Prevalence, Diagnosis, and Treatment of Ovarian Cysts in Bitches and Queens: A Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
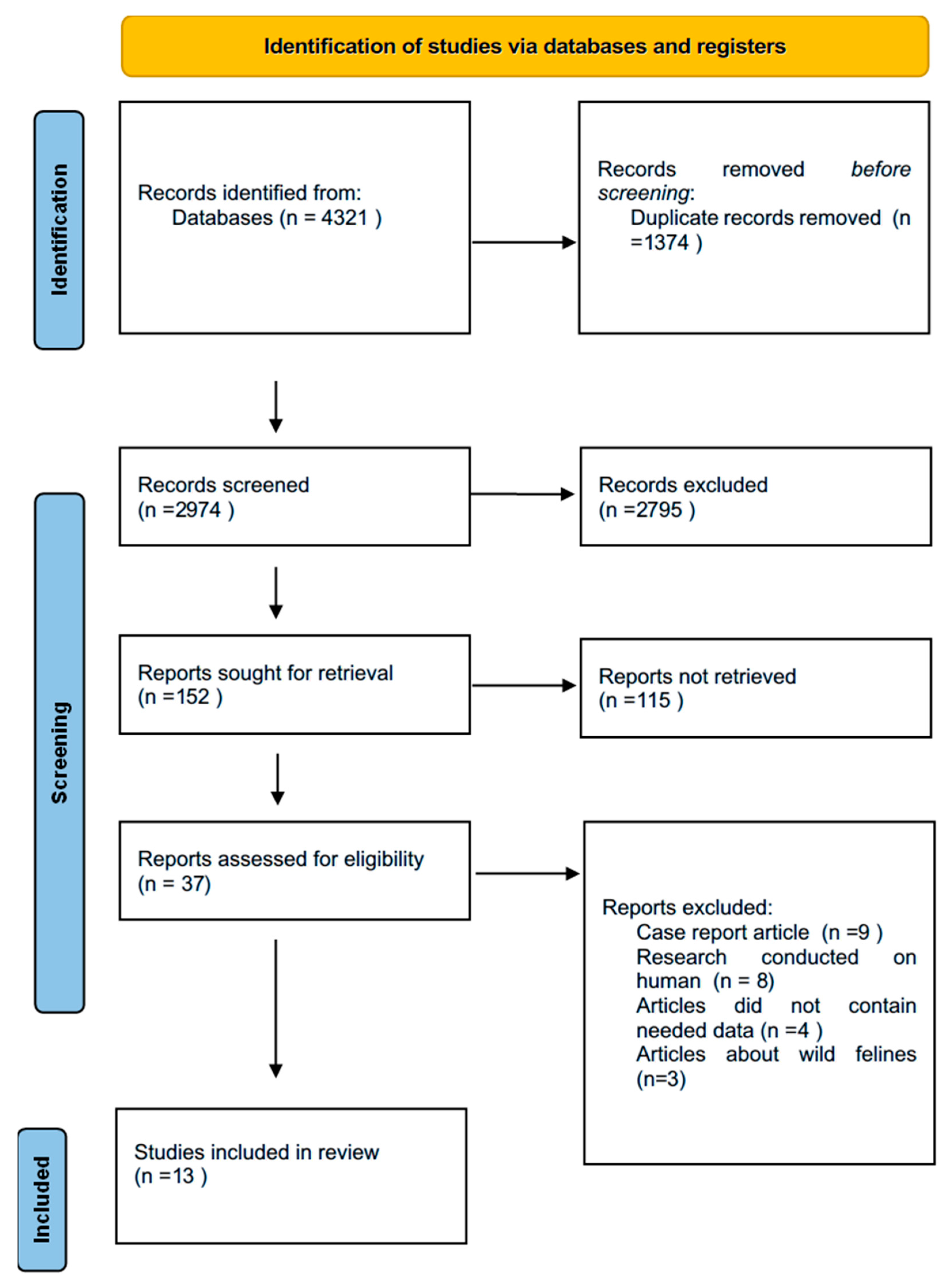
2.1. Study Selection
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Assessment of Study Quality and Risk of Bias
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Included Studies
3.2. Characteristics of the Study Population
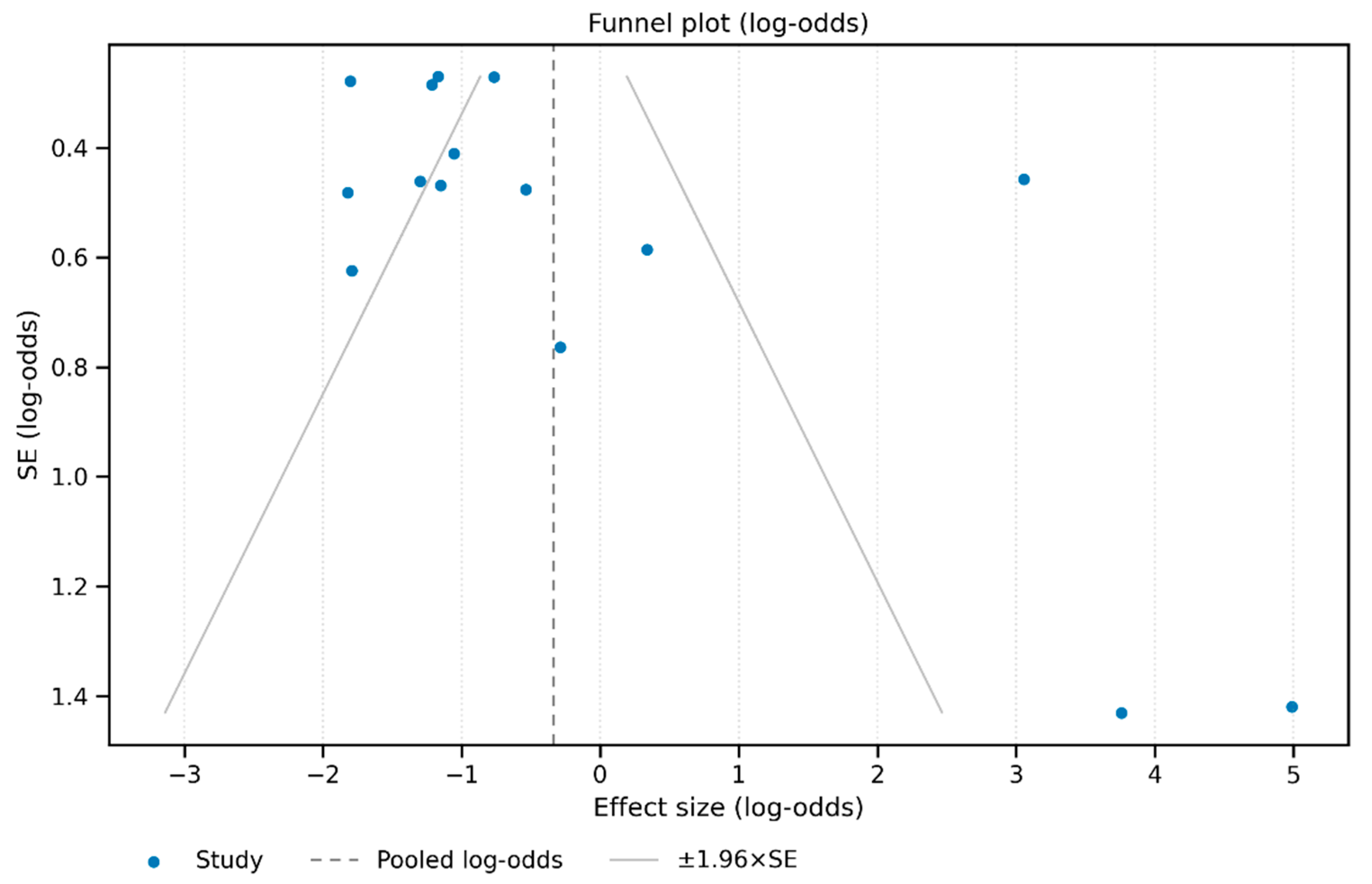
3.3. Risk of Bias and GRADE Evaluation
3.4. Ovarian Cysts
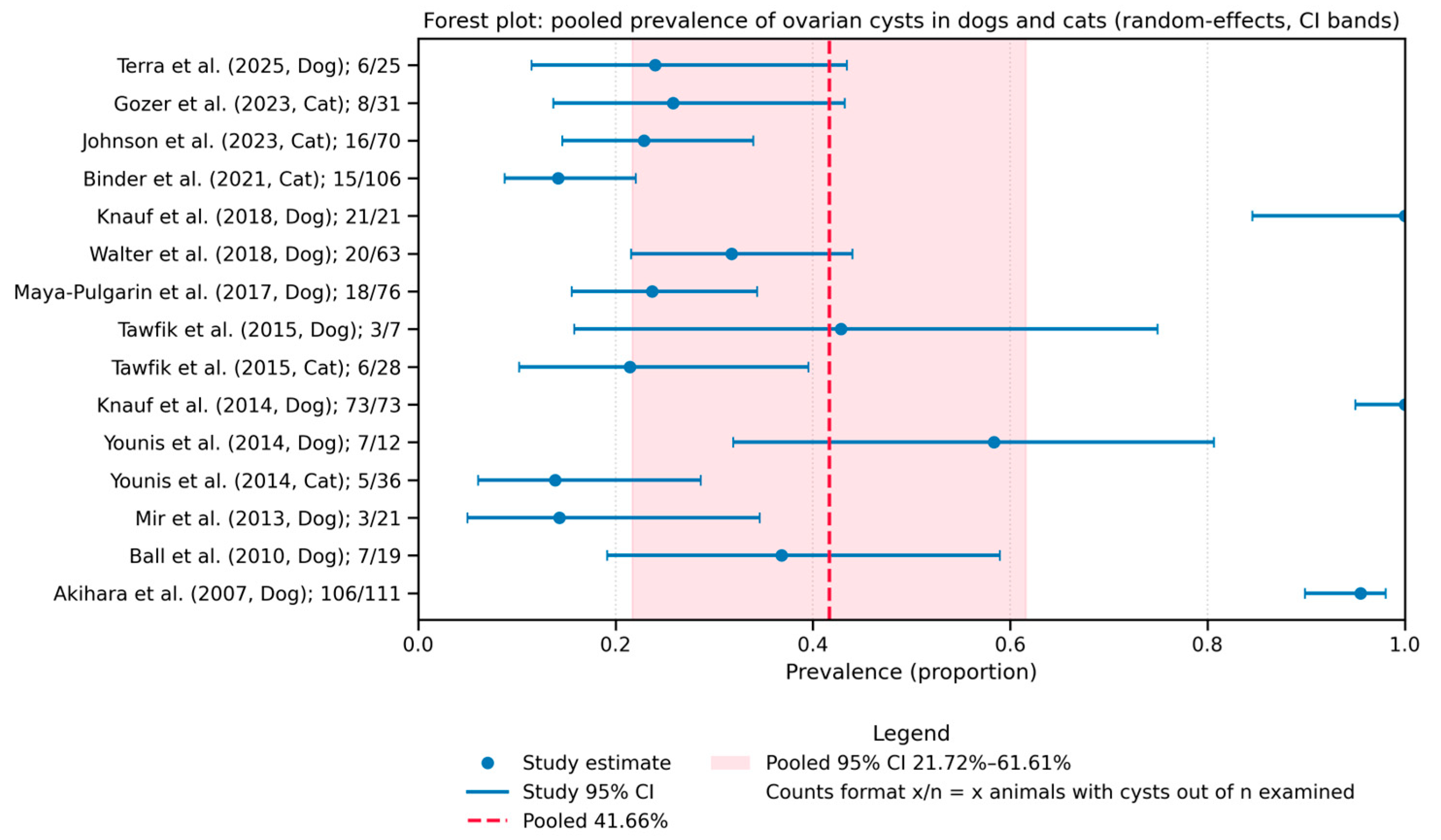
3.4.1. Prevalence of Ovarian Cysts
3.4.2. Species Comparison: Dog vs. Cat
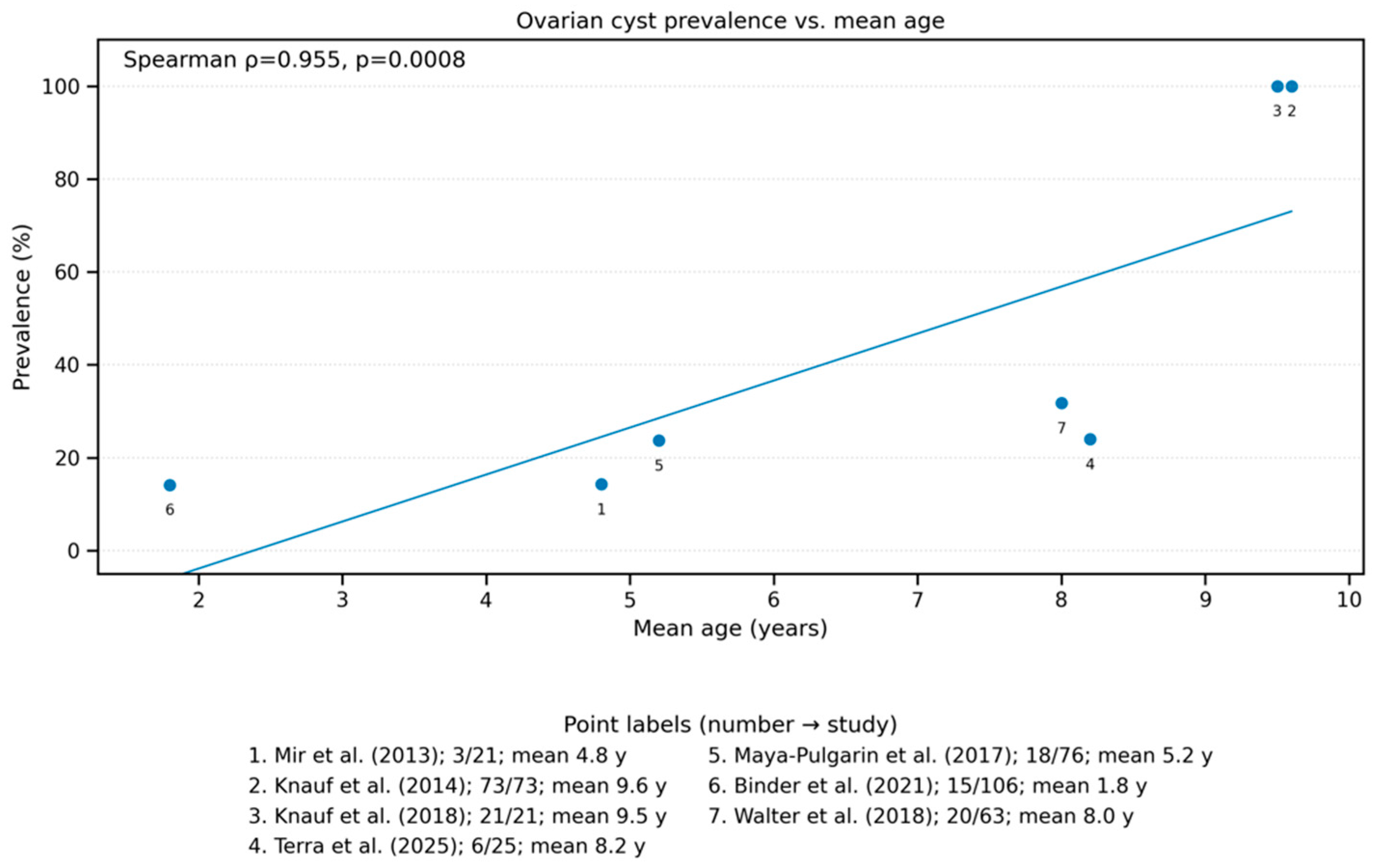
3.4.3. Association with Age
3.4.4. Most Common Cyst Types
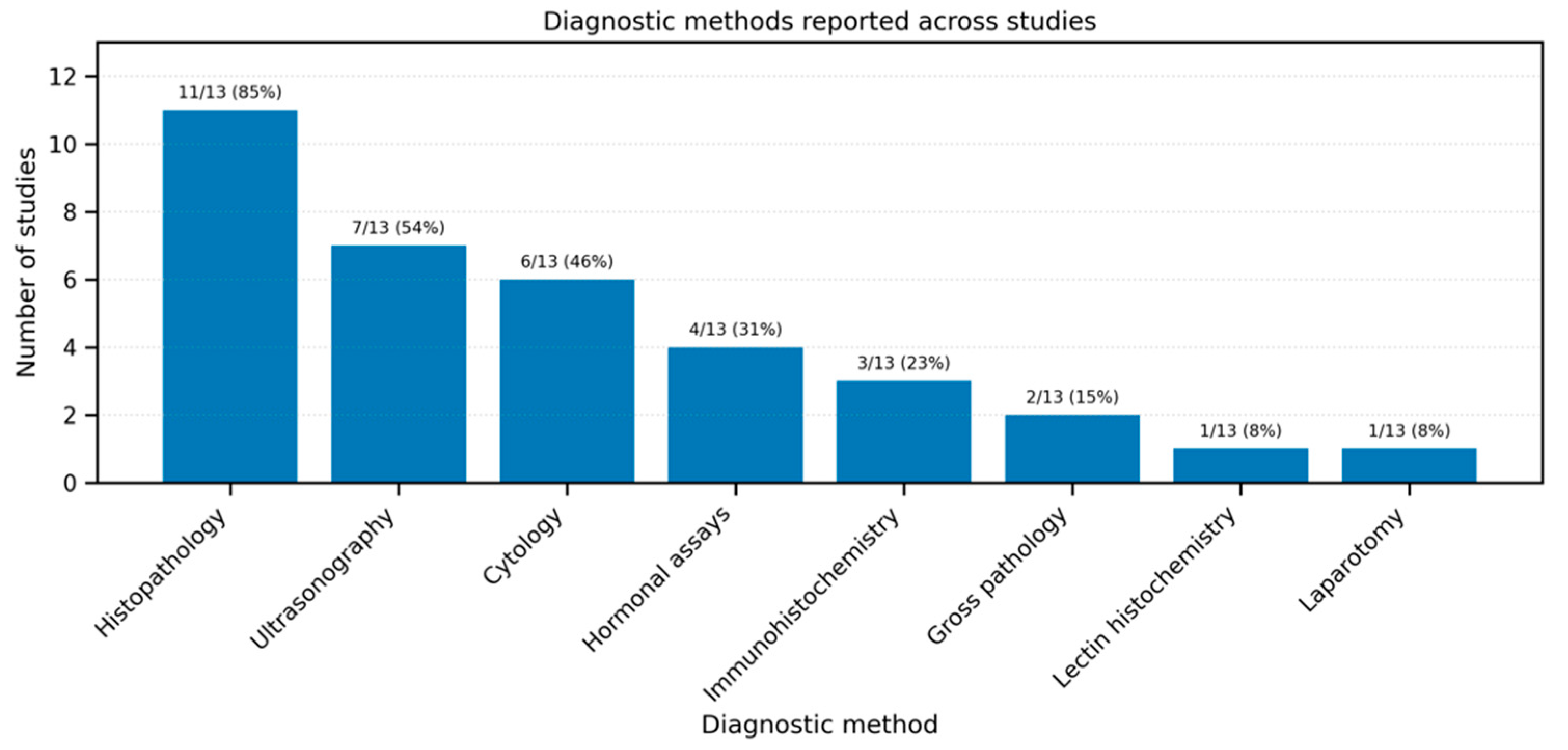
3.5. Diagnosis
3.6. Treatment of Ovarian Cysts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMH | Anti-Müllerian Hormone |
| CI | Confidence Interval |
| GnRH | Gonadotropin-Releasing Hormone |
| hCG | Human Chorionic Gonadotropin |
| NOS | Newcastle–Ottawa Scale |
| OR | Odds Ratio |
| OVH | Ovariohysterectomy |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| RCT | Randomized Controlled Trial |
| RoB-2 | Cochrane Risk of Bias 2 |
| RR | Risk Ratio |
References
