2021, 100, 100809. [Google Scholar] [CrossRef]
- Marion, J.; Biernat, M.; Thomas, F.; Savary, G.; Le Breton, Y.; Zabielski, R.; Le Huerou-Luron, I.; Le Dividich, J. Small intestine growth and morphometry in piglets weaned at 7 days of age. effects of level of energy intake. Reprod. Nutr. Dev. 2002, 42, 339–354. [Google Scholar] [CrossRef]
- Mahdavi, R.; Harsini, S.G.; Piray, A.H. The effect of reducing dietary energy on performance, intestinal morphology and intestinal peptide and amino acid transporters in broiler chicks. Vet. Med. Sci. 2024, 10, e1364. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Gong, L.; Wang, J.; Fu, J.; Lv, Z.; Zhou, L.; Li, X.; Liu, Q.; Xia, P.; et al. Mannanase improves the growth performance of broilers by alleviating inflammation of the intestinal epithelium and improving intestinal microbiota. Anim. Nutr. 2024, 16, 376–394. [Google Scholar] [CrossRef]
- Yu, Y.; Zi, Y.; Fu, R.; Fu, B.; Li, C.; Lv, Y.; Li, Z.; Wang, H.; Leng, J. Effects of dietary energy levels on microorganisms and short-chain fatty acids of rumen and tight junction proteins in Honghe Yellow cattle. Front. Microbiol. 2024, 15, 1335818. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yan, X.; An, J.; Wang, X.; Mi, H.; Liu, F. Modified Jiaoqi Powder enhances epithelial autophagy against TNF-triggered apoptosis in chronic ulcerative colitis. Phytomedicine 2025, 136, 155996. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Cao, T.; Ipemba, E.; Bakala, G.B.; Leveut, L.G.D.; Peng, W.; Ji, F.; Li, H.; Xu, L.; Wu, H. Effects of dietary supplementation with bile acids on growth performance, antioxidant capacity, lipid metabolism, and cecal microbiota of Danzhou chickens. Poult. Sci. 2025, 104, 105276. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.-R.; Yu, J.; Choi, S.-J. Hydrothermal treatment enhances antioxidant activity and intestinal absorption of rutin in tartary buckwheat flour extracts. Foods 2020, 9, 8. [Google Scholar] [CrossRef]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radical Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Gebicka, L.; Krych-Madej, J. The role of catalases in the prevention/promotion of oxidative stress. J. Inorg. Biochem. 2019, 197, 110699. [Google Scholar] [CrossRef]
- Tu, J.; Kang, M.; Zhao, Q.; Xue, C.; Bi, C.; Dong, N. Oleanolic acid improves antioxidant capacity and the abundance of Faecalibacterium prausnitzii in the intestine of broilers. Poult. Sci. 2024, 103, 104340. [Google Scholar] [CrossRef]
- Kim, Y.E.; Kim, J. ROS-scavenging therapeutic hydrogels for modulation of the inflammatory response. ACS Appl. Mater. Interfaces 2022, 14, 23002–23021. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, H.; Jiang, Y.; Wang, J.; Wu, D.; Wu, C.; Che, L.; Lin, Y.; Zhuo, Y.; Luo, Z.; et al. Chromium propionate supplementation to energy- and protein-reduced diets reduces feed consumption but improves feed conversion ratio of yellow-feathered male broilers in the early period and improves meat quality. Poult. Sci. 2024, 103, 103260. [Google Scholar] [CrossRef]