- Knauf, Y.; Köhler, K.; Knauf, S.; Wehrend, A. Histological Classification of Canine Ovarian Cyst Types with Reference to Medical History. J. Vet. Sci. 2018, 19, 725. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, J.K.; Patra, M.K.; Singh, L.K.; Saxena, A.C.; De, U.K.; Singh, V.; Mathesh, K.; Kumar, H.; Krishnaswamy, N. Ovarian Cysts in the Bitch: An Update. Top. Companion Anim. Med. 2021, 43, 100511. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.D.; Root Kustritz, M.V.; Olson, P.S. Canine and Feline Theriogenology; Saunders: Philadelphia, PA, USA, 2001; ISBN 0721656072. [Google Scholar]
- Knauf, Y.; Bostedt, H.; Failing, K.; Knauf, S.; Wehrend, A. Gross Pathology and Endocrinology of Ovarian Cysts in Bitches. Reprod. Domest. Anim. 2014, 49, 463. [Google Scholar] [CrossRef] [PubMed]
- Meftakh, I.A.; Rybin, E.V.; Proshkin, V.M. Current Aspects of the Problem of Ovarian Cysts in Bitches of Reproductive Age. Leg. Regul. Vet. Med. 2024, 3, 56–59. [Google Scholar] [CrossRef]
- Knauf, Y.; Failing, K.; Knauf, S.; Wehrend, A. Treatment of Bitches with Ovarian Cysts Using Human Chorionic Gonadotropin-Releasing Hormone Analogon. Tierärztliche Praxis. Ausg. K Kleintiere/Heimtiere 2013, 41, 93–100. [Google Scholar]
- Pires, M.A.; Payan-Carreira, R. Ovarian Cysts in Dogs’ Practice. In Advances in Medicine and Biology. Chapter 4; Berhardt, L.V., Ed.; Nova Science Publishers: New York, NY, USA, 2016; Volume 94, ISBN 9781634841856. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Terra, J.P.; Almeida, T.B.; Rezende, L.P.O.; Barbeito, C.G.; Acuña, F.; Blume, G.R.; Eloi, R.S.A.; Oliveira, L.B.; Santos, A.L.R.M.; Sant’Ana, F.J.F. Lectin-Histochemical Pattern on the Cystic and Neoplastic Ovaries of Bitches. Reprod. Domest. Anim. 2025, 60, e70075. [Google Scholar] [CrossRef]
- Gozer, A.; Bahan, O.; Dogruer, G.; Kutlu, T. Serum Antimüllerian Hormone Concentrations in Female Cats. Relation with Ovarian Remnant Syndrome, Ovarian Cysts and Gonadectomy Status. Theriogenology 2023, 200, 106–113. [Google Scholar] [CrossRef]
- Johnson, A.K.; Jandrlich, D.; Joiner, K.; Martin, D.R. Observations about Declining Fertility in a Feline Breeding Colony. Theriogenology 2023, 212, 111–116. [Google Scholar] [CrossRef]
- Binder, C.; Reifinger, M.; Aurich, J.; Aurich, C. Histopathological Findings in the Uteri and Ovaries of Clinically Healthy Cats Presented for Routine Spaying. J. Feline Med. Surg. 2021, 23, 770–776. [Google Scholar] [CrossRef]