- Wang, M.; Huang, H.; Wang, L.; Yin, L.; Yang, H.; Chen, C.; Zheng, Q.; He, S. Tannic acid attenuates intestinal oxidative damage by improving antioxidant capacity and intestinal barrier in weaned piglets and IPEC-J2 cells. Front. Nutr. 2022, 9, 1012207. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Camara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Zeng, Z.; Xu, X.; Zhang, Q.; Li, P.; Zhao, P.; Li, Q.; Liu, J.; Piao, X. Effects of essential oil supplementation of a low-energy diet on performance, intestinal morphology and microflora, immune properties and antioxidant activities in weaned pigs. Anim. Sci. J. 2015, 86, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Von Schwartzenberg, R.J.; Bisanz, J.E.; Lyalina, S.; Spanogiannopoulos, P.; Ang, Q.Y.; Cai, J.; Dickmann, S.; Friedrich, M.; Liu, S.-Y.; Collins, S.L.; et al. Caloric restriction disrupts the microbiota and colonization resistance. Nature 2021, 595, 272–277. [Google Scholar] [CrossRef]
- Gao, L.; Li, J.; Zhou, Y.; Huang, X.; Qin, X.; Du, G. Effects of baicalein on cortical proinflammatory cytokines and the intestinal microbiome in senescence accelerated mouse prone 8. ACS Chem. Neurosci. 2018, 9, 1714–1724. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, H.; Zhang, Y.; Gu, J.; Zhang, X.; Gong, X.; Hao, Z. Comprehensive effect of carbon tetrachloride and reversal of gandankang formula in mice liver: Involved in oxidative stress, excessive inflammation, and intestinal microflora. Antioxidants 2022, 11, 2234. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Su, Y.; Hu, K.; Li, D.; Guo, H.; Xie, Z. Microbial-transferred metabolites of black tea theaflavins by human gut microbiota and their impact on antioxidant capacity. Molecules 2023, 28, 5871. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, Y.; Zhang, X.; Feng, Y.; Li, C.; Ge, Y.; Yang, Y.; Su, J.; Chu, X. The effects of degraded polysaccharides from Acanthopanax senticosus on growth, antioxidant and immune effects in broiler chicks based on intestinal flora. Poult. Sci. 2025, 104, 104933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-J.; Qu, H.-L.; Zhao, N.; Wang, J.; Wang, X.-Y.; Hai, R.; Li, B. Assessment of causal direction between gut microbiota and inflammatory bowel disease: A mendelian randomization analysis. Front. Genet. 2021, 12, 631061. [Google Scholar] [CrossRef]
- Mohebali, N.; Ekat, K.; Kreikemeyer, B.; Breitrueck, A. Barrier protection and recovery effects of gut commensal bacteria on differentiated intestinal epithelial cells in vitro. Nutrients 2020, 12, 2251. [Google Scholar] [CrossRef]
- Yang, R.; Shan, S.; Shi, J.; Li, H.; An, N.; Li, S.; Cui, K.; Guo, H.; Li, Z. Coprococcus eutactus, a potent probiotic, alleviates colitis via acetate-mediated IgA response and microbiota restoration. J. Agric. Food. Chem. 2023, 71, 3273–3284. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Jung, D.-H.; Park, C.-S. Important roles of Ruminococcaceae in the human intestine for resistant starch utilization. Food Sci. Biotechnol. 2024, 33, 2009–2019. [Google Scholar] [CrossRef]
- Tian, H.; Wen, Z.; Chen, J.; Zhao, C.; Yang, C.; Guo, Y.; Sun, B. Alleviation of DSS-induced ulcerative colitis by pomelo peel polysaccharides: Exploration for the potential mechanism from comprehensive analysis of multi-omics. Food Biosci. 2025, 65, 105955. [Google Scholar] [CrossRef]
- Xing, R.; Du, P.; Wang, Z.; Fan, Z.; Wang, L.; Huang, Y.; Chen, W.; Si, X. Porcine bile acids improve antioxidant status and immune function by increasing hungatella abundance with different protein level diets in late-laying hens. Animals 2025, 15, 500. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, J.; Cai, Z.; Zhou, K.; Chang, L.; Bai, Y.; Ma, Y. Prevotella induces the production of Th17 cells in the colon of mice. J. Immunol. Res. 2020, 2020, 9607328. [Google Scholar] [CrossRef] [PubMed]