- Walter, B.; Coelfen, A.; Jäger, K.; Reese, S.; Meyer-Lindenberg, A.; Aupperle-Lellbach, H. Anti-Muellerian Hormone Concentration in Bitches with Histopathologically Diagnosed Ovarian Tumours and Cysts. Reprod. Domest. Anim. 2018, 53, 784–792. [Google Scholar] [CrossRef]
- Maya-Pulgarin, D.; Gonzalez-Dominguez, M.S.; Aranzazu-Taborda, D.; Mendoza, N.; Maldonado-Estrada, J.G. Histopathologic Findings in Uteri and Ovaries Collected from Clinically Healthy Dogs at Elective Ovariohysterectomy: A Cross-Sectional Study. J. Vet. Sci. 2017, 18, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, M.F.; Oda, S.S.; El-Neweshy, M.S.; El-Manakhly, E.S.M. Pathological Study on Female Reproductive Affections in Dogs and Cats at Alexandria Province, Egypt. Alex. J. Vet. Sci. 2015, 46, 74. [Google Scholar] [CrossRef]
- Younis, M.; Mohammed, F.F.; Ragab, R.S.; Gohar, H.M. Ultrasonography and Pathological Evaluation of Cystic Endometrial Hyperplasia Pyometra Complex in Bitches and Queens with Related Ovarian Alterations. Glob. Vet. 2014, 13, 60–67. [Google Scholar] [CrossRef]
- Mir, F.; Fontaine, E.; Albaric, O.; Greer, M.; Vannier, F.; Schlafer, D.H.; Fontbonne, A. Findings in Uterine Biopsies Obtained by Laparotomy from Bitches with Unexplained Infertility or Pregnancy Loss: An Observational Study. Theriogenology 2013, 79, 312–322. [Google Scholar] [CrossRef]
- Ball, R.L.; Birchard, S.J.; May, L.R.; Threlfall, W.R.; Young, G.S. Ovarian Remnant Syndrome in Dogs and Cats: 21 Cases (2000–2007). J. Am. Vet. Med. Assoc. 2010, 236, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Akihara, Y.; Shimoyama, Y.; Kawasako, K.; Komine, M.; Hirayama, K.; Kagawa, Y.; Omachi, T.; Matsuda, K.; Okamoto, M.; Kadosawa, T.; et al. Immunohistochemical Evaluation of Canine Ovarian Cysts. J. Vet. Med. Sci. 2007, 69, 1033–1037. [Google Scholar] [CrossRef][Green Version]
- Arlt, S.P.; Haimerl, P. Cystic Ovaries and Ovarian Neoplasia in the Female Dog—A Systematic Review. Reprod. Domest. Anim. 2016, 51, 3–11. [Google Scholar] [CrossRef]
- Sontas, B.H.; Milani, C.; Romagnoli, S.; Bertolini, G.; Caldin, M.; Caliari, D.; Zappulli, V.; Mollo, A. A Huge Ovarian Cyst in a Hysterectomized Bitch. Reprod. Domest. Anim. 2011, 46, 1107–1111. [Google Scholar] [CrossRef]
- Arlt, S.P.; Spankowsky, S.; Heuwieser, W. Follicular Cysts and Prolonged Oestrus in a Female Dog after Administration of a Deslorelin Implant. N. Z. Vet. J. 2011, 59, 87–91. [Google Scholar] [CrossRef]
- Robert Weedon, G.; Root Kustritz, M.V.; Bushby, P. Influence of Spay–Neuter Timing on Health. In High-Quality, High-Volume Spay and Neuter and Other Shelter Surgeries; Wiley: Hoboken, NJ, USA, 2019; pp. 509–520. [Google Scholar] [CrossRef]