- Saied, A.A.; Bryan, L.K.; Bolin, D.C. Ulcerative, granulomatous glossitis and enteritis caused by Rhodococcus equi in a heifer. J. Vet. Diagn. Investig. 2019, 31, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Boopathy, R. Methanogenesis from furfural by defined mixed cultures. Curr. Microbiol. 2002, 44, 406–410. [Google Scholar] [CrossRef]
- MacFabe, D.F. Enteric short-chain fatty acids: Microbial messengers of metabolism, mitochondria, and mind: Implications in autism spectrum disorders. Microb. Ecol. Health Dis. 2015, 26, 28177. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, X.; Deng, Q.; Huang, F. Rapeseed polysaccharides alleviate overweight induced by high-fat diet with regulation of gut microbiota in rats. Oil Crop. Sci. 2021, 6, 192–200. [Google Scholar] [CrossRef]
- Li, W.; Lin, X.; Liang, H.; Wu, Z.; Wang, M.; Sun, J.; Li, X.; He, W.; Gao, X.; Hu, T.; et al. Genomic and functional diversity of the human-derived isolates of Faecalibacterium. Front. Microbiol. 2024, 15, 1379500. [Google Scholar] [CrossRef]
- Gu, Q.; Xia, C.; Liu, N.; Chen, Z.; Zhou, Q.; Li, P. Lactobacillus plantarum ZJ316 alleviates ulcerative colitis by inhibiting inflammation and regulating short-chain fatty acid levels and the gut microbiota in a mouse model. Food Funct. 2023, 14, 3982–3993. [Google Scholar] [CrossRef]
- Notting, F.; Pirovano, W.; Sybesma, W.; Kort, R. The butyrate-producing and spore-forming bacterial genus Coprococcus as a potential biomarker for neurological disorders. Gut Microbiome 2023, 4, e16. [Google Scholar] [CrossRef]
- Wu, R.; Fu, W.; Wan, M.; Wu, W.; Yao, Y.; Li, W. Effect of polysaccharide from ganoderma atrum on hyperglycemia, blood lipid and gut microbiota of diabetic rats. Food Sci. 2022, 43, 91–102. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Hou, M.; Gao, T.; Sun, J.; Luo, H.; Wang, F.; Zhong, F.; Ma, A.; Cai, J. Lactobacillus casei improve anti-tuberculosis drugs-induced intestinal adverse reactions in rat by modulating gut microbiota and short-chain fatty acids. Nutrients 2022, 14, 1668. [Google Scholar] [CrossRef]
- Yun, S.I.; Ohta, Y. Removal of volatile fatty acids with immobilized Rhodococcus sp B261. Bioresour. Technol. 2005, 96, 41–46. [Google Scholar] [CrossRef]
- Liu, H.; Lu, H.; Wang, Y.; Yu, C.; He, Z.; Dong, H. Unlocking the power of short-chain fatty acids in ameliorating intestinal mucosal immunity: A new porcine nutritional approach. Front. Cell. Infect. Microbiol. 2024, 14, 1449030. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-J.; Xing, J.H.; Yan, Q.-S.; Zou, B.-S.; Wang, Y.-J.; Niu, T.-M.; Yu, T.; Huang, H.-B.; Zhang, D.; Zhang, S.-M.; et al. The acetic acid produced by lactobacillus species regulates immune function to alleviate pedv infection in piglets. Probiotics Antimicrob. Proteins 2024, 1–18. [Google Scholar] [CrossRef]
- Connolly, K.R.; Sweeney, T.; Kiernan, D.P.; Round, A.; Ryan, M.T.; Gath, V.; Maher, S.; Vigors, S.; O’Doherty, J.V. The role of propionic acid as a feed additive and grain preservative on weanling pig performance and digestive health. Anim. Feed Sci. Technol. 2025, 321, 116237. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, Q.; Qin, J.; Zhao, Q.; Shi, B. Resveratrol alleviates oxidative stress induced by oxidized soybean oil and improves gut function via changing gut microbiota in weaned piglets. J. Anim. Sci. Biotechnol. 2023, 14, 54. [Google Scholar] [CrossRef]