- Graham, L. ACOG Releases Guidelines on Management of Adnexal Masses. Am. Fam. Physician 2008, 77, 1320–1323. [Google Scholar]
| Base | Keywords |
|---|---|
| PubMed | (“Ovarian Cysts”[MeSH] OR “ovarian cyst” OR “ovarian cysts”) AND (“dog” OR “bitch” OR “female dog”) AND (“prevalence” OR “diagnosis” OR “ultrasound” OR “treatment” OR “surgery”) |
| (“Ovarian Cysts”[MeSH] OR “ovarian cyst” OR “ovarian cysts”) AND (“cat” OR “queen” OR “female cat”) AND (“prevalence” OR “diagnosis” OR “ultrasound” OR “treatment” OR “surgery”) | |
| Ovarian Cysts[MeSH] OR “ovarian cyst” OR “ovarian cysts”) AND (“dog” OR “bitch” OR “female dog”) | |
| (“Ovarian Cysts”[MeSH] OR “ovarian cyst” OR “ovarian cysts”) AND (“cat” OR “queen” OR “female cat”) | |
| canine ovarian cysts treatment | |
| feline ovarian cysts treatment | |
| Scopus | TITLE-ABS-KEY(“ovarian cyst” OR “ovarian cysts” OR “cystic ovary”) AND TITLE-ABS-KEY(dog OR dogs OR “female dog” OR bitch OR canine OR canines OR cat OR cats OR queen OR queens OR feline OR felines) AND TITLE-ABS-KEY(prevalence OR diagnosis OR ultrasound OR histopathology OR treatment OR therapy OR surgery) |
| Google scholar | “ovarian cysts” AND (surgery OR “ovariohysterectomy” OR “hormonal treatment”) AND (dog OR bitch) |
| “ovarian cysts” AND (surgery OR “ovariohysterectomy” OR “hormonal treatment”) AND (cat OR queen) |
| No. | Author | Year | Country | Species | Number of Animals | Number of Animals with Ovarian Cysts | Follicular Cyst | Corpus Luteum Cyst | Rete Ovarii Cyst | Cysts of Subsurface Epithelial Structures | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Terra et al. [9] | 2025 | Brazil | Dog | 25 | 6 | 3 | 3 | 0 | 0 | 0 |
| 2 | Gozer et al. [10] | 2023 | Türkiye | Cat | 31 | 8 | No data | No data | No data | No data | No data |
| 3 | Johnson et al. [11] | 2023 | USA | Cat | 70 | 16 | No data | No data | No data | No data | No data |
| 4 | Binder et al. [12] | 2021 | Austria | Cat | 106 | 15 | 4 | 1 | 2 | 0 | 8 |
| 5 | Knauf et al. [1] | 2018 | Germany | Dog | 21 | 21 | 32 | 7 | 20 | 37 | 7 |
| 6 | Walter et al. [13] | 2018 | Germany | Dog | 63 | 20 | 8 | 7 | 0 | 5 | 0 |
| 7 | Maya-Pulgarin et al. [14] | 2017 | Columbia | Dog | 76 | 24 | 14 | 0 | 0 | 4 | 0 |
| 8 | Tawfik et al. [15] | 2015 | Egypt | Dog | 7 | 3 | 43 | 1 | 1 | 1 | 0 |
| Cat | 28 | 6 | 21 | 6 | 0 | 0 | |||||
| 9 | Knauf et al. [4] | 2014 | Germany | Dog | 73 | 73 | No data | No data | No data | No data | No data |
| 10 | Younis et al. [16] | 2014 | Egypt | Dog | 12 | 7 | 58 | 7 | 0 | 0 | 0 |