- Inagaki, A.; Sakata, T. Dose-dependent stimulatory and inhibitory effects of luminal and serosal n-butyric acid on epithelial cell proliferation of pig distal colonic mucosa. J. Nutr. Sci. Vitaminol. 2005, 51, 156–160. [Google Scholar] [CrossRef]
- Mishiro, T.; Kusunoki, R.; Otani, A.; Ansary, M.M.U.; Tongu, M.; Harashima, N.; Yamada, T.; Sato, S.; Amano, Y.; Itoh, K.; et al. Butyric acid attenuates intestinal inflammation in murine DSS-induced colitis model via milk fat globule-EGF factor 8. Lab. Investig. 2013, 93, 834–843. [Google Scholar] [CrossRef]
- De Keyser, K.; Dierick, N.; Kanto, U.; Hongsapak, T.; Buyens, G.; Kuterna, L.; Vanderbeke, E. Medium-chain glycerides affect gut morphology, immune- and goblet cells in post-weaning piglets: In vitro fatty acid screening with Escherichia coli and in vivo consolidation with LPS challenge. J. Anim. Physiol. Anim. Nutr. 2019, 103, 221–230. [Google Scholar] [CrossRef]
- Kovanda, L.; Rengman, S.; Tawde, S.; Pos, J.; Park, S.; Sun, S.H.; Park, J.; Kim, K.; Li, X.D.; Liu, Y.H. Dietary glycerides of valerate ameliorate diarrhea and impact intestinal physiology and serum biomarkers in weaned piglets infected with enterotoxigenic Escherichia coli F18. J. Anim. Sci. 2024, 102, skae322. [Google Scholar] [CrossRef] [PubMed]


| Items 1 | CG | EY1 | EY2 | EY3 |
|---|---|---|---|---|
| Ingredients | ||||
| Corn | 52.85 | 54.4 | 55.93 | 57.47 |
| Soybean meal | 9.08 | 8.75 | 8.43 | 8.10 |
| Wheat bra | 32.10 | 32.10 | 32.10 | 32.10 |
| Soybean oil | 3.64 | 2.42 | 1.21 | 0 |
| Stone powder | 1.30 | 1.30 | 1.30 | 1.30 |
| NaCl | 0.20 | 0.20 | 0.20 | 0.20 |
| L-Lysin (98%) | 0.03 | 0.03 | 0.03 | 0.03 |
| Compound premix 2 | 0.50 | 0.50 | 0.50 | 0.50 |
| Mold inhibitor | 0.30 | 0.30 | 0.30 | 0.30 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutrition levels 3 | ||||
| Net energy levels (MJ/kg) | 9.82 | 9.57 | 9.32 | 9.07 |
| Crude protein | 13.52 | 13.52 | 13.52 | 13.52 |
| Crude fiber | 3.74 | 3.77 | 3.8 | 3.84 |
| Crude fat | 6.94 | 5.80 | 4.67 | 3.53 |
| Ash | 2.79 | 2.79 | 2.78 | 2.77 |
| Calcium | 0.53 | 0.53 | 0.53 | 0.53 |
| Phosphorus | 0.54 | 0.55 | 0.55 | 0.55 |
| Lysine | 0.68 | 0.68 | 0.68 | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Wu, H.; Chai, H.; Zhao, D.; Peng, W.; Ji, F.; Zhang, L.; Lv, R. Moderate Reduction in Dietary Net Energy Level Enhances Intestinal Health in Tunchang Pigs via Gut Microbiota Modulation. Animals 2025, 15, 2836. https://doi.org/10.3390/ani15192836
Yu X, Wu H, Chai H, Zhao D, Peng W, Ji F, Zhang L, Lv R. Moderate Reduction in Dietary Net Energy Level Enhances Intestinal Health in Tunchang Pigs via Gut Microbiota Modulation. Animals. 2025; 15(19):2836. https://doi.org/10.3390/ani15192836
Chicago/Turabian StyleYu, Xilong, Hongzhi Wu, Haoliang Chai, Dexin Zhao, Weiqi Peng, Fengjie Ji, Lidong Zhang, and Renlong Lv. 2025. "Moderate Reduction in Dietary Net Energy Level Enhances Intestinal Health in Tunchang Pigs via Gut Microbiota Modulation" Animals 15, no. 19: 2836. https://doi.org/10.3390/ani15192836
APA StyleYu, X., Wu, H., Chai, H., Zhao, D., Peng, W., Ji, F., Zhang, L., & Lv, R. (2025). Moderate Reduction in Dietary Net Energy Level Enhances Intestinal Health in Tunchang Pigs via Gut Microbiota Modulation. Animals, 15(19), 2836. https://doi.org/10.3390/ani15192836