| Cat | 36 | 5 | 14 | 4 | 0 | 1 | 0 | ||||
| 11 | Mir et al. [17] | 2013 | France | Dog | 21 | 3 | 3 | 0 | 0 | 0 | 0 |
| 12 | Ball et al. [18] | 2010 | USA | Dog | 19 | 7 | 7 | 0 | 0 | 0 | 0 |
| Cat | 2 | ||||||||||
| 13 | Akihara et al. [19] | 2007 | Japan | Dog | 111 | 106 | 26 | 0 | 12 | 57 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domrazek, K.; Kondratek, K.; Tobolewski, F.; Jurka, P. Prevalence, Diagnosis, and Treatment of Ovarian Cysts in Bitches and Queens: A Meta-Analysis. Animals 2025, 15, 2800. https://doi.org/10.3390/ani15192800
Domrazek K, Kondratek K, Tobolewski F, Jurka P. Prevalence, Diagnosis, and Treatment of Ovarian Cysts in Bitches and Queens: A Meta-Analysis. Animals. 2025; 15(19):2800. https://doi.org/10.3390/ani15192800
Chicago/Turabian StyleDomrazek, Kinga, Katarzyna Kondratek, Filip Tobolewski, and Piotr Jurka. 2025. "Prevalence, Diagnosis, and Treatment of Ovarian Cysts in Bitches and Queens: A Meta-Analysis" Animals 15, no. 19: 2800. https://doi.org/10.3390/ani15192800
APA StyleDomrazek, K., Kondratek, K., Tobolewski, F., & Jurka, P. (2025). Prevalence, Diagnosis, and Treatment of Ovarian Cysts in Bitches and Queens: A Meta-Analysis. Animals, 15(19), 2800. https://doi.org/10.3390/ani15192800





